Abstract
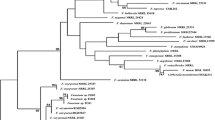
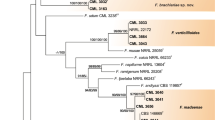
In recent years, ambrosia beetles, Euwallacea interjectus, have emerged as important pests of exotic Populus in poplar cultivations from Jiangsu, China. However, Fusarium mutualists of E. interjectus within the Ambrosia Fusarium Clade (AFC) remain unclear. In the investigation of Fusarium mutualists, 36 isolates were obtained from five geographically separated sites in Jiangsu Province and Jiangxi Province. Three novel species of AFC were identified based on morphological and multi-gene phylogenetic analysis; three novel species of AFC were identified. These new species are described here as Fusarium gannanense (AF-25), Fusarium populicola (AF-26), and Fusarium tumidispermus (AF-27). Fusarium populicola may be a pathogen of poplar and exhibited common to poplar cultivations in Jiangsu Province. The other two species were recovered from three locations in Jiangxi Province. The distribution of mutualists of E. interjectus within AFC is associated with the geographical distance among the samples with samples from the same region sharing the same mutualist. The present study augments our knowledge on the mutualists of Euwallacea within AFC in the native geographic range.














Similar content being viewed by others
Code availability
Not applicable.
Change history
15 September 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11557-022-01839-4
References
Aoki T, Kasson MT, Berger MC, Freeman S, Geiser DM, O’Donnell K (2018) Fusarium oligoseptatum sp. nov., a mycosymbiont of the ambrosia beetle Euwallacea validus in the Eastern US and typification of F. ambrosium. Fungal Syst Evol 1:23–39. https://doi.org/10.3114/fuse.2018.01.03
Aoki T, Liyanage PNH, Konkol JL, Ploetz RC, Smith JA, Kasson MT, Freeman S, Geiser DM, O’Donnell K (2021) Three novel Ambrosia Fusarium Clade species producing multiseptate “dolphin-shaped” conidia, and an augmented description of Fusarium kuroshium. Mycologia 113(5):1089–1109. https://doi.org/10.1080/00275514.2021.1923300
Aoki T, Smith JA, Kasson MT, Freeman S, Geiser DM, Geering ADW, O’Donnell K (2019) Three novel Ambrosia Fusarium Clade species producing clavate macroconidia known (F. floridanum and F. obliquiseptatum) or predicted (F. tuaranense) to be farmed by Euwallacea spp. (Coleoptera: Scolytinae) on woody hosts. Mycologia 111(6):919–935. https://doi.org/10.1080/00275514.2019.1647074
Aoki T, Smith JA, Mount LL, Geiser DM, O’Donnell K (2013) Fusarium torreyae sp. nov., a pathogen causing canker disease of Florida torreya (Torreya taxifolia), a critically endangered conifer restricted to northern Florida and southwestern Georgia. Mycologia 105(2):312–319. https://doi.org/10.3852/12-262
Brayford D (1987) Fusarium bungicourtii sp. nov., and its relationship to F. tumidum and F. tumidum var. coeruleum. Trans Br Mycol Soc 89:347–351
Carrillo JD, Dodge C, Stouthamer R, Eskalen A (2020) Fungal symbionts of the polyphagous and Kuroshio shot hole borers (Coleoptera: Scolytinae, Euwallacea spp.) in California can support both ambrosia beetle systems on artificial media. Symbiosis 80(2):155–168. https://doi.org/10.1007/s13199-019-00652-0
Carrillo JD, Rugman-Jones PF, Husein D, Stajich JE, Kasson MT, Carrillo D, Stouthamer R, Eskalen A (2019) Members of the Euwallacea fornicatus species complex exhibit promiscuous mutualism with ambrosia fungi in Taiwan. Fungal Genet Biol 133:103269. https://doi.org/10.1016/j.fgb.2019.103269
Cognato AI, Hoebeke ER, Kajimura H, Smtih SM (2015) History of the exotic ambrosia beetles Euwallacea interjectus and Euwallacea validus (Coleoptera: Curculionidae: Xyleborini) in the United States. J Econ Entomol 108(3):1129–1135. https://doi.org/10.1111/efp.12124
