Abstract
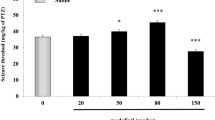
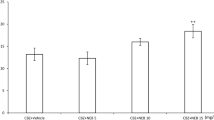
N-(2-hydroxyethyl) nicotinamide nitrate (nicorandil), a nitrate that activates adenosine triphosphate (ATP)-sensitive potassium (KATP) channels, is generally used in the treatment of angina and offers long-term cardioprotective effects. It has been reported that several KATP channel openers can effectively alleviate the symptoms of seizure. The purpose of this study was to investigate the improvement in seizures induced by nicorandil. In this study, seizure tests were used to evaluate the effect of different doses of nicorandil by analysing seizure incidence, including minimal clonic seizure and generalised tonic–clonic seizure. We used a maximal electroshock seizure (MES) model, a metrazol maximal seizure (MMS) model and a chronic pentylenetetrazol (PTZ)-induced seizure model to evaluate the effect of nicorandil in improving seizures. Each mouse in the MES model was given an electric shock, while those in the nicorandil group received 0.5, 1, 2, 3 and 6 mg/kg of nicorandil by intraperitoneal injection, respectively. In the MMS model, the mice in the PTZ group and the nicorandil group were injected subcutaneously with PTZ (90 mg/kg), and the mice in the nicorandil group were injected intraperitoneally with 1, 3 and 5 mg/kg nicorandil, respectively. In the chronic PTZ-induced seizure model, the mice in the PTZ group and the nicorandil group were injected intraperitoneally with PTZ (40 mg/kg), and the mice in the nicorandil group were each given 1 and 3 mg/kg of PTZ at a volume of 200 nL. Brain slices containing the hippocampus were prepared, and cell-attached recording was used to record the spontaneous firing of pyramidal neurons in the hippocampal CA1 region. Nicorandil (i.p.) significantly increased both the maximum electroconvulsive protection rate in the MES model and the seizure latency in the MMS model. Nicorandil infused directly onto the hippocampal CA1 region via an implanted cannula relieved symptoms in chronic PTZ-induced seizures. The excitability of pyramidal neurons in the hippocampal CA1 region of the mice was significantly increased after both the acute and chronic administration of PTZ. To a certain extent, nicorandil reversed the increase in both firing frequency and proportion of burst spikes caused by PTZ (P < 0.05). Our results suggest that nicorandil functions by downregulating the excitability of pyramidal neurons in the hippocampal CA1 region of mice and is a potential candidate for the treatment of seizures.





Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this article.
Abbreviations
- NMDA:
-
N-methyl-D-aspartic acid
- EEG:
-
Electroencephalogram
- GABA:
-
γ-Aminobutyric acid
- ATP:
-
Adenosine triphosphate
- KATP channels:
-
ATP-sensitive potassium channels
- mitoKATP channels:
-
Mitochondrial KATP channel
- Nicorandil:
-
N-(2-hydroxyethyl) nicotinamide nitrate
- NO:
-
Nitric oxide
- GABAA :
-
γ-Aminobutyric acid type A
- DMSO:
-
Dimethylsulfoxide
- MES:
-
Maximal electroshock seizure model
- MMS:
-
Metrazol maximal seizure model
- PTZ:
-
Pentylenetetrazol
- GTCS:
-
Generalized tonic clonic seizure
- MCS:
-
Minimal clonic seizure
- ACSF:
-
Rtificial cerebrospinal fluid
- ISI:
-
Interval between adjacent spikes
References
Lin JJ, Mula M, Hermann BP (2012) Uncovering the neurobehavioural comorbidities of epilepsy over the lifespan. Lancet 380(9848):1180–1192. https://doi.org/10.1016/S0140-6736(12)61455-X
Pitkänen A, Löscher W, Vezzani A, Becker AJ, Simonato M, Lukasiuk K et al (2016) Advances in the development of biomarkers for epilepsy. Lancet Neurol 15(8):843–856. https://doi.org/10.1016/S1474-4422(16)00112-5
