Summary
The Winteraceae comprise 100 – 130 species in five to nine genera. The genus Zygogynum is currently thought to have a Papuasian-Pacific distribution and is only known from the western Pacific islands (New Caledonia, Vanuatu and the Solomon Islands), Australia and New Guinea, including the far western Bird’s Head Peninsula, but is not yet recorded from islands to the west of the Sahul Plate and the biogeographic boundary of Lydekker’s Line. Here, two new species of Zygogynum are described from west of both Wallace’s and Lydekker’s Lines. Zygogynum moluccanum is described from the Moluccan islands of Indonesia, and Z. sundaicum, a new species and generic record for the Sunda shelf, is described from the northern part of the Malaysian State of Sarawak in Borneo. In addition to descriptions and illustrations of the new species, the generic delimitation, fossil record and potential dispersal agents of Zygogynum are discussed.
Similar content being viewed by others
Introduction
Winteraceae are a family of aromatic trees and shrubs in the order Canellales within the early diverging angiosperm group informally termed the ‘magnoliids’ (Vink 1993a; Stevens 2001). Members of the family are generally found in moist montane tropical environments with most species native to South-East Asia and Australasia. Generic and species limits are yet to be conclusively resolved, and either five genera containing c. 100 species (Stevens 2001 onwards; POWO 2022) are recognised or nine genera with 130 species (Guymer 2007); the Stevens and POWO limits are followed here.
The family has its greatest diversity in Australasia and the Pacific with the two largest genera being Zygogynum Baill. s.l. (45 species, New Guinea to New Caledonia and Queensland) and Tasmannia R.Br. ex DC. (36 species, Malesia to the Solomon Islands, centred on Papuasia). Pseudowintera Dandy, with four species, is endemic to New Zealand, and Drimys J.R.Forst. & G.Forst. is restricted to Central and South America with seven species. The family is absent from mainland Africa but the monotypic genus Takhtajania M.A.Baranova & J.-F.Leroy is found on Madagascar.
Members of the family are often found as understorey components of tropical montane forest, but in New Guinea Tasmannia can reach alpine habitats to c. 4200 m elevation, and Zygogynum c. 3200 m (Johns et al. 2006; Utteridge 2021). The family is placed together with Canellaceae within the Canellales, and members have spirally arranged simple, exstipulate leaves and flowers with free parts including several, usually free, superior carpels that become a compound fruit (comprised of ‘berry-lets’) at maturity; in addition, plants often have an aromatic (peppery) smell when crushed.
This study focuses on the genus Zygogynum and describes two new extant species from Borneo and Seram, islands either side of traditional biogeographic boundaries — the Wallace and Lydekker Lines — indicating the different composition of taxa, primarily faunal (Ali & Heaney 2021), between the western Asian assemblages of the Sunda Shelf and the Australasian ones of the Sahul Shelf. These are the first contemporary records of the genus outside Papuasia and the Pacific and suggest Long Distance Dispersal (LDD) and establishment events.
Delimitation of Zygogynum
Within the Winteraceae, Zygogynum has been considered similar to the genera Bubbia Tiegh. and Exospermum Tiegh. on account of its terminal inflorescence (rather than lateral in Tasmannia); however, the limits of these three genera have been uncertain.
Initially, Zygogynum was considered an endemic genus restricted to New Caledonia, distinct in having the carpels united into an ovary (Vink 1985), whereas Bubbia, with a distribution from Malesia (New Guinea) and northern Australia to New Caledonia, was distinguished from Zygogynum on account of the free, not fused, carpels. Finally, Exospermum, with a single species endemic to New Caledonia, has coherent to free carpels. Vink (1985), in his comprehensive review of material of all three genera, found intermediates between free and fused carpels in representative species from each genus, and placed all three in an enlarged Zygogynum — the earliest available name. Recently, Guymer (2007) in the Flora of Australia, retained the Australian species as Bubbia, but molecular studies by Marquínez et al. (2009) and Pratt (2013) supported the retention of Vink’s taxonomy with Bubbia included in a larger Zygogynum. The most recent phylogeny of Thomas et al. (2014), using two internal transcribed spacer (ITS) data sets and a trnL-F data set, agreed with the relationships in Marquínez et al. (2009), and the enlarged concept of Zygogynum including Bubbia and Exospermum is followed here.
Zygogynum is clearly defined by its inflorescence position and calyx. Members of Zygogynum have a terminal inflorescence and, after flowering, the twig elongates sympodially from a vegetative bud in the axil of a cataphyll subtending the inflorescence axis. In addition, the calyx is relatively thick, rarely calyptrate and encloses the bud and remains persistent below the fruit. In Tasmannia, the only other genus of Winteraceae in Australasia, the inflorescences are lateral, the branch elongates monopodially from a terminal vegetative bud, and its calyx is somewhat thinner (described as sub-menbranaceous or papyraceous), completely enclosing the bud, calyptrate and usually caducous. The two new species described here are unequivocally members of the genus Zygogynum due to the terminal inflorescence with no accompanying terminal vegetative bud and the persistent calyx below the fruit.
Diagnostic characters for species of Zygogynum
There is no recent revision of the genus, no infrageneric classification and the group lacks a phylogeny with comprehensive sampling across the distributional range. The most recent phylogeny of the group included only 10 of the 45 accepted species and showed that the Australian species formerly placed in Bubbia form a distinct clade within a group including other Zygogynum species and some previously placed in Exospermum (Thomas et al. 2014). Without a species-level phylogeny there are no monophyletic groups within the genus to scrutinise for putative morphological synapomorphies and the evolution of character traits. It is useful, therefore, to examine the characters used by previous workers to delimit species and as diagnostic characters in identification keys.