Cognato AI, Sari G, Smith SM, Beaver RA, Li Y, Hulcr J, Jordal BH, Kajimura H, Lin CS, Pham TH, Singh S, Sittichaya W (2020) The essential role of taxonomic expertise in the creation of DNA databases for the identification and delimitation of Southeast Asian ambrosia beetle species (Curculionidae: Scolytinae: Xyleborini). Front Ecol Evol 8:27. https://doi.org/10.3389/fevo.2020.00027
Fraedrich SW, Harrington TC, Best GS (2015) Xyleborus glabratus attacks and systemic colonization by Raffaelea lauricola associated with dieback of Cinnamomum camphora in the southeastern United States. For Pathol 45(1):60–70
Freeman S, Sharon M, Maymon M, Mendel Z, Protasov A, Aoki T, Eskalen A, O’Donnell K (2013) Fusarium euwallaceae sp. nov.—a symbiotic fungus of Euwallacea sp., an invasive ambrosia beetle in Israel and California. Mycologia 105(6):1595–1606. https://doi.org/10.3852/13-066
Hughes MA, Riggins JJ, Koch FH, Cognato AI, Anderson C, Formby JP, Dreaden TJ, Ploetz RC, Smith JA (2017) No rest for the laurels: symbiotic invaders cause unprecedented damage to southern USA forests. Biol Invasions 19(7):2143–2157
Hulcr J, Dunn RR (2011) The sudden emergence of pathogenicity in insect–fungus symbioses threatens naive forest ecosystems. Proc R Soc B Biol Sci 278(1720):2866–2873. https://doi.org/10.1098/rspb.2011.1130
Hulcr J, Stelinski LL (2017) The ambrosia symbiosis: from evolutionary ecology to practical management. Annu Rev Entomol 62:285–303. https://doi.org/10.1146/annurev-ento-031616-035105
Hulcr J, Black A, Prior K, Chen CY, Li HF (2017) Studies of ambrosia beetles (Coleoptera: Curculionidae) in their native ranges help predict invasion impact. Fla Entomol 100(2):257–261. https://doi.org/10.1653/024.100.0219
Jiang ZR, Kinoshita S, Sasaki O, Cognato AI, Kajimura H (2019) Non-destructive observation of the mycangia of Euwallacea interjectus (Blandford) (Coleoptera: Curculionidae: Scolytinae) using X-ray computed tomography. Entomol Sci 22(2):173–181. https://doi.org/10.1111/ens.12353
Jiang ZR, Masuya H, Kajimura H (2021) Novel symbiotic association between Euwallacea ambrosia beetle and Fusarium fungus on fig trees in Japan. Front Microbiol 12:725210. https://doi.org/10.3389/fmicb.2021.725210
Joseph R, Keyhani NO (2021) Fungal mutualisms and pathosystems: life and death in the ambrosia beetle mycangia. Appl Microbiol Biotechnol 105(9):3393–3410. https://doi.org/10.1007/s00253-021-11268-0
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14(6):587–589. https://doi.org/10.1038/nmeth.4285
Kasson MT, O’Donnell K, Rooney AP, Sink S, Ploetz RC, Ploetz JN, Konkol JL, Carrillo D, Freeman S, Mendel Z, Smith JA, Black AW, Hulcr J, Bateman C, Stefkova K, Campbell PR, Geering ADW, Dann EK, Eskalen A et al (2013) An inordinate fondness for Fusarium: phylogenetic diversity of fusaria cultivated by ambrosia beetles in the genus Euwallacea on avocado and other plant hosts. Fungal Genet Biol 56:147–157. https://doi.org/10.1016/j.fgb.2013.04.004
Kurtzman CP, Robnett CJ (1997) Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5'end of the large-subunit (26S) ribosomal DNA gene. J Clin Microbiol 35(5):1216–1223
Landi L, Braccini CL, Knížek M, Pereyra VA, Marvaldi AE (2019) A newly detected exotic ambrosia beetle in Argentina: Euwallacea interjectus (Coleoptera: Curculionidae: Scolytinae). Fla Entomol 102(1):240–242. https://doi.org/10.1653/024.102.0141
Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B (2017) PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol Biol Evol 34(3):772–773. https://doi.org/10.1093/molbev/msw260
Leslie JF, Summerell BA (2006) The Fusarium laboratory manual. Blackwell Publishing, Ames
Li Y, Ruan Y, Kasson MT, Stanley EL, Gillett CPDT, Johnson AJ, Zhang M, Hulcr J (2018) Structure of the ambrosia beetle (Coleoptera: Curculionidae) mycangia revealed through micro-computed tomography. J Insect Sci 18(5):1–13. https://doi.org/10.1093/jisesa/iey096