Dehkordi HT, Bijad E, Saghaei E, Korrani MS, Amini-Khoei H (2022) Chronic stress but not acute stress decreases the seizure threshold in PTZ-induced seizure in mice: role of inflammatory response and oxidative stress. Naunyn Schmiedebergs Arch Pharmacol. https://doi.org/10.1007/s00210-022-02364-7
Sokolova TV, Zabrodskaya YM, Litovchenko AV, Paramonova NM, Kasumov VR, Kravtsova SV et al (2022) Relationship between neuroglial apoptosis and neuroinflammation in the epileptic focus of the brain and in the blood of patients with drug-resistant epilepsy. Int J Mol Sci 23(20):12561. https://doi.org/10.3390/ijms232012561
Henshall DC, Simon RP (2005) Epilepsy and apoptosis pathways. J Cereb Blood Flow Metab 25(12):1557–1572. https://doi.org/10.1038/sj.jcbfm.9600149
Haj-Mirzaian A, Ramezanzadeh K, Tafazolimoghadam A, Kazemi K, Nikbakhsh R, Nikbakhsh R et al (2019) Protective effect of minocycline on LPS-induced mitochondrial dysfunction and decreased seizure threshold through nitric oxide pathway. Eur J Pharmacol. 858:172446. https://doi.org/10.1016/j.ejphar.2019.172446
Vezzani A, French J, Bartfai T, Baram TZ (2011) The role of inflammation in epilepsy. Nat Rev Neurol 7(1):31–40. https://doi.org/10.1038/nrneurol.2010.178
Akyuz E, Polat AK, Eroglu E, Kullu I, Angelopoulou E, Paudel YN (2021) Revisiting the role of neurotransmitters in epilepsy: an updated review. Life sci 265:118826. https://doi.org/10.1016/j.lfs.2020.118826
Morimoto K, Fahnestock M, Racine RJ (2004) Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol 73(1):1–60. https://doi.org/10.1016/j.pneurobio.2004.03.009
Bourdillon P, Apra C, Guénot M, Duffau H (2017) Similarities and differences in neuroplasticity mechanisms between brain gliomas and nonlesional epilepsy. Epilepsia 58(12):2038–2047. https://doi.org/10.1111/epi.13935
Patel DC, Tewari BP, Chaunsali L, Sontheimer H (2019) Neuron-glia interactions in the pathophysiology of epilepsy. Nat Rev Neurosci 20(5):282–297. https://doi.org/10.1038/s41583-019-0126-4
Hepp Y, Salles A, Carbo-Tano M, Pedreira ME, Freudenthal R (2016) Surface expression of NMDA receptor changes during memory consolidation in the crab Neohelice granulata. Learn Mem 23(8):427–434. https://doi.org/10.1101/lm.041707.116
Amini-Khoei H, Kordjazy N, Haj-Mirzaian A, Amiri S, Haj-Mirzaian A, Shirzadian A et al (2018) Anticonvulsant effect of minocycline on pentylenetetrazole-induced seizure in mice: involvement of nitric oxide and N-methyl-d-aspartate receptor. Can J Physiol Pharmacol 96(8):742–750. https://doi.org/10.1139/cjpp-2017-0673
Rahimi-Madiseh M, Lorigooini Z, Boroujeni SN, Taji M, Amini-Khoei H (2022) The role of the NMDA receptor in the anticonvulsant effect of ellagic acid in pentylenetetrazole-induced seizures in male Mice. Behav Neurol 2022:9015842. https://doi.org/10.1155/2022/9015842
Gooshe M, Tabaeizadeh M, Aleyasin AR, Mojahedi P, Ghasemi K, Yousefi F et al (2017) Levosimendan exerts anticonvulsant properties against PTZ-induced seizures in mice through activation of nNOS/NO pathway: role for KATP channel. Life Sci 168:38–46. https://doi.org/10.1016/j.lfs.2016.11.006
Amiri S, Haj-Mirzaian A, Amini-Khoei H, Shirzadian A, Rahimi-Balaei M, Razmi A et al (2016) Lithium attenuates the proconvulsant effect of adolescent social isolation stress via involvement of the nitrergic system. Epilepsy Behav 61:6–13. https://doi.org/10.1016/j.yebeh.2016.04.035
Muñoz-López M (2015) Past, present, and future in hippocampal formation and memory research. Hippocampus 25(6):726–730. https://doi.org/10.1002/hipo.22452
Fogwe LA, Reddy V, Mesfin FB. Neuroanatomy, Hippocampus. 2022 Jul 18. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