Van Tieghem (1900) used the branching of the inflorescence primary rays (equivalent to a partial inflorescence) to recognise three sections, but Smith (1943: 141) considered this character of “no more than specific value”. Smith (1943) employed many characters in his key to New Guinea Zygogynum (treated then as Bubbia), but the following were used throughout the key to divide the species into smaller groups: leaf size (‘small’ up to 14 cm long vs ‘larger’ from 14 – 40 cm long), angle of divergence of secondary veins (leaving the midrib at 50° – 75° vs 70° – 85°), petal number (4 – 5 vs 5 – 10) and stigma position (apical vs apical and ventral). Vink (1977), in his revision of Zygogynum in the Old World (the genus was considered endemic to New Caledonia at that time), noted that specific delimitation in the genus is rather difficult with many overlapping characters. In his key to species, Vink (1977: 222) primarily used petal fusion (outer petals free or connate in bud), pollen (solitary or in tetrads), number of stigmas (fewer, or more, than 10), midrib shape (triangular or rounded below), pedicel length and the lower leaf colour (stomata and/or unspecialised cells white). More recently, Vink (2016) used additional characters in New Guinea, including the length of the stigma extension across the apex of the carpel and the number of carpels; no key to the genus in New Guinea, nor discussion of diagnostic characters, was given.
Materials and Methods
The new species of Zygogynum were described from herbarium specimens at BO, K and SAR (herbarium acronyms follow Thiers, continuously updated); specimens of the new species and related taxa, including types, were examined in CANB, E and online portals including specimen images from Global Plants JSTOR (http://plants.jstor.org/ — which includes specimens from A, BM, BRI, GH, MEL, M, P, S) and the BioPortal of Naturalis Biodiversity Center (http://bioportal.naturalis.nl/ — which includes AMD, L, U and WAG). Measurements were made from herbarium specimens and rehydrated material for the floral descriptions; terminology for shapes follows Systematics Association Committee (1962) and leaf and venation terminology follows Hickey (1979). The conservation assessments of the species were undertaken using IUCN categories of threat (see IUCN 2012, 2016) and the Extent of Occurrence (EOO) and Area of Occupancy (AOO) were estimated using the GeoCat tool (Bachman et al. 2011).
Species accounts
Zygogynum moluccanum Utteridge & Rustiami sp. nov. Type: Indonesia, Maluku Province, Seram, Manusela National Park, north side of Gunong Binaia [Binaiya; 3°10'24"S 129°27'18"E], 2000 m elev., 12 Sept. 1987 (fl.), Argent C87178 (holotype BO! [BO1959130]; isotypes E! [E00156739], K-2 sheets!).
http://www.ipni.org/urn:lsid:ipni.org:names:77301110-1
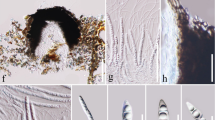
Tree to 15 m tall; bark smooth; entirely glabrous. Branchlets stout, brown. Leaves spirally arranged, evenly spaced along the branchlets; petiole 1.65 – 2.1 cm long, flat above, rounded below, brown; blade (elliptic –) obovate, (8.5 –) 13 – 18 × 4.5 – 5.7 cm, coriaceous, brown to ginger-brown below, apex obtuse to rounded, base cuneate, decurrent along the upper part of the petiole, margins entire and slightly revolute when dry; midrib sunken above, distinctly raised and triangular in cross-section below; secondary veins 16 – 21 pairs, widely spreading at 80° – 85° with midrib, straight or curved, loop-forming branches present towards the margin with veins joining the superadjacent secondary at 60° – 75° and enclosed by loop-forming tertiary arches, very conspicuous on the adaxial surface, somewhat obscure on the abaxial surface; tertiary veins random to orthogonal reticulate. Inflorescences of 4 primary rays, 2.7 – 4.6 cm long, 3 – 8 flowered, axes with (0 –) 2 – 3 secondary branches with 2 – 3 dichasially arranged flowers per branch. Pedicels 5 – 15 mm long. Calyx rupturing into usually 3 triangular segments, 1 – 3 mm long. Torus c. 1 mm high. Petals: outer petals completely fused or free at the very apex only, splitting into c. 5 segments; inner petals c. 10, oblong to obovate, apex rounded, 4.5 – 6.5 × 1.5 – 2 mm. Stamens c. 22, 1 – 1.8 mm long; pollen in tetrads. Carpel 1, obovoid, 3 – 3.5 × 3 – 3.5 mm, with conspicuous longitudinal ridges impressed from the stamens; stigmatic crest running the length of the carpel apex, not descending; ovules c. 28. Fruitlet (from Kostermans 1266) red when mature, globose, c. 11.6 × 12.3 mm; outer layer of rather thin fruit wall with small clusters of brachysclereids, pulpa not or protruding only slightly between seeds. Seeds 12, obovoid, c. 5 × 3 × 1.2 mm; testa black, smooth. Fig. 1.
recognition. Zygogynum moluccanum is unique in the genus by the combination of almost completely fused outer petals and a solitary carpel.
distribution. Endemic to the Moluccan islands of Indonesia — Ambon, Halmahera, Morotai and Seram. Map 1.
Distribution of Zygogynum: circles show previously described species of Zygogynum (incl. Bubbia) from GBIF (2018); triangles show the localities of Z. moluccanum and the black square in northern Borneo shows the locality of Z. sundaicum.
specimens examined. indonesia. maluku province: Ambon Island, Waai [c. 03°33'50"S, 128°18'37"E], 400 m, 9 July 1959 (fr.), Kuswata & Soepadmo 271 (BO); Seram Island, southern side of Central Seram: en route from Wae Tapakasitam (3°13'S 129°37'E) to Huhunya (Hoale Pass, 3°14'S 129°38'E) along a trail to Hoale Pass, 1300 – 1730 m, 21 Dec. 1996 (fl.), Kato et al. 940 (BO, K, TI n.v.). maluku utara province: Halmahera Island, Gn. Sembilan [near the road from Desa Gamsungi to Tosoa, c. 01°18'N, 127°31'E], 600 m, 28 Sept. 1951 (fl.), Pleyte 300 (BO!); Morotai Island, G. Para2 [= Parapara, c. 2°11'35"N 128°28'20"E], 900 m, 26 May 1949 (fl.), Kostermans 1134 (BO!); ibid., 1000 m, 28 May 1949 (fr.), Kostermans 1266 (BO!).
