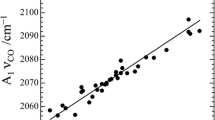
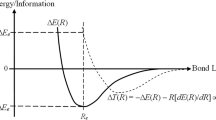
Abstract
The formation of electron donor-acceptor complexes is studied with global and local charge transfer partitionings. The 1-parabola model is applied to the bromination reaction of alkenes and the correlations found between the global and local charge transferred with the transition energy of the charge transfer bands and the kinetic rate constants indicate that the nucleophilic attack of alkenes to bromine is the electronic process controlling the reactivity in the formation of the electron donor-acceptor complexes in this reaction. The 2-parabolas model is used in studying the nitrosation of aromatic compounds where colorful electron donor-acceptor complexes are formed. In this case, and like previous applications of the 2-parabolas model, the consistent usage of the model mandates the explicit consideration of reaction conditions in preparing the reactants to have a direction of electron transfer that is consistent with the chemical potential differences. For the nitrosation reaction this implies considering the nitrosonium cation as the charge acceptor. Both applications support that the charge transferred predicted from chemical reactivity models can be used as a scale to measure the nucleophilicity in reactivity trends.

ᅟ










Similar content being viewed by others
References
Pearson RG (1989) Absolute electronegativity and hardness - applications to organic-chemistry. J Organomet Chem 54:1423–1430. https://doi.org/10.1021/jo00267a034
Pearson RG (1990) Hard and soft acids and bases - the evolution of a chemical concept. Coord Chem Rev 100:403–425. https://doi.org/10.1016/0010-8545(90)85016-l
Parr RG, Yang W (1994) Density functional theory of atoms and molecules. International series of monographs on chemistry. Oxford University Press, New York
Chermette H (1999) Chemical reactivity indexes in density functional theory. J Comput Chem 20:129–154. https://doi.org/10.1002/(Sici)1096-987x(19990115)20:1<129::Aid-Jcc13>3.0.Co;2-A
Parr RG, Von Szentpaly L, Liu SB (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924. https://doi.org/10.1021/ja983494x
Ayers PW, Levy M (2000) Perspective on "density functional approach to the frontier-electron theory of chemical reactivity". Theor Chem Acc 103:353–360
Parr RG, Yang W (1984) Perspective on "density functional approach to the frontier-electron theory of chemical reactivity". J Am Chem Soc 106:4049–4050
Ayers PW, Parr RG (2000) Variational principles for describing chemical reactions: the Fukui function and chemical hardness revisited. J Am Chem Soc 122:2010–2018. https://doi.org/10.1021/ja9924039
Geerlings P, De Proft F, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 103:1793–1873. https://doi.org/10.1021/cr990029p
Ayers PW, Anderson JSM, Bartolotti LJ (2005) Perturbative perspectives on the chemical reaction prediction problem. Int J Quantum Chem 101:520–534. https://doi.org/10.1002/qua.20307
Chattaraj PK, Sarkar U, Roy DR (2006) Electrophilicity index. Chem Rev 106:2065–2091. https://doi.org/10.1021/cr040109f
Chattaraj PK, Roy DR (2007) Update 1 of: electrophilicity index. Chem Rev 107:PR46–PR74. https://doi.org/10.1021/cr078014b
Gázquez JL (2008) Perspectives on the density functional theory of chemical reactivity. J Mex Chem Soc 52:3–10
Chattaraj PK (2009) Chemical reactivity theory: a density functional view. CRC, Boca Raton
Liu SB (2009) Conceptual density functional theory and some recent developments. Acta Phys Chim Sin 25:590–600. https://doi.org/10.3866/Pku.Whxb20090332
Roos G, Geerlings P, Messens J (2009) Enzymatic catalysis: the emerging role of conceptual density functional theory. J Phys Chem B 113:13465–13475. https://doi.org/10.1021/jp9034584
Chattaraj PK, Giri S, Duley S (2011) Update 2 of: electrophilicity index. Chem Rev 111:PR43–PR75. https://doi.org/10.1021/cr100149p
Orozco-Valencia AU, Vela A (2012) The electrodonating and electroaccepting powers in atoms. J Mex Chem Soc 56:294–301