Baulac M (2015) MTLE with hippocampal sclerosis in adult as a syndrome. Rev Neurol (Paris) 171(3):259–266. https://doi.org/10.1016/j.neurol.2015.02.004
Vermeulen RJ (2022) Cognitive, academic, executive and psychological functioning in children with spastic motor type cerebral palsy: influence of extent, location, and laterality of brain lesions. Eur J Paediatr Neurol 38:A1. https://doi.org/10.1016/j.ejpn.2022.04.007
Wang RY, Yang YR, Chang HC (2022) The SDF1-CXCR4 axis is involved in the hyperbaric oxygen therapy-mediated neuronal cells migration in transient brain ischemic rats. Int J Mol Sci 23(3):1780. https://doi.org/10.3390/ijms23031780
Andoh M, Ikegaya Y, Koyama R (2020) Microglia modulate the structure and function of the hippocampus after early-life seizures. J Pharmacol Sci 144(4):212–217. https://doi.org/10.1016/j.jphs.2020.09.003
Cimbalnik J, Pail M, Klimes P, Travnicek V, Roman R, Vajcner A et al (2020) Cognitive processing impacts high frequency intracranial EEG activity of human hippocampus in patients with pharmacoresistant focal epilepsy. Front Neurol 11:578571. https://doi.org/10.3389/fneur.2020.578571
Joëls M (2009) Stress, the hippocampus, and epilepsy. Epilepsia 50(4):586–597. https://doi.org/10.1111/j.1528-1167.2008.01902.x
Silverman RB (2018) Design and mechanism of GABA aminotransferase inactivators treatments for epilepsies and addictions. Chem Rev 118(7):4037–4070. https://doi.org/10.1021/acs.chemrev.8b00009
Rogawski MA, Löscher W (2004) The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nat Med 10(7):685–692. https://doi.org/10.1038/nm1074
Paggio Angela, Checchetto Vanessa, Campo Antonio, Menabò Roberta, Di Marco Giulia, Di Lisa Fabio, Szabo Ildiko, Rizzuto Rosario, De Stefani Diego (2019) Identification of an ATP-sensitive potassium channel in mitochondria. Nature 572(7771):609–613. https://doi.org/10.1038/s41586-019-1498-3
Salgado-Puga K, Rodríguez-Colorado J, Prado-Alcalá RA, Peña-Ortega F (2017) Subclinical doses of ATP-sensitive potassium channel modulators prevent alterations in memory and synaptic plasticity induced by amyloid-β. J Alzheimers Dis 57(1):205–226. https://doi.org/10.3233/JAD-160543
Wang ZP, Zhang ZH, Zeng YM, Jiang S, Wang SQ, Wang S (2006) Protective effect of sevoflurane preconditioning on oxygen-glucose deprivation injury in rat hippocampal slices: the role of mitochondrial K(ATP) channels. Sheng Li Xue Bao 58(3):201–206
César IC, Godin AM, Araujo DP, Oliveira FC, Menezes RR, Santos JR et al (2014) Synthesis, antinociceptive activity and pharmacokinetic profiles of nicorandil and its isomers. Bioorg Med Chem 22(9):2783–2790. https://doi.org/10.1016/j.bmc.2014.03.011
Malysheva AM, Martsevich SIu, Ginzburg ML (2011) Cardioprotective characteristics of the drug nicorandil in patients with coronary heart disease. Ter Arkh 83(9):14–9
Markham A, Plosker GL, Goa KL (2000) Nicorandil. An updated review of its use in ischaemic heart disease with emphasis on its cardioprotective effects. Drugs 60(4):955–74. https://doi.org/10.2165/00003495-200060040-00007
Tarkin JM, Kaski JC (2016) Vasodilator therapy: nitrates and nicorandil. Cardiovasc Drugs Ther 30(4):367–378. https://doi.org/10.1007/s10557-016-6668-z
Amini-Khoei H, Kordjazy N, Haj-Mirzaian A, Amiri S, Haj-Mirzaian A et al (2018) Anticonvulsant effect of minocycline on pentylenetetrazole-induced seizure in mice: involvement of nitric oxide and N-methyl-d-aspartate receptor. Can J Physiol Pharmacol 96(8):742–750. https://doi.org/10.1139/cjpp-2017-0673
Heurteaux C, Bertaina V, Widmann C, Lazdunski M (1993) K+ channel openers prevent global ischemia-induced expression of c-fos, c-jun, heat shock protein, and amyloid beta-protein precursor genes and neuronal death in rat hippocampus. Proc Natl Acad Sci USA 90(20):9431–9435. https://doi.org/10.1073/pnas.90.20.9431
