Abstract
A series of high-quality single crystals of the formula NaxK1−xAlSiO4 were synthesized using a high temperature hydrothermal method. This enabled the detailed single crystal study of four examples of this class of compounds, namely KAlSiO4, Na0.10K0.90AlSiO4 Na3KAl4Si4O16 and NaAlSiO4. The potassium-containing species all had fully ordered AlO4 and SiO4 tetrahedral sites that led to formation of polar acentric structures. In contrast NaAlSiO4 displayed the unusual feature of an exceptionally large and complex unit cell along with complete disordering of the Al and Si sites. This led to the formation of a centrosymmetric structure, that is also a new polymorph of the NaAlSiO4 composition. The polymorphism of hydrothermal KAlSiO4 was also examined in light of the crystal’s synthetic and thermal histories. The study also revealed a structural sensitivity toward the degree of Na/K substitution in the lattice. The strong tendency to form polar acentric structures makes understanding these structures of great interest. These detailed structures resolved a considerable degree of previous structural ambiguity within this nominally simple class of compounds.
Graphical Abstract
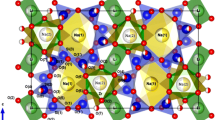
Structural subtleties are examined in the nepheline–kalsilite series of NaxK1−xAlSiO4, revealing changes in the resulting structure according to synthetic method, thermal history, and alkali metal substitution.









Similar content being viewed by others
Data Availability
CCDC 2106945–2106948 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre and FIZ Karlsruhe via www.ccdc.cam.ac.uk/data_request/cif. The authors declare that all other data supporting the findings of this study are available within the article and its Supplementary Information files.
References
Palmer DC (1994) Stuffed derivatives of the silica polymorphs. Rev Mineral Geochem 29(1):83–122
Takada A, Glaser KJ, Bell RG, Catlow CRA (2018) Molecular dynamics study of tridymite. IUCrJ 5(3):325–334. https://doi.org/10.1107/S2052252518004803
Buerger MJ (1954) The stuffed derivatives of the silica structures. Am Mineral 39:600–614
Morris RV, Vaniman DT, Blake DF, Gellert R, Chipera SJ, Rampe EB, Ming DW, Morrison SM, Downs RT, Treiman AH, Yen AS, Grotzinger JP, Achilles CN, Bristow TF, Crisp JA, Marais DJD, Farmer JD, Fendrich KV, Frydenvang J, Graff TG, Morookian J-M, Stolper EM, Schwenzer SP (2016) Silicic volcanism on mars evidenced by tridymite in high-SiO2 sedimentary rock at gale crater. Proc Natl Acad Sci USA 113(26):7071–7076. https://doi.org/10.1073/pnas.1607098113
Hippler B, Böhm H (1989) Structure investigation on sodium-nephelines. Z Kristallogr Cryst Mater 187:39–53
Brown WL, Cesbron F (1972) Trinepheline; a new synthetic modification in the nepheline group. Z Kristallogr Cryst Mater. https://doi.org/10.1524/zkri.1972.136.16.468
Klaska KH (1974) Strukturuntersuchugen an Tribymitabkommlingen. PhD Thesis, Universitat Hamburg, Germany
von Jarchow O, Reese HH, Saalfeld H (1966) Hydrothermalsynthesen von Zeolithen Der Sodalith-Und Cancrinitgruppe. Neues Jahrb Mineral Mh 10:289–297
Selker P, Bartsh HH, Klaska R (1985) Struktur Und Hydrothermalsynthesen von NaAlSiO4-Modifikationen. Z Kristallogr 170:175–176
Kahlenberg V, Bohm H (1998) Crystal structure of hexagonal trinepheline—a new synthetic NaAlSiO4 modification. Am Mineral 83:631–637
Vulic P, Kahlenberg V, Konzett J (2008) On the existence of a Na-deficient monoclinic trinepheline with composition Na7.85Al7.85Si8.15O32. Am Mineral 93(7):1072–1079. https://doi.org/10.2138/am.2008.2702