Parr RG, Donnelly RA, Levy M, Palke WE (1978) Electronegativity - density functional viewpoint. J Chem Phys 68:3801–3807
Parr RG, Pearson RG (1983) Absolute hardness-companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516. https://doi.org/10.1021/Ja00364a005
Parr RG, Yang WT (1984) Density functional-approach to the frontier-electron theory of chemical-reactivity. J Am Chem Soc 106:4049–4050
Yang WT, Parr RG (1985) Hardness, softness, and the fukui function in the electronic theory of metals and catalysis. Proc Natl Acad Sci. U S A 82:6723–6726
Mulliken RS (1952) Molecular compounds and their spectra. 2. J Am Chem Soc 74:811–824. https://doi.org/10.1021/ja01123a067
Foster R (1980) Electron donor-acceptor complexes. J Phys Chem 84:2135–2141. https://doi.org/10.1021/j100454a006
Sennikov PG, Egorochkin AN (1982) Electronic spectroscopy of complexes with a charge-transfer as a method of studying intramolecular interactions in organic and hetero-organic compounds. Usp Khim 51:561–585
Kochi JK (1991) Charge-transfer excitation of molecular-complexes in organic and organometallic chemistry. Pure Appl Chem 63:255–264. https://doi.org/10.1351/pac199163020255
Fukuzumi S, Kochi JK (1982) Transition-state barrier for electrophilic reactions - solvation of charge-transfer ion-pairs as the unifying factor in alkene addition and aromatic-substitution with bromine. J Am Chem Soc 104:7599–7609. https://doi.org/10.1021/ja00390a035
Rosokha SV, Kochi JK (2002) The preorganization step in organic reaction mechanisms. Charge-transfer complexes as precursors to electrophilic aromatic substitutions. J Organomet Chem 67:1727–1737. https://doi.org/10.1021/jo011072r
Vasilyev AV, Lindeman SV, Kochi JK (2002) Molecular structures of the metastable charge-transfer complexes of benzene (and toluene) with bromine as the pre-reactive intermediates in electrophilic aromatic bromination. New J Chem 26:582–592. https://doi.org/10.1039/b110169m
Lenoir D (2003) The electrophilic substitution of arenes: Is the pi complex a key intermediate and what is its nature? Angewandte Chemie 42:854–857. https://doi.org/10.1002/anie.200390231
Piedras A, Gomez B, Carmona-Espindola J, Arroyo R, Gázquez JL (2016) Intramolecular charge transfer model in fluorescence processes. Theor Chem Accounts 135:9. https://doi.org/10.1007/s00214-016-1997-3
Gázquez JL, Cedillo A, Vela A (2007) Electrodonating and electroaccepting powers. J Phys Chem A 111:1966–1970. https://doi.org/10.1021/jp065459f
Perdew JP, Parr RG, Levy M, Balduz JL (1982) Density-functional theory for fractional particle number-derivative discontinuities of the energy. Phys Rev Lett 49:1691–1694. https://doi.org/10.1103/PhysRevLett.49.1691
Yang WT, Zhang YK, Ayers PW (2000) Degenerate ground states and a fractional number of electrons in density and reduced density matrix functional theory. Phys Rev Lett 84:5172–5175. https://doi.org/10.1103/PhysRevLett.84.5172
Ayers PW (2008) The dependence on and continuity of the energy and other molecular properties with respect to the number of electrons. J Math Chem 43:285–303. https://doi.org/10.1007/s10910-006-9195-5
Pearson RG (1963) Hard and soft acids and bases. J Am Chem Soc 85:3533–3539. https://doi.org/10.1021/ja00905a001
Pearson RG (1968) Hard and soft acids and bases HSAB.1. Fundamental principles. J Chem Educ 45:581–587
Pearson RG (1968) Hard and soft acids and bases HSAB.2. Underlying theories. J Chem Educ 45:643–648
Chattaraj PK, Lee H, Parr RG (1991) HSAB principle. J Am Chem Soc 113:1855–1856. https://doi.org/10.1021/ja00005a073
Chattaraj PK, Ayers PW (2005) The maximum hardness principle implies the hard/soft acid/base rule. J Chem Phys 123:2. https://doi.org/10.1063/1.2011395
Ayers PW, Parr RG, Pearson RG (2006) Elucidating the hard/soft acid/base principle: a perspective based on half-reactions. J Chem Phys 124:194107. https://doi.org/10.1063/1.2196882