habitat. Collected from lowland and montane forests, from 600 – 2000 m elevation. Ambon and Seram are within the Seram Rain Forests Ecoregion and Morotai and Halmahera are within the Halmahera Rain Forests Ecoregion (Wikramanayake et al. 2002), both of which are broadly defined to include lowland evergreen, semi-evergreen and montane forests.
preliminary conservation assessment. Zygogynum moluccanum is currently known from six collections from five locations, with an EOO of 80,019.326 km2 and an AOO of 20 km2 (using the standard grid cell size of 2 km2). As a terrestrial species, the areas of ocean between the islands inflate the EOO, and an approximate Terrestrial Area of Habitat (AOH — see Brooks et al. 2019), estimated from the land area of the islands, is 37,960 km2. The EOO and AOH are both larger than any of the IUCN threatened thresholds, whilst the AOO of 20 km2, together with the species being known from no more than five locations, would indicate a threat status of Vulnerable. However, on Seram, both Gunung Binaiya and Hoale Pass are still forested according to satellite imagery (Google Earth 2022a) and, in addition, the species was described as a “common tree” in the field notes of Argent C87178. Sitting within the Wallacea biodiversity hotspot (Myers et al. 2000), the Seram Rain Forest Ecoregion is stated to have a “relatively stable” conservation status (Wikramanayake et al. 2002). In addition, although large areas are disturbed and/or converted in the lowlands, there are still extensive areas of forest on hilly areas above elevations of c. 200 m at the Ambon, Halmahera and Morotai localities (Google Earth 2022b); we note, however, that the Ambon, Halmahera and Morotai specimens were collected in the 1940s and 1950s. Further collections and observations of the species are needed for a full assessment, including the habitat requirements, number of mature individuals in the population and ecology (including the status of dispersal agents). The species is provisionally assigned a conservation rating of Least Concern (LC).
phenology. Collected in flower in May, Sept. and Dec., and in fruit in May and July.
etymology. Named for the Moluccas, or ‘Spice Islands’, comprising the islands included in the Indonesian Provinces of Maluku and Maluku Utara. The origin of the name of the Moluccas is from the Arabic ‘al-Muluk’ meaning ‘of the kings’ referring to the four sultanates, or kingdoms, of the northern islands (Brown 2009).
notes. Zygogynum moluccanum is unique in the genus in its combination of almost completely fused outer petals, c. 10 inner petals and a solitary carpel, with the latter character particularly diagnostic. In the specimens of Z. moluccanum, the carpel is clearly solitary in all the flowers that can be examined (n = 24), and there is only a single stigmatic crest on each carpel, discounting the possibility of fusion of two or more carpels (as found in those members of Zygogynum previously placed in Exospermum). The flowers were described as “greenish white with [a] peculiar ‘fishy’ smell” (fide Argent C87178). Only a single fruiting specimen is known, Kostermans 1266 (BO), and the fruitlets are clearly globose when dry but have split irregularly across the apex in several places obscuring the stigmatic crest.
In most Zygogynum species, there is an outer ‘whorl’ of petals which can be free or fused for part, or nearly all, of their length (e.g., see Vink 1977, 1985: Figs 1a & e). Where fused for most of their length, the outer 'whorl' of petals usually consists of four fused decussate petals (Vink 1977), and at anthesis they can rupture regularly or irregularly; fused outer petals is a useful character for identification. If the outer petals can separate along predetermined rupture lines, then regularly shaped ‘petals’ will form, but if rupture lines are lacking, then irregularly shape ‘petals’ will form. Vink (2016) noted that in the New Guinea species of Zygogynum the outer petals are usually free, but in New Caledonia several species have the outer petals connate for up to half their length. Zygogynum moluccanum is similar to the New Caledonian species Z. pauciflorum (Baker f.) Vink in having connate petals with thinner apical lobes and rupturing at anthesis, but differs in having compound inflorescences with several flowers (Z. pauciflorum has a single flower on each inflorescence), comparatively shorter pedicels, less than 15 mm long (Z. pauciflorum has pedicels 18 – 52 mm long) and the solitary carpel (Z. pauciflorum has 1 – 3 carpels). Of the New Guinean species, the petals are only fused in Z. pachyanthum (A.C.Sm.) Vink, but that species has 3 – 4 carpels fused at anthesis and then becoming free in fruit.
Several species of Zygogynum can have one to several carpels, and solitary carpels were a diagnostic character for several new species from New Guinea described by Vink (2016). Two species from New Guinea always have solitary carpels: Z. ledermannii (Diels) Vink (endemic to the Sepik region of Papua New Guinea) and Z. megacarpum (A.C.Sm.) Vink (endemic to the western part of the Central Cordillera in Indonesian New Guinea) but both differ from Z. moluccanum in the petal number and morphology (see Table 1). Several other species have very few carpels and occasionally a single carpel, but these all have fewer petals which are free: Z. longifolium (A.C.Sm.) Vink (New Guinea), Z. queenslandianum (Vink) Vink (Australia), Z. schramii Vink (New Guinea) and Z. semecarpoides (F.Muell.) Vink (Australia); see Table 1 for differences.
In New Caledonia, many species can have solitary carpels, but there are only three with outer petals fused for a significant length which could be confused with Zygogynum moluccanum (see Vink 1993b). These taxa, Z. cristatum Vink, Z. pauciflorum (Baker f.) Vink and Z. stipitatum Baill., all have one to several carpels and numerous stamens, in addition, the fusion of the outer petals is not as complete as in Z. moluccanum; further differences are given in Table 2.
On Seram, Zygogynum moluccanum is another addition to the flora of Gunung Binaiya, the highest peak on this island rising to 3027 m. Several endemic new species have been described from there including the tree ferns Alsophila binayana (M.Kato) Lehnert & Coritico and Sphaeropteris pukuana (M.Kato) Lehnert & Coritico that form tree fern savanna up to 2700 m (Ebihara et al. 2012; Coritico et al. 2017), as well as Poa opinata Veldkamp, an interesting, but expected, ‘stepping-stone’ taxon, filling a gap between the genus in Borneo and New Guinea (Veldkamp 2017). In addition, several new species of fern are currently being prepared for description from the mountain (Ebihara et al. 2012); Gunung Binaiya clearly merits further exploration.