Balassone G, Kahlenberg V, Altomare A, Mormone A, Rizzi R, Saviano M, Mondillo N (2014) Nephelines from the Somma-Vesuvius Volcanic Complex (Southern Italy): crystal-chemical, structural and genetic investigations. Mineral Petrol 108(1):71–90. https://doi.org/10.1007/s00710-013-0290-6
Gregorkiewitz M (1984) Crystal structure and Al/Si-ordering of a synthetic nepheline. Bull minéral 107(3–4):499–507
Merlino S, Franco E, Mattia C, Pasero M, De Gennaro M (1985) The crystal structure of panunzite (natural tetrakalsilite). Neues Jahrb Mineral 7:322–328
Cellai D, Bonazzi P, Carpenter MA (1997) Natural kalsilite KAlSiO4 with P31c symmetry: crystal structure and twinning. Am Mineral 82:276–279
Andou Y, Kawahara A (1984) The refinement of the structure of synthetic kalsilite. Mineral J 12(4):153–161. https://doi.org/10.2465/minerj.12.153
Becerro AI, Escudero A, Mantovani M (2009) The hydrothermal conversion of kaolinite to kalsilite: influence of time, temperature, and pH. Am Mineral 94(11–12):1672–1678. https://doi.org/10.2138/am.2009.3284
Dollase WA, Freeborn WP (1977) The structure of KAlSiO4 with P63mc symmetry. Am Mineral 62:420–421
Gregorkiewitz M, Li Y, White TJ, Withers RL, Sobrados I (2008) The structure of “Orthorhombic” KAlSiO4–O1: evidence for Al–Si order from MAS NMR data combined with Rietveld refinement and electron microscopy. Can Mineral 46(6):1511–1526. https://doi.org/10.3749/canmin.46.6.1511
Khomyakov AP, Nechelyustov GN, Sokolova E, Bonaccorsi E, Merlino S, Pasero M (2002) Megakalsilite, a new polymorph of KAlSiO4 from the Khibina alkaline massif, Kola Peninsula, Russia: mineral description and crystal structure. Can Mineral 40(3):961–970. https://doi.org/10.2113/gscanmin.40.3.961
Byrappa K, Yoshimura M (2001) Handbook of hydrothermal technology: a technology for crystal growth and materials processing. Noyes Publications, Norwich
Halasyamani PS, Poeppelmeier KR (1998) Noncentrosymmetric oxides. Chem Mater 10(10):2753–2769. https://doi.org/10.1021/cm980140w
Tran TT, Yu H, Rondinelli JM, Poeppelmeier KR, Halasyamani PS (2016) Deep ultraviolet nonlinear optical materials. Chem Mater 28(15):5238–5258. https://doi.org/10.1021/acs.chemmater.6b02366
Winkler HGF (1947) On the synthesis of nepheline. Am Mineral 32:131–136
Iwasaki F, Iwasaki H (2002) Historical review of quartz crystal growth. J Cryst Growth 237:820–827. https://doi.org/10.1016/S0022-0248(01)02043-7
Thompson RJ (2007) Crystal clear: the struggle for reliable communications technology in World War II. IEEE Press, Wiley-Interscience, Hoboken
Becker P (1998) Borate materials in nonlinear optics. Adv Mater 10(13):979–992. https://doi.org/10.1002/(SICI)1521-4095(199809)10:13%3c979::AID-ADMA979%3e3.0.CO;2-N
Terry RJ, Vinton D, McMillen CD, Kolis JW (2018) A cesium rare-earth silicate Cs3RESi6O15 (RE=Dy–Lu, Y, In): the parent of an unusual structural class featuring a remarkable 57 Å unit cell axis. Angew Chem Int Ed 57(8):2077–2080. https://doi.org/10.1002/anie.201708798
McMillen DC, Kolis WJ (2016) Hydrothermal synthesis as a route to mineralogically-inspired structures. Dalton Trans 45(7):2772–2784. https://doi.org/10.1039/C5DT03424H
Fulle K, Sanjeewa LD, McMillen CD, Kolis JW (2017) Crystal chemistry and the role of ionic radius in rare earth tetrasilicates: Ba2RE2Si4O12F2 (RE = Er3+–Lu3+) and Ba2RE2Si4O13 (RE = La3+–Ho3+). Acta Crystallogr B 73(5):907–915. https://doi.org/10.1107/S2052520617009544
Mann JM, McMillen CD, Kolis JW (2015) Crystal chemistry of alkali thorium silicates under hydrothermal conditions. Cryst Growth Des 15(6):2643–2651. https://doi.org/10.1021/cg5017164
Lee C-S, Lin C-H, Wang S-L, Lii K-H (2010) [Na7UIVO2(UVO)2(UV/VIO2)2Si4O16]: a mixed-valence uranium silicate. Angew Chem Int Ed 49(25):4254–4256. https://doi.org/10.1002/anie.201001095