Wong JC, Dutton SB, Collins SD, Schachter S, Escayg A (2016) Huperzine A provides robust and sustained protection against induced Seizures in Scn1a Mutant Mice. Front Pharmacol 7:357. https://doi.org/10.3389/fphar.2016.00357
Heubl M, Zhang J, Pressey JC, Al Awabdh S, Renner M, Gomez-Castro F et al (2017) GABAA receptor dependent synaptic inhibition rapidly tunes KCC2 activity via the Cl–sensitive WNK1 kinase. Nat Commun 8(1):1776. https://doi.org/10.1038/s41467-017-01749-0
Malik AR, Szydlowska K, Nizinska K, Asaro A, van Vliet EA, Popp O et al (2019) SorCS2 controls functional expression of amino acid transporter EAAT3 and protects neurons from oxidative stress and epilepsy-induced pathology. Cell Rep 26(10):2792-2804.e6. https://doi.org/10.1016/j.celrep.2019.02.027
Wu D, Bacaj T, Morishita W, Goswami D, Arendt KL, Xu W, Chen L, Malenka RC, Südhof TC (2017) Postsynaptic synaptotagmins mediate AMPA receptor exocytosis during LTP. Nature 544(7650):316–321. https://doi.org/10.1038/nature21720
Li X, Zhu H, Sun X, Zuo F, Lei J, Wang Z et al (2016) Human neural stem cell transplantation rescues cognitive defects in APP/PS1 model of Alzheimer’s Disease by enhancing neuronal connectivity and metabolic activity. Front Aging Neurosci 8:282. https://doi.org/10.3389/fnagi.2016.00282
Sun W, Li XL, Tang CZ, An L (2018) Acute Low Alcohol Disrupts hippocampus-striatum neural correlate of learning strategy by inhibition of PKA/CREB pathway in rats. Front Pharmacol 9:1439. https://doi.org/10.3389/fphar.2018.01439
Liu ZL, Xu RX, Jiang XD, Yin Z, Luo CY, Du MX et al (2003) Effect of kainate on the synaptic transmission in hippocampal CA1 region. Di Yi Jun Yi Da Xue Xue Bao 23(7):652–654
Shen KZ, Wu YN, Munhall AC, Johnson SW (2016) AMP kinase regulates ligand-gated K-ATP channels in substantia nigra dopamine neurons. Neuroscience 330:219–228. https://doi.org/10.1016/j.neuroscience.2016.06.001
Sharma NK, Kaur S, Goel RK (2020) Exploring the ameliorative role of α7 neuronal nicotinic acetylcholine receptor modulation in epilepsy and associated comorbidities in post-PTZ-kindled mice. Epilepsy Behav 103(Pt A):106862. https://doi.org/10.1016/j.yebeh.2019.106862
Van Bogaert P, De Tiège X, Vanderwinden JM, Damhaut P, Schiffmann SN, Goldman S (2001) Comparative study of hippocampal neuronal loss and in vivo binding of 5-HT1a receptors in the KA model of limbic epilepsy in the rat. Epilepsy Res 47(1–2):127–139. https://doi.org/10.1016/s0920-1211(01)00301-1
Gröticke I, Hoffmann K, Löscher W (2007) Behavioral alterations in the pilocarpine model of temporal lobe epilepsy in mice. Exp Neurol 207(2):329–349. https://doi.org/10.1016/j.expneurol.2007.06.021
Thapliyal S, Babu K (2018) Pentylenetetrazole (PTZ)-induced convulsion assay to determine GABAergic defects in caenorhabditis elegans. Bio Protoc. https://doi.org/10.21769/BioProtoc.2989
Yamada K, Ji JJ, Yuan H, Miki T, Sato S, Horimoto N et al (2001) Protective role of ATP-sensitive potassium channels in hypoxia-induced generalized seizure (New York, N.Y.). Science 292(5521):1543–6. https://doi.org/10.1126/science.1059829
Rubio C, Luna R, Rosiles A, Rubio-Osornio M (2020) Caloric restriction and ketogenic diet therapy for epilepsy: a Molecular approach involving Wnt pathway and KATP channels. Front Neurol. 11:584298. https://doi.org/10.3389/fneur.2020.584298
Yahil S, Wozniak DF, Yan Z, Mennerick S, Remedi MS (2021) Cognitive deficits and impaired hippocampal long-term potentiation in KATP-induced DEND syndrome. Proc Natl Acad Sci U S A. 118(45):e2109721118. https://doi.org/10.1073/pnas.2109721118