Zygogynum sundaicum Utteridge sp. nov. Type: Malaysia, Sarawak, Limbang Division, Murud [03°54'18"N, 115°29'18"E], Sg. Rabatek, 1 May 2002 (fr.), Yahud et al. S 88260 (holotype SAR-image seen!; isotype K!).
http://www.ipni.org/urn:lsid:ipni.org:names:77301111-1
Tree c. 10 m tall, c. 12 cm diam.; bark smooth, grey; entirely glabrous. Branchlets stout, dark reddish-brown to black. Leaves spirally arranged, evenly spaced along the branchlets; petiole 1.6 – 1.8 cm long, flat above, rounded below, winged distally from the lamina extending along the petiole, dark brown; blade obovate, 15 – 19.5 × 5.25 – 7.25 cm, coriaceous, brown to ginger-brown below, apex obtuse, base cuneate, decurrent along the upper part of the petiole, margins entire and slightly revolute when dry; midrib sunken above, distinctly raised and rounded below; secondary veins 13 – 17 pairs, widely spreading at 80° – 85° with midrib, straight or curved, loop-forming branches present towards the margin with veins joining the superadjacent secondary at a right angle and enclosed by loop-forming tertiary arches, very conspicuous on the adaxial surface, conspicuous to obscure on the abaxial surface; tertiary veins random to orthogonal reticulate. Inflorescences of up to 5 primary rays, 3.5 – 10.5 cm long, 14 – 30-flowered, axes with 3 – 4 secondary branches with 3 – 5 dichasially arranged flowers per branch. Pedicels (in fruit) 5 – 7.5 mm long. Flowers n.v., only fruits seen. Calyx 1 – 1.5 mm long. Carpels 4 – 8 (counted from scars in fruit). Fruitlets pale green, globose but somewhat laterally compressed, 7 – 9.5 × 6.5 – 7.5 mm, stipe 1 – 1.5 mm; stigmatic crest ventral (–subapical), 2 – 3.5 mm long; outer layer of rather thin fruit wall with small clusters of brachysclereids, pulpa protruding only slightly between seeds. Seeds 5 – 8, obovoid, c. 4 × 1.2 × 2 mm; testa dark brown-black, smooth. Fig. 2.
recognition. Zygogynum sundaicum is unique in the following combination of characters: obovate leaves that are relatively narrow being less than 7.25 cm wide, widely spreading secondary veins, dense, clustered stomata on the lower leaf surface covering the secondary and tertiary veins, the few primary rays in each inflorescence having only up to 4 rays, fruitlets with a short stigma, 2 – 3.5 mm long, on the ventral (– subapical) side, and obovoid seeds with a smooth testa.
distribution. Borneo, known only from the type locality in the Malaysian state of Sarawak. Map 1.
habitat. “Near the river, alt. 1120 m” fide Yahud et al. S 88260.
preliminary conservation assessment. Known only from the type, this species is assigned as Data Deficient. The island of Borneo is part of the Sundaland Biodiversity Hotspot (Myers et al. 2000) and Sarawak has lost eighty percent of its primary forest over the last forty years (Gaveau et al. 2016). However, Zygogynum sundaicum is currently known from the Mount Murud area which sits within the Pulong Tau National Park (UNEP-WCMC 2021), and still has extensive areas of forest cover.
phenology. Collected in fruit in May.
etymology. Named for Sundaland, the biogeographic region of South-East Asia comprised primarily of the Sunda Shelf — the extension of the Asia continental plate including the Malay Peninsula and the islands of Borneo, Java and Sumatra.
notes. There are few obvious diagnostic characters in the genus, but a combination of vegetative (particularly the leaf size and venation) and inflorescence morphology can be used as diagnostic characters for Zygogynum sundaicum as outlined in ‘Recognition’. This species does not easily key out in the last treatment of the genus by Smith (1943). Using Smith’s key to the New Guinean taxa, the only key for the Malesian region currently available, it would key on leaf size and venation to either to Z. argenteum (A.C.Sm.) Vink (as Bubbia argentea A.C.Sm. in Smith 1943: 150) or Z. calothyrsum (Diels) Vink (as B. calothyrsa (Diels) A.C.Sm. in Smith 1943: 150); the latter being one of the most widespread species of Zygogynum in New Guinea. The new species described here is not placed satisfactorily with this group because they are characterised by a strictly apical stigma position (Z. sundaicum has a ventral-subapical stigma). In addition, Z. sundaicum differs in the 13 – 17 pairs of secondary veins diverging at an angle of 80° – 85° (Z. argenteum: 15 – 20 pairs of secondary veins diverging at an angle of 70° – 80°; Z. calothyrsum: up to 25 pairs at an angle of 90°), and in the inflorescence with only 5 peduncles (primary rays) up to 7.5 cm long (Z. argenteum: 10 – 13 peduncles, each up to 9 cm long; Z. calothyrsum: up to 8 peduncles, up to 12 cm long). Based on Smith (1943), an additional candidate species for comparison, based on leaf size, inflorescence branching and stigma morphology, would be Z. umbellatum (Ridl.) Vink (as Bubbia idenburgensis A.C.Sm. in Smith (1943) but considered within Z. umbellatum see Vink 2016: 49), a species scattered, but rare, throughout New Guinea. Zygogynum sundaicum differs from Z. umbellatum in the angle of secondary nerve divergence (Z. umbellatum: 55° – 75°) and the number of carpels (Z. umbellatum: 8 – 22).
Outside of Malesia, Zygogynum sundaicum is most similar to Z. semecarpoides (F.Muell.) Vink from north-eastern Queensland in the leaf shape, inflorescence length, and number of flowers and carpels, but that species is a larger and more robust plant differing in the larger leaves usually 13 – 36 cm long, with longer pedicels up to 13 mm long, and larger fruits usually 12 – 15 mm long with 10 – 12 seeds per apocarp/drupelet. The fruitlets are probably immature, but seed dimensions are taken from the largest fruitlet.