Terry RJ, McMillen CD, Chen X, Wen Y, Zhu L, Chumanov G, Kolis JW (2018) Hydrothermal single crystal growth and second harmonic generation of Li2SiO3, Li2GeO3 and Li2Si2O5. J Cryst Growth 493:58–64. https://doi.org/10.1016/j.jcrysgro.2018.02.028
Bruker (2015) APEX3. Bruker AXS, Madison
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr C 71(1):3–8. https://doi.org/10.1107/S2053229614024218
Momma K, Izumi F (2011) VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J Appl Crystallogr 44(6):1272–1276. https://doi.org/10.1107/S0021889811038970
Tuttle OF, Smith JV (1958) The nepheline–kalsilite system; II, phase relations. Am J Sci 256(8):571–589. https://doi.org/10.2475/ajs.256.8.571
Bannister FA, Hey MH (1931) A chemical, optical, and X-ray study of nepheline and kaliophilite. Mineral Mag 22(134):569–608. https://doi.org/10.1180/minmag.1931.022.134.03
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A 32(5):751–767. https://doi.org/10.1107/S0567739476001551
Kawahara A, Andou Y, Marumo F, Okuno M (1987) The crystal structure of high temperature form of kalsilite (KAlSiO4) at 950°C. Mineral J 13(5):260–270. https://doi.org/10.2465/minerj.13.260
Cellai D, Gesing T, Wruck B, Carpenter AM (1999) X-ray study of the trigonal → hexagonal phase transition in metamorphic kalsilite. Am Mineral. https://doi.org/10.2138/am-1999-11-1223
Simmons WB, Peacor DR (1972) Refinement of the crystal structure of a volcanic nepheline. Am Mineral 57(11–12):1711–1719
Foreman N, Peacor DR (1970) Refinement of the nepheline structure at several temperatures. Z Kristallogr Cryst Mater 132(1–6):45–70. https://doi.org/10.1524/zkri.1970.132.16.45
Buerger MJ, Klein GE, Donnay G (1954) Determination of the crystal structure of nepheline. Am Mineral 39:805–818
Antao SM, Hassan I (2010) Nepheline: structure of three samples from the Bancroft area, Ontario, obtained using synchrotron high-resolution powder X-ray diffraction. Can Mineral 48(1):69–80. https://doi.org/10.3749/canmin.48.1.69
Hassan I, Antao SM, Hersi AAM (2003) Single-crystal XRD, TEM, and thermal studies of the satellite reflections in nepheline. Can Mineral 41(3):759–783. https://doi.org/10.2113/gscanmin.41.3.759
Henderson CMB, Roux J (1977) Inversions in sub-potassic nephelines. Contrib Mineral Petrol 61(3):279–298. https://doi.org/10.1007/BF00376702
Henderson CMB, Thompson AB (1980) The low-temperature inversion in sub-potassic nephelines. Am Mineral 65:970–980
Schneider H, Flörke OW, Stoeck R (1994) The NaAlSiO4 nepheline–carnegieite solid-state transformation. Z Kristallogr Cryst Mater 209(2):113–117
Liebau F (1985) Structural chemistry of silicates: structure, bonding, and classification. Springer, Berlin
Gatta GD, Angel RJ, Carpenter MA (2010) Low-temperature behavior of natural kalsilite with P31c symmetry: an in situ single-crystal X-ray diffraction study. Am Mineral 95(7):1027–1034. https://doi.org/10.2138/am.2010.3478
Gatehouse BM (1989) Structure of CsAlTiO4—a compound with TiO4 tetrahedra. Acta Crystallogr C 45(11):1674–1677. https://doi.org/10.1107/S010827018900418X
Acknowledgements
We are indebted to the National Science Foundation NSF-DMR1808371 for financial support of the synthesis and crystal growth.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CDM is an Associate Editor with Journal of Chemical Crystallography, and was excluded from the peer review process.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Terry, R.J., McMillen, C.D. & Kolis, J.W. Hydrothermal Single Crystal Growth and Structural Investigation of the Nepheline and Kalsilite Stuffed Tridymite Species. J Chem Crystallogr 53, 25–37 (2023). https://doi.org/10.1007/s10870-022-00940-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-022-00940-6