Ayers PW (2007) The physical basis of the hard/soft acid/base principle. Faraday Discuss 135:161–190. https://doi.org/10.1039/b606877d
Chattaraj PK, Ayers PW, Melin J (2007) Further links between the maximum hardness principle and the hard/soft acid/base principle: insights from hard/soft exchange reactions. PCCP 9:3853–3856. https://doi.org/10.1039/b705742c
Moens J, Jaque P, De Proft F, Geerlings P (2008) The study of redox reactions on the basis of conceptual DFT principles: EEM and vertical quantities. J Phys Chem A 112:6023–6031. https://doi.org/10.1021/jp711652a
Chamorro E, Duque-Norena M, Perez P (2009) A comparison between theoretical and experimental models of electrophilicity and nucleophilicity. Theochem J Mol Struct 896:73–79. https://doi.org/10.1016/j.theochem.2008.11.009
Chattaraj PK, Chakraborty A, Giri S (2009) Net electrophilicity. J Phys Chem A 113:10068–10074. https://doi.org/10.1021/jp904674x
Chattaraj PK, Giri S (2009) Electrophilicity index within a conceptual DFT framework. In: Webb GA (ed) Annual reports on the progress of chemistry 2009, vol 105, Sect. C: physical chemistry, p 13. https://doi.org/10.1039/b802832j
De Proft F, Chamorro E, Perez P, Duque M, De Vleeschouwer F, Geerlings P (2009) Spin-polarized reactivity indices from density functional theory: theory and applications. Chemical modelling: applications and theory, vol 6. https://doi.org/10.1039/b812888j
Martinez A (2009) Donator acceptor map of psittacofulvins and anthocyanins: are they good antioxidant substances? J Phys Chem B 113:4915–4921. https://doi.org/10.1021/jp8102436
Martinez A (2009) Donator-acceptor map and work function for linear polyene-conjugated molecules. A density functional approximation study. J Phys Chem B 113:3212–3217. https://doi.org/10.1021/jp8106364
Chattaraj PK, Duley S (2010) Electron affinity, electronegativity, and electrophilicity of atoms and ions. J Chem Eng Data 55:1882–1886. https://doi.org/10.1021/je900892p
David J, Guerra D, Hadad CZ, Restrepo A (2010) Structure and Reactivity of the (Au6Pt)-Au-1 Clusters. J Phys Chem A 114:10726–10731. https://doi.org/10.1021/jp106544w
Domingo LR, Perez P (2011) The nucleophilicity N index in organic chemistry. Org Biomol Chem 9:7168–7175. https://doi.org/10.1039/c1ob05856h
Torres-Garcia E, Galano A, Rodriguez-Gattorno G (2011) Oxidative desulfurization (ODS) of organosulfur compounds catalyzed by peroxo-metallate complexes of WOx-ZrO2: thermochemical, structural, and reactivity indexes analyses. J Catal 282:201–208. https://doi.org/10.1016/j.jcat.2011.06.010
Martínez-González E, Frontana C (2014) Employment of electrodonating capacity as an index of reactive modulation by substituent effects: application for electron-transfer-controlled hydrogen bonding. J Organomet Chem 79:1131–1137. https://doi.org/10.1021/jo402565t
Miranda-Quintana RA, Gonzaleza MM, Ayers PW (2016) Electronegativity and redox reactions. PCCP 18:22235–22243. https://doi.org/10.1039/c6cp03213c
Xu BB, Li YZ, Song P, Ma FC, Sun MT (2017) Photoactive layer based on T-shaped benzimidazole dyes used for solar cell: from photoelectric properties to molecular design. Sci Rep 7:45688. https://doi.org/10.1038/srep45688
Orozco-Valencia AU, Gázquez JL, Vela A (2017) Global and local partitioning of the charge transferred in the Parr-Pearson model. J Phys Chem A 121:4019–4029. https://doi.org/10.1021/acs.jpca.7b01765
Orozco-Valencia U, Gázquez JL, Vela A (2017) Donation and back-donation analyzed through a charge transfer model based on density functional theory. J Mol Model 23:207–215. https://doi.org/10.1007/s00894-017-3368-y
Orozco-Valencia U, Gázquez JL, Vela A (2018) Reactivity of indoles through the eyes of a charge-transfer partitioning analysis. Acta Phys Chim Sin 34:692–698. https://doi.org/10.3866/pku.whxb201801121
Orozco-Valencia U, Gázquez JL, Vela A (2018) Role of reaction conditions in the global and local two parabolas charge transfer model. J Phys Chem A 122:1796–1806. https://doi.org/10.1021/acs.jpca.7b12001