Discussion
Regardless of the application of different generic names, none of the species within Zygogynum s.l. (incl. Bubbia and Exospermum) have been reported from Wallacea or Sundaland, with the genus only recorded to the east of Lydekker’s Line — the demarcation line running along the western edge of New Guinea. For example, Joyce et al. (2020a) list 13 species of Zygogynum, all restricted to New Guinea and, because they follow Australian taxonomy, five species of Bubbia from Australia only. Lydekker’s Line “indicates the very different nature of the New Guinea flora” (Raes & van Welzen 2009) but, at the generic level, New Guinea has been shown to be more closely aligned with core Malesia rather than the Pacific including Bougainville and the Solomon Islands (Marsh et al. 2009; Joyce et al. 2020b).
Members of the Winteraceae, however, do extend into western Malesia, with the genus Tasmannia recorded from Sulawesi, the Philippines and Borneo, demonstrating that the family has dispersed across extensive water bodies. Wong (1996), who treated the family for the Tree Flora of Sabah and Sarawak, noted that in “Borneo (all territories), Drimys [then the accepted name for Old World Tasmannia] is the sole genus” for the family.
This paper is the first definitive record of the putatively Sahulian and Pacific genus Zygogynum west of Lydekker’s and Wallace’s Lines. There are at least two possible scenarios to explain the contemporary distribution of these eastern two species: i) they are relicts at the very fringe of a more extensive distribution that has now contracted, or ii) are the consequence of relatively recent dispersal events from the centre of species diversity in Papuasia and the Pacific. These two scenarios are briefly discussed below, with the caveat that the genus has not been comprehensively sampled for a contemporary phylogeny.
Relicts from a range contraction?
Zygogynum is known from several fossil records, including leaf material attributed to the genus from the Early Miocene (c. 20 Ma) in Otago, New Zealand (Pole 1996), and pollen of the Z. queenslandianum-type from a similar age in the Cape of Good Hope, South Africa (Coetzee & Praglowski 1988). Most recently, Liang et al. (2018) described Zygogynum poratus Liang & Zhou, based on fossil leaves from Central Yunnan from the Middle Miocene (c. 16 – 11.6 Mya). Liang et al. (2018) postulate that “the distribution of Zygogynum was much wider in the Miocene than at present” and “Zygogynum migrated into tropical China from Gondwana through transoceanic dispersal during the Middle Miocene because of the collision of Gondwanaland and Laurasia during the Late Tertiary”.
At least three possibilities arise from the description of the fossil Zygogynum from Yunnan: 1) the record is a misidentification, 2) the genus was extremely widespread in the Miocene and later contracted to a ‘core’ Papuasian-Pacific area, or 3) there were ad hoc LDD events between eastern Malesia and Yunnan with subsequent extinction. The leaf material, especially the general leaf architecture, is poorly preserved and it is important to note that Liang et al. (2018) describe the lower surface of the leaves of the fossil with “sparse unicellular short hairs (approximately 10 – 25 μm) and the hair bases are 6 – 20 μm”; these are clearly shown in their SEM figures (Liang et al. 2018: Fig. 5F). However, hairs are not found in Zygogynum. Vink (1977: 220), in his meticulous analysis of the morphology of the genus in the strict sense, noted “Hairs or distinct papillae were not found in this genus”, and subsequently for Zygogynum treated in a broad sense for the Flore de la Nouvelle-Calédonie, Vink (1993b: 91) describes the genus as “Plantes entièrement glabres, rarement pourvues de papilles dans l'inflorescence”1Footnote 1. In addition, Liang et al. (2018: Tab. 1) also record Zygogynum as having a modern day distribution (‘Recent’) in South America, Africa, Asia and Oceania, but give no references to corroborate the South America and Africa records. Further observations, especially of more definitive floral or fruit fossils that can be assigned without doubt to Zygogynum, are needed to better understand the Miocene distribution of the genus, as the assignment of Yunnan leaf fossil to Zygogynum appears erroneous.
Recent dispersal?
Modern day Malesia comprises three distinct zones: the Sunda continental shelf in the west (made up of parts of continental South-East Asia including mainland Malaysia, as well as Borneo, Sumatra and Java), the numerous islands of Wallacea in the centre (including Sulawesi, Sumba, Timor etc.) and the Sahul continental shelf in the east (New Guinea and Australia); this area is termed the Sunda-Sahul Convergence Zone (Joyce et al. 2020b).
It is most likely that members of the genus have moved outward from Australia as the clade including Zygogynum is estimated to have originated in Australia following its separation from Drimys in South America in the Palaeogene (c. 23 – 65 Mya; Thomas et al. 2014). Without an accurate fossil record or detailed phylogeny with comprehensive sampling of the New Guinea species, it is difficult to place the arrival of Zygogynum in New Guinea and estimate when ancestors of Z. moluccanum and Z. sundaicum crossed Lydekker’s Line to Wallacea and Sundaland. In addition, postulated LDD events are more likely to occur from a large continent to smaller continental fragments and oceanic islands (Crisp et al. 2009).
There are at least three potential LDD models for the distribution of the two new Zygogynum species to the west of Lydekker’s Line. The first invokes movement from Australia to New Guinea followed by a period of radiation on New Guinea after which LDD events establish the genus in the Moluccas and Borneo. The second scenario is one of LDD to the Moluccas and Borneo directly from Australia. Postulated dates for these scenarios include the formation of New Guinea, beginning with an eastern landmass from c. 35 – 30 Mya, followed by further orogeny from c. 15 Mya and rapid uplift of the Central Ranges which reached their current elevation at c. 5 Mya (van Ufford & Cloos 2005). The final one is of one or several LDD events from Australia to the early land masses formed in the outer Melanesian arc, and species achieving their current distribution through movement of these land masses into their contemporary position and subsequent dispersal.