Lynch SC, Twizeyimana M, Mayorquin JS, Kayim M, Kasson MT, Thu PQ, Bateman C, Rugman-Jones P, Hulcr J, Stouthamer R, Eskalen A (2016) Identification, pathogenicity and abundance of Paracremonium pembeum sp. nov. and Graphium euwallaceae sp. nov.—two newly discovered mycangial associates of the polyphagous shot hole borer (Euwallacea sp.) in California[J]. Mycologia 108(2):313–329. https://doi.org/10.3852/15-063
Lynn KM, Wingfield MJ, Durán A, Marincowitz S, Oliveira LS, De Beer ZW, Barnes I (2020) Euwallacea perbrevis (Coleoptera: Curculionidae: Scolytinae), a confirmed pest on Acacia crassicarpa in Riau, Indonesia, and a new fungal symbiont; Fusarium rekanum sp. nov. Antonie Van Leeuwenhoek 113(6):803–823. https://doi.org/10.1007/s10482-020-01392-8
Lynn KM, Wingfield MJ, Durán A, Oliveira LS, de Beer ZW, Barnes I (2021) Novel Fusarium mutualists of two Euwallacea species infesting Acacia crassicarpa in Indonesia. Mycologia 113(3):536–558. https://doi.org/10.1080/00275514.2021.1875708
Mayers CG, Harrington TC, Masuya H, Jordal BH, McNew DL, Shih HH, Roets F, Kietzka GJ (2020) Patterns of coevolution between ambrosia beetle mycangia and the Ceratocystidaceae, with five new fungal genera and seven new species. Persoonia 44(1):41–66. https://doi.org/10.3767/persoonia.2020.44.02
Na F, Carrillo JD, Mayorquin JS, Ndinga-Muniania C, Stajich JE, Stouthamer R, Huang YT, Lin YT, Chen CY, Eskalen A (2018) Two novel fungal symbionts Fusarium kuroshium sp. nov. and Graphium kuroshium sp. nov. of Kuroshio shot hole borer (Euwallacea sp. nr. fornicatus) cause Fusarium dieback on woody host species in California. Plant Dis 102(6):1154–1164
Nguyen LT, Schmidt HA, Von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32(1):268–274. https://doi.org/10.1093/molbev/msu300
Nirenberg H (1981) A simplified method for identifying Fusarium spp. Can J Bot 59:1599–1609
O’Donnell K, Libeskind-Hadas R, Hulcr J, Bateman C, Kasson MT, Ploetz RC, Konkol JL, Ploetz JN, Carrillo D, Campbell A, Duncan RE, Liyanage PNH, Eskalen A, Lynch SC, Geiser DM, Freeman S, Mendel Z, Sharon M, Aoki T et al (2016) Invasive Asian Fusarium–Euwallacea ambrosia beetle mutualists pose a serious threat to forests, urban landscapes and the avocado industry. Phytoparasitica 44(4):435–442. https://doi.org/10.1007/s12600-016-0543-0
O’Donnell K, Sink S, Libeskind-Hadas R, Hulcr J, Kasson MT, Ploetz RC, Konkol JL, Ploetz JN, Carrillo D, Campbell A, Duncan RE, Liyanage PNH, Eskalen A, Na F, Geiser DM, Bateman C, Freeman S, Mendel Z, Sharon M et al (2015) Discordant phylogenies suggest repeated host shifts in the Fusarium–Euwallacea ambrosia beetle mutualism. Fungal Genet Biol 82:277–290. https://doi.org/10.1016/j.fgb.2014.10.014
O'Donnell K, Cigelnik E, Nirenberg HI (1998) Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 90(3):465–493. https://doi.org/10.1080/00275514.1998.12026933
O'Donnell K, Sarver BAJ, Brandt M, Chang DC, Noble-Wang J, Park BJ, Sutton DA, Benjamin L, Lindsley M, Padhye A, Geiser DM, Ward TJ (2007) Phylogenetic diversity and microsphere array-based genotyping of human pathogenic fusaria, including isolates from the multistate contact lens-associated US keratitis outbreaks of 2005 and 2006. J Clin Microbiol 45(7):2235–2248. https://doi.org/10.1128/JCM.00533-07
O'Donnell K, Sutton DA, Rinaldi MG, Sarver BAJ, Arunmozhi Balajee S, Schroers HJ, Summerbell RC, Robert VARG, Crous PW, Zhang N, Aoki T, Jung K, Park J, Lee YH, Kang S, Park B, Geiser DM (2010) Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J Clin Microbiol 48(10):3708–3718. https://doi.org/10.1128/JCM.00989-10
Paap T, De Beer ZW, Migliorini D, Nel WJ, Wingfield MJ (2018) The polyphagous shot hole borer (PSHB) and its fungal symbiont Fusarium euwallaceae: a new invasion in South Africa. Australas Plant Pathol 47(2):231–237. https://doi.org/10.1007/s13313-018-0545-0
Ranger CM, Schultz PB, Frank SD, Chong JH, Reding ME (2015) Non-native ambrosia beetles as opportunistic exploiters of living but weakened trees. PLoS One 10(7):e0131496. https://doi.org/10.1371/journal.pone.0131496