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Accounts 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: the PBE0 model. J Chem Phys 110:6158–6170. https://doi.org/10.1063/1.478522
Adamo C, Scuseria GE, Barone V (1999) Accurate excitation energies from time-dependent density functional theory: assessing the PBE0 model. J Chem Phys 111:2889–2899. https://doi.org/10.1063/1.479571
Ernzerhof M, Scuseria GE (1999) Assessment of the Perdew-Burke-Ernzerhof exchange-correlation functional. J Chem Phys 110:5029–5036. https://doi.org/10.1063/1.478401
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, MCX L, Marenich A, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2009) Gaussian 09, revision D.01. Gaussian Inc., Wallingford
Yang W, Mortier WJ (1986) The use of global and local molecular-parameters for the analysis of the gas-phase basicity of amines. J Am Chem Soc 108:5708–5711. https://doi.org/10.1021/ja00279a008
Ayers PW, Morrison RC, Roy RK (2002) Variational principles for describing chemical reactions: Condensed reactivity indices. J Chem Phys 116:8731–8744. https://doi.org/10.1063/1.1467338
Bultinck P, Fias S, Van Alsenoy C, Ayers PW, Carbo-Dorca R (2007) Critical thoughts on computing atom condensed Fukui functions. J Chem Phys 127:11. https://doi.org/10.1063/1.2749518
Hirshfeld FL (1977) Bonded-atom fragments for describing molecular charge-densities. Theor Chim Acta 44:129–138. https://doi.org/10.1007/bf00549096
Glendening ED, Reed AE, Carpenter JE, Weinhold F (1995) NBO Version 3.1. University of Wisconsin, Madison
Reed AE, Weinstock RB, Weinhold F (1985) Natural-population analysis. J Chem Phys 83:735–746. https://doi.org/10.1063/1.449486
Yamada S, Bessho J, Nakasato H, Tsutsumi O (2018) Color tuning donor-acceptor-type azobenzene dyes by controlling the molecular geometry of the donor moiety. Dyes Pigments 150:89–96. https://doi.org/10.1016/j.dyepig.2017.11.002
Ramírez-Ramírez JZ, Vargas R, Garza J, Gázquez JL (2010) Simple charge-transfer model for metallic complexes. J Phys Chem A 114:7945–7951. https://doi.org/10.1021/jp100309c
Miranda-Quintana RA, Ayers PW (2016) Fractional electron number, temperature, and perturbations in chemical reactions. PCCP 18:15070–15080. https://doi.org/10.1039/c6cp00939e
Kim EK, Kochi JK (1991) Charge-transfer structures of aromatic electron donor-acceptor complexes leading to electron-transfer with the electrophilic nitrosonium cation. J Am Chem Soc 113:4962–4974. https://doi.org/10.1021/ja00013a036
Rosokha SV, Kochi JK (2001) Mechanism of inner-sphere electron transfer via charge-transfer (precursor) complexes. Redox energetics of aromatic donors with the nitrosonium acceptor. J Am Chem Soc 123:8985–8999. https://doi.org/10.1021/ja010859w
Acknowledgments
We thank the Cluster Híbrido Xiuhcoatl from Cinvestav and Laboratorio Nacional de Cómputo de Alto Desempeño (LANCAD) for providing computer time for this project. JLG thanks Conacyt for grant 237045, and AV thanks Conacyt for grant Fronteras 867. It is our great honor and pleasure to dedicate this contribution to the celebration of the 60th birthday of our great friend Prof. Pratim Chatarraj, with whom we have always enjoyed enlightening discussions and his deep thoughts about chemical reactivity within DFT. We wish him many more years of a happy, healthy, and scientifically productive life.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper belongs to Topical Collection International Conference on Systems and Processes in Physics, Chemistry and Biology (ICSPPCB-2018) in honor of Professor Pratim K. Chattaraj on his sixtieth birthday
Electronic supplementary material
ESM 1
(PDF 906 kb)
Rights and permissions
About this article
Cite this article
Orozco-Valencia, U., Gázquez, J.L. & Vela, A. Global and local charge transfer in electron donor-acceptor complexes. J Mol Model 24, 250 (2018). https://doi.org/10.1007/s00894-018-3772-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3772-y