The Sahul shelf collided with the Sunda shelf from approximately 25 Mya, with the islands of Wallacea established in their present locations from c. 5 Mya (Hall 2017; Toussaint et al. 2014). Although the formation of New Guinea and the Malesian archipelago is relatively recent in geological time, the modal dates of plant species divergence have been estimated (with a 95% confidence interval) to be 0.75 (0.58 – 0.98) Mya in the super-tree analysis of Hedges et al. (2015), which would be after the proposed dates of the formation of Malesia as we know today. These scenarios also assume that the ancestral or sister taxa to the two western Zygogynum species are from New Guinea and/or Australia but this has yet to be established. Indeed, Zygogynum moluccanum has characters, such as the solitary carpel and calyptrate outer petals that are also seen in some New Caledonian taxa, perhaps suggesting that two independent LDD events have probably occurred.
Dispersal agents — constipated columbids?
Zygogynum fruits are compound/aggregate fruits consisting of few to many ‘berry-lets’, each one derived from an individual carpel after fertilisation, usually up to c. 1.5 cm in height and diameter, and with a fleshy pericarp maturing red or black. Lacking any specific adaptations for wind or water dispersal, Zygogynum fruits are clearly adapted for zoochory: in New Guinea, for example, the genus is recorded as bird dispersed in Stiles (1982: Tab. 2; as Bubbia sp.). In Borneo, various bird families were “frequently observed eating fleshy fruits” of various plant groups, including Drymis [sic] (= Tasmannia piperita (Hook.f) Miers — the only taxon of ‘Drimys’ in Borneo; Kimura et al. 2001). The fruits of Tasmannia piperita are also berry-lets up to 1.5 cm high and c. 8 mm in diameter and become black when ripe (Vink 1970: 315).
Many birds are frugivorous, but of those with broad distributions across SE Asia, rather than restricted to a particular island or region, the Imperial Pigeons (Ducula Hodgson, 1836) are strong fliers, often nomadic following seasonal fruit sources (Goodwin 1983), and ideal candidates for consideration as LDD vectors of zoochorous fruits in Asia (Corlett 2017). Although fruit bats or flying foxes (the Pteropodidae) can travel long distances and retain seeds over long distances, they are precluded as potential dispersal agents of Zygogynum berry-lets because of their narrow gastrointestinal tracts: these can accommodate seeds only up to 2.5 mm in diameter in cynopterine fruit bats (Cynopterus F.Cuvier, 1824), or 5 mm in diameter in the flying foxes (Pteropus Brisson, 1762) (Shilton et al. 1999).
Four species of Imperial Pigeon are found in Borneo, but the Pied Imperial Pigeon, Ducula bicolor (Scopoli, 1786), and the Green Imperial Pigeon, Ducula aenea (L., 1766), have widespread ranges outside Borneo. The Pied Imperial Pigeon extends from the Andamans, though Malesia and along the northern and eastern regions of Australia. It is usually considered a species of offshore islands and coastal habitats, including mangrove, but there are numerous inland records from West Papua, Sulawesi and even in Borneo from Danum Valley (Levatich & Padilla 2019). The Green Imperial Pigeon has a more westerly distribution, extending from the west coast of the Indian Subcontinent, through Indo-China and western Malesia, the Philippines and Sulawesi, with scattered records in the Moluccas and even two from the Arfak Mountains in West Papua (Creuwels 2020). Fruit sizes and the birds that eat them are given for a guild of birds in the Philippines in van Hamann & Curio (1999), including a record of Ducula bicolor eating a broad range of fruits between 19 – 60 mm. Both of these bird taxa are potential vectors for a range of fleshy fruits across the region, however, Baker et al. (1998) note that “the efficiency of birds in long-distance plant dispersal is difficult to assess”, and understanding factors such as the seed retention time in the digestive system and the viability of the seed after defecation or regurgitation will vary between each bird and plant taxon.
Future work
It is hoped that the description of these two species will inspire botanists and ecologists to make further collections and observations of these enigmatic taxa. Improved sampling, especially of Zygogynum sundaicum in Sarawak and adjacent areas in the ‘Heart of Borneo’ — the central part of the island where forests remain intact, will allow the conservation status of the taxa to be properly assessed, reveal ecological interactions and provide contemporary material for molecular and morphological studies. Re-examination of the available fossils would help to understand the distribution of the genus in geologic time, especially during the Miocene. Comprehensive phylogenetic studies are needed to understand the taxonomic relationships of these two species. A specimen level sampling regime using next generation sequencing techniques would clarify generic level questions and address species level taxonomy, especially within New Guinea.
Notes
Plants entirely glabrous, rarely with papillae in the inflorescence.
References
Ali, J. R. & Heaney, L. R. (2021). Wallace's line, Wallacea, and associated divides and areas: history of a tortuous tangle of ideas and labels. Biol. Rev. 96: 922 – 942.
Bachman, S., Moat, J., Hill, A., de la Torre, J. & Scott, B. (2011). Supporting Red List threat assessments with GeoCAT: Geospatial Conservation Assessment Tool. ZooKeys 150: 117 – 126.
Baker, W. J., Coode, M. J. W., Dransfield, J., Dransfield, S., Harley, M. M., Hoffmann, P. & Johns, R. J. (1998). Patterns of distribution of Malesian vascular plants. In: R. Hall & J. D. Holloway (eds), Biogeography and geological evolution of SE Asia, pp. 243 – 258. Backhuys Publishers, Leiden.
Brooks, T. M., Pimm, S. L., Akçakaya, H. R., Buchanan, G. M., Butchart, S. H. M., Foden, W., Hilton-Taylor, C., Hoffmann, M., Jenkins, C. N., Joppa, L., Li, B. V., Menon, V., Ocampo-Peñuela, N. & Rondinini, C. (2019). Measuring Terrestrial Area of Habitat (AOH) and Its Utility for the IUCN Red List. Trends Ecol. Evol. 34: 977 – 986.
Brown, I. (2009). The Territories of Indonesia. Routledge, London.
Coetzee, J. A. & Praglowski, J. (1988). Winteraceae pollen from the Miocene of the southwestern Cape (South Africa) — relationship to modern taxa and phytogeographical significance. Grana 27: 27 – 37.
Coritico, F. P., Amoroso, V. B. & Lehnert, M. (2017). New records, names and combinations of scaly tree ferns (Cyatheaceae) in eastern Malesia. Blumea 62: 92 – 96.