D’Adamo MC, Catacuzzeno L, Di Giovanni G, Franciolini F, Pessia M (2013) K(+) channelepsy: progress in the neurobiology of potassium channels and epilepsy. Front Cell Neurosci 7:134. https://doi.org/10.3389/fncel.2013.00134
Slingerland AS, Hattersley AT (2005) Mutations in the Kir6.2 subunit of the KATP channel and permanent neonatal diabetes: new insights and new treatment. Ann Med 37(3):186–95. https://doi.org/10.1080/07853890510007287
Wang Z, Bian W, Yan Y, Zhang DM (2022) Functional Regulation of K(ATP) Channels and Mutant Insight Into Clinical Therapeutic Strategies in Cardiovascular Diseases. Front Pharmacol 13:868401. https://doi.org/10.3389/fphar.2022.868401
Kong JJ, Zhang DD, Li P, Wei CY, Yu HJ, Zhang H, Zhang W, Wang YF, Cao YP (2015) Nicorandil inhibits oxidative stress and amyloid-β precursor protein processing in SH-SY5Y cells overexpressing APPsw. Int J Clin Exp Med 8(2):1966–1975
Paggio A, Checchetto V, Campo A, Menabò R, Marco GD, Lisa FD, Szabo I et al (2019) Identification of an ATP-sensitive potassium channel in mitochondria. Nature 572(7771):609–613. https://doi.org/10.1038/s41586-019-1498-3
Lukowski R, Cruz Santos M, Kuret A, Ruth P (2022) cGMP and mitochondrial K(+) channels-Compartmentalized but closely connected in cardioprotection. Br J Pharmacol 179(11):2344–2360. https://doi.org/10.1111/bph.15536
Yoshida M, Nakakimura K, Cui YJ, Matsumoto M, Sakabe T (2004) Adenosine A(1) receptor antagonist and mitochondrial ATP-sensitive potassium channel blocker attenuate the tolerance to focal cerebral ischemia in rats. J Cereb Blood Flow Metab 24(7):771–779. https://doi.org/10.1097/01.WCB.0000122742.72175.1B
Yoshihisa A, Sato Y, Watanabe S, Yokokawa T, Sato T, Suzuki S et al (2017) Decreased cardiac mortality with nicorandil in patients with ischemic heart failure. BMC Cardiovasc Disord 17(1):141. https://doi.org/10.1186/s12872-017-0577-3
Liang LN, Zhong X, Zhou Y, Hou ZQ, Hu HR, Zhu FF et al (2017) Cardioprotective effect of nicorandil against myocardial injury following cardiac arrest in swine. Am J Emerg Med 35(8):1082–1089. https://doi.org/10.1016/j.ajem.2017.02.051
Sánchez-Duarte S, Márquez-Gamiño S, Montoya-Pérez R, Villicaña-Gómez EA, Vera-Delgado KS, Caudillo-Cisneros C et al (2021) Nicorandil decreases oxidative stress in slow- and fast-twitch muscle fibers of diabetic rats by improving the glutathione system functioning. J Diabetes Investig 12(7):1152–1161. https://doi.org/10.1111/jdi.13513
Lenz Max, Kaun Christoph, Krychtiuk Konstantin A, Haider Patrick, Brekalo Mira, Maier Nadine et al (2021) Effects of nicorandil on inflammation apoptosis and atherosclerotic plaque progression. Biomedicines. https://doi.org/10.3390/biomedicines9020120
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conception and design: ZJ. Administrative support: LD and XT. Provision of study materials or patients: QJ. Collection and assembly of data: SQ and YL. Data analysis and interpretation: WWP. Manuscript writing: All authors. Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, or publication of this article.
Ethical Approval
This study was conducted in accordance with the declaration of Helsinki.This study was conducted with approval from the Ethics Committee of The Second Hospital of Hebei Medical University (Approval NO. 2020-AE006).
Informed Consent
Written informed consent was obtained from all participants.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, J., Liang, D., Xie, T. et al. Nicorandil Exerts Anticonvulsant Effects in Pentylenetetrazol-Induced Seizures and Maximal-Electroshock-Induced Seizures by Downregulating Excitability in Hippocampal Pyramidal Neurons. Neurochem Res 48, 2701–2713 (2023). https://doi.org/10.1007/s11064-023-03932-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03932-w