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. https://doi.org/10.1093/sysbio/sys029
Roques A, Fan J, Courtial B, Zhang Y, Yart A, Auger-Rozenberg M, Denux O, Kenis M, Baker R, Sun J (2015) Planting sentinel European trees in Eastern Asia as a novel method to identify potential insect pest invaders. PLoS One 10(5):e0120864. https://doi.org/10.1371/journal.pone.0120864
Six DL, Poulsen M, Hansen AK, Wingfield MJ, Roux J, Eggleton P, Slippers B, Paine TD (2011) Anthropogenic effects on interaction outcomes: examples from insectmicrobial symbioses in forest and savanna ecosystems. Symbiosis 53:101–121. https://doi.org/10.1007/s13199-011-0119-1
Smith SM, Beaver RA, Cognato AI (2020) A monograph of the xyleborini (Coleoptera, Curculionidae, Scolytinae) of the Indochinese Peninsula (except Malaysia) and China. ZooKeys 983:1–422. https://doi.org/10.3897/zookeys.983.52630
Stouthamer R, Rugman-Jones P, Thu PQ, Eskalen A, Thibault T, Hulcr J, Wang LJ, Jordal BH, Chen CYU, Cooperband M, Lin CS, Kamata N, Lu SS, Masuya H, Mendel Z, Rabaglia R, Sanguansub S, Shih HH, Sittichaya W, Zong SX (2017) Tracing the origin of a cryptic invader: phylogeography of the Euwallacea fornicatus (Coleoptera: Curculionidae: Scolytinae) species complex. Agric For Entomol 19(4):366–375. https://doi.org/10.1111/afe.12215
Vega FE, Biedermann PHW (2020) On interactions, associations, mycetangia, mutualists and symbiotes in insect-fungus symbioses. Fungal Ecol 44:100909. https://doi.org/10.1016/j.funeco.2019.100909
Wang L, Chen F, Qiu C, Tang J, Ding X, Ren J (2021a) Identification and risk analyses of Euwallacea interjectus. J Nanjing For Univ (Nat Sci Ed) 45(5):201–208
Wang Z, Li Y, Ernstsons AS, Sun R, Hulcr J, Gao L (2021b) The infestation and habitat of the ambrosia beetle Euwallacea interjectus (Coleoptera: Curculionidae: Scolytinae) in the riparian zone of Shanghai, China. Agric For Entomol 23(1):104–109. https://doi.org/10.1111/afe.12405
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322
Yin H, Huang FS, Li Z (1984) Economic insect fauna of China. Fasc. 29. Coleoptera: Scolytidae. Science Press, Beijing 203p
Zhang D, Gao F, Jakovlić I, Zou H, Zhang J, Li WX, Wang GT (2020) PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol Ecol Resour 20(1):348–355. https://doi.org/10.1111/1755-0998.13096
Acknowledgements
The authors sincerely thank Dinggen Ji and Jinhua Yang for the assistance on collection of the study samples and Nan Jiang, Fan He, and Zichun Li for the assistance on experiment of the laboratory studies. Special thanks to Jiao He and Lichao Wang for their help on phenotypic characterization of the new species. Special thanks to Professor Changlu Wang (Department of Entomology, Rutgers University) for revising the text.
Funding
This work was supported by the Forestry Science, Technology Innovation and Promotion Projects of Jiangsu (LYKJ-Dongtai [2021] no.1) and the Forest Development Special Planning Projects of Jiangsu.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, sampling, collecting, DNA isolation, and preparation for sequencing were performed by Shengchang Lai and Chen Zhao. Data analyses and interpretation were performed by Shengchang Lai and Lulu Dai. You Li supported scientific guidance for studies and writing. The first draft of the manuscript was written by Shengchang Lai, and all authors commented on previous versions of the manuscript. All authors read, edited, and approved the final manuscript. Dejun Hao and Lulu Dai supervised the study and reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Roland Kirschner
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lai, S., Zhao, C., Li, Y. et al. Three novel Fusarium mutualists of ambrosia beetle Euwallacea interjectus in China. Mycol Progress 21, 70 (2022). https://doi.org/10.1007/s11557-022-01820-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-022-01820-1