Corlett, R. T. (2017). Frugivory and seed dispersal by vertebrates in tropical and subtropical Asia: An update. Global Ecol. Conserv. 11: 1 – 22.
Creuwels, J. (2020). Naturalis Biodiversity Center (NL) - Aves. Naturalis Biodiversity Center. Occurrence dataset https://doi.org/10.15468/dxmzbz accessed via GBIF.org on 31 May 2020. https://www.gbif.org/occurrence/2434080392
Crisp, M. D., Arroyo, M. T. K., Cook, L. G., Gandolfo, M. A., Jordan, G. J., McGlone, M. S., Weston, P. H., Westoby, M., Wilf, P. & Linder, H. P. (2009). Phylogenetic biome conservatism on a global scale. Nature 458: 754 – 756.
Ebihara, A., Fraser-Jenkins, C. R., Parris, B. S., Zhang, X.-C., Yang, Y.-H., Chiou, W.-L., Chang, H.-M., Lindsay, S., Middleton, D., Kato, M., Praptosuwiryo, T. N., Amoroso, V. B., Barcelona, J. F., Ranil, R. H. G., Park, C.-H., Murakami, N. & Hoya, A. (2012). 2012 Rare and Threatened Pteridophytes of Asia 1. An enumeration of narrowly distributed taxa. Bull. Natl. Mus. Nat. Sci., Tokyo, B, 38: 93 – 119.
Gaveau, D. L. A., Sheil, D., Husnayaen, Salim, M. A., Arjasakusuma, S., Ancrenaz, M., Pacheco, P. & Meijaard, M. (2016). Rapid conversions and avoided deforestation: examining four decades of industrial plantation expansion in Borneo. Sci. Reports 6: art. 32017.
GBIF.org (20 August 2018). GBIF Occurrence Download. https://doi.org/10.15468/dl.2w0avu
Goodwin (1983). Pigeons and doves of the world, 3rd edition. Cornell University Press, Ithaca, New York.
Google Earth. (2022a). Gunung Binaiya. Retrieved from https://www.google.com/maps/place/Gunung+Binaiya/@-3.1654699,129.4401796,51944m/data=!3m1!1e3!4m5!3m4!1s0x2d6a490fa2bd37d7:0x6423c278c76847c3!8m2!3d-3.1733394!4d129.4549942
Google Earth. (2022b). Maluku Islands, Indonesia. Retrieved from https://www.google.com/maps/place/Maluku+Islands/@-3.1137678,125.2027303,1680915m/data=!3m2!1e3!4b1!4m5!3m4!1s0x2d6f7f356749030f:0x1c5346360ea19f41!8m2!3d-2.8646166!4d129.5765974
Guymer, G. P. (2007). Flora of Australia. Winteraceae to Platanaceae, Vol. 2. CSIRO Publishing, Melbourne.
Hall, R. (2017). Southeast Asia: new views of the geology of the Malay Archipelago. Annual Rev. Earth Planet. Sci. 45: 331 – 358.
Hedges, S. B., Marin, J., Suleski, M., Paymer, M. & Kumar, S. (2015). Tree of life reveals clock-like speciation and diversification. Molec. Biol. Evol. 32: 835 – 845.
Hickey, L. J. (1979). A revised classification of the architecture of dicotyledonous leaves. In: C. R. Metcalfe & L. Chalk (eds), Anatomy of the dicotyledons, pp. 25 – 39. Clarendon Press, Oxford.
IUCN (2012). IUCN Red List Categories and Criteria: Version 3.1. Second edition. IUCN, Gland and Cambridge.
IUCN Standards and Petition Subcommittee (2016). Guidelines for using the IUCN Red List Categories and Criteria. Version 11. Prepared by the Standards and Petitions Subcommittee of the IUCN Species Survival Commission. http://www.iucnredlist.org/documents/RedListGuidelines.pdf [Accessed 20 Sept. 2020].
Johns, R. J., Edwards, P. J., Utteridge, T. M. A. & Hopkins, H. F. (2006). A Guide to the Alpine and Subalpine Flora of Mount Jaya. Royal Botanic Gardens, Kew.
Joyce, E. M., Thiele, K. R., Slik, J. W. F. & Crayn, D. M. (2020a). Checklist of the vascular flora of the Sunda-Sahul Convergence Zone v1.1. James Cook University. (dataset). https://doi.org/10.25903/5ea0dd85f8ea9
____, ____, ____ & ____(2020b). Plants will cross the lines: climate and available land mass are the major determinants of phytogeographical patterns in the Sunda–Sahul convergence zone. Biol. J. Linn. Soc. 132: 374 – 387.
Kimura, K., Yumoto, T. & Kihachiro, K. (2001). Fruiting phenology of fleshy-fruited plants and seasonal dynamics of frugivorous birds in four vegetation zones on Mt. Kinabalu, Borneo. J. Trop. Ecol. 17: 833 – 858.
Levatich, T. & Padilla, F. (2019). EOD — eBird Observation Dataset. Cornell Lab of Ornithology. Occurrence dataset https://doi.org/10.15468/aomfnb accessed via GBIF.org on 31 May 2020. https://www.gbif.org/occurrence/1760991167
Liang, X. Q., Lu, P., Zhang, J. W., Su, T. & Zhou, Z. K. (2018). First fossils of Zygogynum from the Middle Miocene of Central Yunnan, Southwest China, and their palaeobiogeographic significance. Palaeoworld 27: 399 – 409. https://doi.org/10.1016/j.palwor.2018.05.003
Marquínez, X., Lohmann, L. G., Salatino, M. L. F., Salatino, A. & Gonzalez, F. (2009). Generic relationships and dating of lineages in Winteraceae based on nuclear (ITS) and plastid (rpS16 and psbA-trnH) sequence data. Molec. Phylogenet. Evol. 53: 435 – 449.
Marsh, S. T., Brummitt, N. A., de Kok, R. P. J. & Utteridge, T. M. A. (2009). Large-scale patterns of plant diversity and conservation priorities in South East Asia. Blumea 54: 103 – 108.
