Abstract
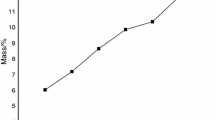
A santabarbaraite mineral sample from Italy has been examined by thermal analysis, scanning electron microscopy with energy dispersive spectroscopy and vibrational spectroscopy. Chemical composition shows a Fe phosphate phase with minor amounts of Mn, Mg, K and Na were also observed. The santabarbaraite mineral was characterized by thermal analysis. Thermogravimetric analysis of the santabarbaraite mineral were obtained by using TA Instruments Inc. Q50 high-resolution TGA operating at a 10 °C min−1 ramp with data sample interval of 0.50 s pt−1 from room temperature to 1000 °C in a high-purity flowing nitrogen atmosphere (100 cm3 min−1). Two mass loss steps are observed at 105 and 364.8 °C and are attributed to dehydration and dehydroxylation. Not all phosphate units are identical in the structure of santabarbaraite. This is reflected in the width of both the Raman and infrared bands. Two strong broad Raman bands observed at 1007 and 1095 cm−1 are assigned to the phosphate ν 1 symmetric and ν 3 antisymmetric stretching mode. Raman bands observed at 561, 592 and 630 cm−1 are assigned to the ν 4 out of plane bending modes of the phosphate units. The observation of multiple bands supports the concept of non-equivalent phosphate units in the structure. Broad Raman bands observed at 3544 and 3611 cm−1 are attributed to the OH stretching vibrations of the hydroxyl units. Vibrational spectroscopy enables subtle details of the molecular structure of santabarbaraite to be determined. Thermal analysis characterises the stability of the mineral.







Similar content being viewed by others
References
Chukanov NV. Mineralogical almanac famous mineral localities series Kerch iron–ore basin. 2005. p. 112.
Pratesi G, Cipriani C, Giuli G, Birch WD. Santabarbaraite: a new amorphous phosphate mineral. Eur J Miner. 2003;15(1):185–92. doi:10.1127/0935-1221/2003/0015-0185.
Kolitsch U, Bernhardt H, Lengauer CL, Blass G, Tillmanns E. Allanpringite, Fe3(PO4)2(OH)3·5H2O, a new ferric iron phosphate from Germany, and its close relation to wavellite. Eur J Miner. 2006;18(6):793–801. doi:10.1127/0935-1221/2006/0018-0793.
Capitelli F, Ventura GD, Bellatreccia F, Sodo A, Saviano M, Ghiara MR, et al. Crystal-chemical study of wavellite from Zbirov, Czech Republic. Miner Mag. 2014;78:1057–70.
Liu Q, Zhang S, Cheng H, Wang D, Li X, Hou X, et al. Thermal behavior of kaolinite-urea intercalation complex and molecular dynamics simulation for urea molecule orientation. J Therm Anal Calorim. 2014;. doi:10.1007/s10973-014-3646-1.
Cheng H, Liu Q, Zhang S, Wang S, Frost RL. Evolved gas analysis of coal-derived pyrite/marcasite. J Therm Anal Calorim. 2014;116(2):887–94. doi:10.1007/s10973-013-3595-0.
Theiss FL, Ayoko GA, Frost RL. Thermogravimetric analysis of selected layered double hydroxides. J Therm Anal Calorim. 2013;112(2):649–57. doi:10.1007/s10973-012-2584-z.
Frost RL, Xi Y. Thermogravimetric analysis of the copper silicate mineral dioptase Cu6[Si6O18]·6H2O. J Therm Anal Calorim. 2013;112(2):615–9. doi:10.1007/s10973-012-2599-5.
Frost RL, Xi Y. Thermoanalytical study of the minerals apophyllite-(KF) KCa4Si8O20F·8H2O and apophyllite-(KOH) KCa4Si8O20(F, OH)·8H2O. J Therm Anal Calorim. 2013;112(2):607–14. doi:10.1007/s10973-012-2615-9.
Frost RL, Palmer SJ, Pogson RE. Thermal stability of crandallite CaAl3(PO4)2(OH)5·(H2O). J Therm Anal Calorim. 2012;107(3):905–9. doi:10.1007/s10973-011-1578-6.
Frost RL, Palmer SJ, Pogson RE. Thermal stability of newberyite Mg(PO3OH)·3H2O. J Therm Anal Calorim. 2012;107(3):1143–6. doi:10.1007/s10973-011-1593-7.
Frost RL, Palmer SJ, Pogson R. Thermal stability of the cave’ mineral ardealite Ca2(HPO4)(SO4)·4H2O. J Therm Anal Calorim. 2012;107(2):549–53. doi:10.1007/s10973-011-1458-0.
Lopez A, Frost RL, Xi Y, Scholz R. Vibrational spectroscopic characterization of the sulphate-carbonate mineral burkeite: implications for evaporites. Spectrosc Lett. 2014;47(7):564–70. doi:10.1080/00387010.2013.825273.
Lopez A, Frost RL, Xi Y, Scholz R. A vibrational spectroscopic study of the sulfate mineral glauberite. Spectrosc Lett. 2014;47(10):740–5. doi:10.1080/00387010.2013.841254.
Lopez A, Frost RL, Xi Y, Scholz R. Vibrational spectroscopic characterization of the arsenate mineral barahonaite: implications for the molecular structure. Spectrosc Lett. 2014;47(7):571–8. doi:10.1080/00387010.2013.825274.
Breitinger DK, Belz HH, Hajba L, Komlosi V, Mink J, Brehm G, et al. Combined vibrational spectra of natural wardite. J Mol Struct. 2004;706(1–3):95–9. doi:10.1016/j.molstruc.2004.01.039.
Acknowledgements
The financial and infra-structure support of the Discipline of Nanotechnology and Molecular Science, Science and Engineering Faculty of the Queensland University of Technology, is gratefully acknowledged. The Australian Research Council (ARC) is thanked for funding the instrumentation. The authors would like to acknowledge the Center of Microscopy at the Universidade Federal de Minas Gerais (http://www.microscopia.ufmg.br) for providing the equipment and technical support for experiments involving electron microscopy. R. Scholz thanks to CNPq—Conselho Nacional de Desenvolvimento Científico e Tecnológico (Grant Nos. 306287/2012-9 and 402852/2012-5) and PROPP/UFOP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frost, R.L., Scholz, R., Ruan, X. et al. A thermogravimetric, scanning electron microscope and vibrational spectroscopic study of the phosphate mineral santabarbaraite from Santa Barbara mine, Tuscany, Italy. J Therm Anal Calorim 124, 639–644 (2016). https://doi.org/10.1007/s10973-015-5183-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-5183-y