Myers, N., Mittermeier, R., Mittermeier, C. G., da Fonseca, G. A. B. & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403: 853 – 858.
Pole, M. (1996). Plant macrofossils from the Foulden Hills Diatomite (Miocene), Central Otago, New Zealand. J. Roy. Soc. New Zealand 26: 1 – 39.
POWO (2022). Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet; http://www.plantsoftheworldonline.org/ [Retrieved 10 Jan. 2022].
Pratt, S. J. (2013). Evolution of the genera Vitex (Lamiaceae) and Zygogynum (Winteraceae) on New Caledonia. Thesis submitted in partial fulfilment of the requirements for the degree of Master of Science in Biological Sciences at The University of Waikato.
Raes, N. & van Welzen, P. C. (2009). The demarcation and internal division of Flora Malesiana: 1857 – present. Blumea 54: 6 – 8.
Shilton, L. A., Altringham, J. D., Compton, S. G. & Whittaker, R. J. (1999). Old World fruit bats can be long-distance seed dispersers through extended retention of viable seeds in the gut. Proc. R. Soc. London, Ser. B, Biol. Sci. 266: 219 – 223.
Smith, A. C. (1943). Taxonomic notes on the Old World species of Winteraceae. J. Arnold Arbor. 24: 119 – 164.
Stevens, P. F. (2001 onwards). Angiosperm phylogeny website. Missouri Botanical Garden, St Louis, MO. Available at: http://www.mobot.org/MOBOT/research/APweb/.
Stiles, E. W. (1982). Fruit Flags: Two Hypotheses. Amer. Naturalist 120: 500 – 509.
Systematics Association Committee (1962). IIa. Terminology of simple symmetrical plane shapes (Chart 1a)*. Taxon 11: 245 – 247. https://doi.org/10.2307/1217034 [Addendum]
Thiers, B. (continuously updated). Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/science/ih/
Thomas, N., Bruhl, J. J., Ford, A. & Weston, P. H. (2014). Molecular dating of Winteraceae reveals a complex biogeographical history involving both ancient Gondwanan vicariance and long-distance dispersal. J. Biogeogr. 41: 894 – 904.
Toussaint, E. F. A., Hall, R., Monaghan, M. T., Sagata, K., Ibalim, S., Shaverdo, H. V., Volger, A. P., Poris, J. & Balke, M. (2014). The towering orogeny of New Guinea as a trigger for arthropod megadiversity. Nature Commun. 5: 4001.
UNEP-WCMC (2021). Protected Area Profile for Pulong Tau National Park from the World Database of Protected Areas, November 2021. Available at: www.protectedplanet.net
Utteridge, T. M. A. (2021). Winteraceae. In: T. M. A. Utteridge & L. V. S. Jennings (eds), Trees of New Guinea, pp. 46 – 48. Royal Botanic Gardens, Kew.
van Hamann, A. & Curio, E. (1999). Interactions among frugivores and fleshy fruit trees in a Philippine submontane rainforest. Conservation Biol. 13: 766 – 773.
van Tieghem, P. (1900). Sur les dicotylédones du groupe des homoxylées. J. Bot. 14: 259 – 297, 330 – 361.
van Ufford, A. Q. & Cloos, M. (2005). Cenozoic tectonics of New Guinea. Amer. Assoc. Petr. Geol. B 89: 119 – 140.
Veldkamp, J. F. (2017). Poa opinata (Gramineae), a new species from G. Binaiya, Ceram, Moluccas, Indonesia. Reinwardtia 16: 73 – 75.
Vink, W. (1970). The Winteraceae of the Old World I. I. Pseudowintera and Drimys — Morphology and taxonomy. Blumea 18: 225 – 354.
____(1977). The Winteraceae of the Old World II. Zygogynum — morphology and taxonomy. Blumea 23: 219 – 250.
____(1985). The Winteraceae of the Old World V. Exospermum links Bubbia to Zygogynum. Blumea 31: 39 – 55.
____(1993a). Winteraceae. In: K. Kubitzki, J. G. Rohwer & V. Bittrich (eds), Families and Genera of Vascular Plants. Vol. 2: 630 – 638. Springer-Verlag, Berlin.
____(1993b). Winteraceae. Flore de la Nouvelle-Calédonie et Dépendances 19: 90 – 175. Muséum National d’Histoire Naturelle, Paris.
____(2016). The Winteraceae of the Old World. VIII. Some Zygogynum species from New Guinea. Blumea 61: 41 – 50.
Wikramanayake, E., Dinerstein, E., Loucks, C. J., Olson, D. M., Morrison, J., Lamoreux, J., McKnight, M. & Hedao, P. (2002). Terrestrial Ecoregions of the Indo-Pacific: A Conservation Assessment. Island Press, Washington, DC.
Wong, K. M. (1996). Winteraceae. In: E. Soepadmo, K. M. Wong & L.G. Saw (eds), Tree Flora of Sabah and Sarawak Vol. 2: 403 – 407. FRIM, Sabah Forestry Department and Sarawak Forestry Department, Malaysia.
Acknowledgements
We would like to thank Nur Safinas Binti Jelani at SAR who very kindly supplied an image of the type of Zygogynum sundaicum. TU would like to thank the Centre for Australian National Biodiversity Research and National Research Collections Australia (CSIRO) for facilitating his visit to the Australian National Herbarium to work on the New Guinea collections, especially Brendan Lepschi for his unwavering assistance and valuable help whilst at CANB; Liam Trethowan (K) kindly assisted with the distribution data. Hein van Grouw, Senior Curator, Bird Group, Dept. of Life Sciences at the Natural History Museum provided useful information about pigeons. We thank the two reviewers for carefully reading through the manuscript and the useful suggestions which have greatly improved the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Utteridge, T.M.A., Rustiami, H. Out of New Guinea? Two new species of Zygogynum (Winteraceae) extend the genus west of Lydekker’s and Wallace’s Lines. Kew Bull 77, 759–771 (2022). https://doi.org/10.1007/s12225-022-10041-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12225-022-10041-4