Abstract
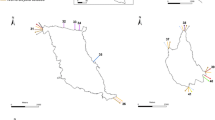
The present study describes the bioinvasion of the vermetid gastropod Eualetes tulipa of the Brazilian coast. The species was first reported in the Pacific Ocean, off the coast of Panama (since the 1840s); however, the type locality was not specified in the original species description. Since then, E. tulipa has been introduced to Hawaii, the Caribbean Sea, southeastern Florida and India. In Brazil, the first documented occurrence was in 2005, at Ceará State, northeast Brazil, and later in 2009 it was registered at Rio de Janeiro State, southeast Brazil, 3000 km from the previous location. Nowadays, they are not only found growing on artificial substrates but also along sandstone fringing reefs and rocky reefs coexisting with the native species Petaloconchus varians. The impact on the native benthic community is unknown; however, studies have suggested impacts such as competing for space with fouling communities (E. tulipa, Venezuela), and causing deleterious effects on corals (Ceraesignum maximum, French Polynesia). The possibility of spread through Brazilian endemic areas (e.g. Abrolhos Marine National Park), is a legitimate cause for concern as a result of oil industry shipping further distributing this non-indigenous species. E. tulipa has a continuous year-long reproduction and fast settlement, within 24 h of hatching. This reproductive mode allows for the highly successful invasion and establishment to new areas following maritime transport or natural rafting, predicting a rapidly widespread distribution and invasion of Brazilian and International waters.






Similar content being viewed by others
References
Antaq (2016) Agência Nacional de Transportes Aquaviários (National Agengy of Waterborne Transport). http://www.antaq.gov.br/portal/Estatisticas_Anuarios.asp. Accessed 28 January 2016
Bezerra DF (2010) Distribuição da malacofauna em pilares dos terminais portuários do Ceará-Brasil, com ênfase no bivalve invasor Isognomom bicolor. Dissertation, Universidade Federal do Ceará, Fortaleza, Brazil
Bieler R (1995, 1995) Vermetid gastropods from Sao Miguel, Azores: comparative anatomy, systematic position and biogeographic affiliation. Açoreana (Supp):173–192
Bieler R (1996) Mörch’s worm-snail taxa (Caenogastropoda: Vermetidae, Siliquariidae, Turritellidae). Am Malacol Bull 13(1/2):23–35
Bieler R (2015) Vermetus panamensis. In: MolluscaBase (2015). Accessed through: World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id=732945 on 2017-02-06
Bieler R, Petit RE (2011) Catalogue of recent and fossil “worm-snail” taxa of the families Vermetidae, Siliquariidae, and Turritellidae (Mollusca: Caenogastropoda). Zootaxa 2948:1–103 http://www.mapress.com/zootaxa/2011/f/zt02948p103.pdf
Birkeland C (1977) The importance of the rate of biomass accumulation in early successional stages of benthic communities to the survival of coral recruits. In: Taylor DL (ed) Proceedings of the Third International Coral Reef Symposium, Miami, Florida, Volume 1: Biology. Rosenstiel School of Marine and Atmospheric Research, pp 15–21
Birkeland C (1987) Nutrient availability as a major determinant of differences among coastal hard-substratum communities in different regions of the tropics. In: Birkeland C (ed) Comparison between Atlantic and Pacific tropical marine coastal ecosystems: community structure, ecological processes, and productivity. UNESCO Reports in Marine Science, 46, pp 43–98
Breves A, Skinner LF (2014) First record of the vermetid Petaloconchus varians (d’Orbigny, 1841) on floating marine debris at Ilha Grande, Rio de Janeiro, Brazil. JICZM 14(1):159–161. doi:10.5894/rgci457
Carbonel C (1998) Modelling of upwelling in the coastal area of Cabo Frio (Rio de Janeiro-Brazil). Braz J Oceanogr 46:1–17. doi:10.1590/S1679-87591998000100001
Carlton JT (1996) Biological invasions and cryptogenic species. Ecology 77(6):1653–1655 http://www.jstor.org/stable/2265767
Carlton JT (1999) Molluscan invasions in marine and estuarine communities. Malacologia 41:439–454
Carlton JT, Eldredge LG (2009) The Introduced and Cryptogenic Marine and Estuarine Animals and Plants of the Hawaiian Archipelago. Bishop Museum Bulletin in Cultural and Environmental Studies 4. Bernice P Bishop Museum, Bishop Museum Press, Honolulu. http://hbs.bishopmuseum.org/pubs-online/pdf/bces4.pdf. Accessed 20 March 2016
Chenu, J.C. (1843–1845) G[enus] Vermetus. Illustrations Conchyliologiques. [no text; pl. 1, 1843, pls. 2–5, 1844a; pl. 5*, 1845a—see Literature Note 4 herein]
Coelho-Souza SA, Pereira GC, Coutinho R, Guimarães JRD (2013) Yearly variation of bacterial production in the Arraial do Cabo protection area (Cabo Frio upwelling region): an evidence of anthropogenic pressure. Braz J Microbiol 44(4):1349–1357
Coles SL, DeFelice RC, Eldredge LG (2002) Nonindigenous marine species at Waikiki and Hawaii Kai, Oahu, Hawaii. Final Report prepared for the David and Lucile Packard Foundation and the State of Hawaii Department of Land and Natural Resources. Bishop Museum Technical Report N°25, Honolulu, Hawaii. http://hbs.bishopmuseum.org/pdf/waikiki.pdf. Accessed 20 March 2016
Coles SL, Kandel FLM, Reath PA, Longenecker K, Eldredge LG (2006) Rapid assessment of Nonindigenous marine species on coral reefs in the main Hawaiian Islands. Pac Sci 60(4):483–507. doi:10.1353/psc.2006.0026
Coles SL, Eldredge LG (2002) Nonindigenous species introductions on coral reefs: a need for information. Pac Sci 56:191–209 http://hdl.handle.net/10125/2650
Collin R, Salazar MZ (2010) Temperature-mediated plasticity and genetic differentiation in egg size and hatching size among populations of Crepidula (Calyptraeidae: Gastropoda). Biol J Linn Soc 99:489–499. doi:10.1111/j.1095-8312.2009.01388.x
Cortés J (2012) Marine biodiversity of an Eastern tropical Pacific oceanic island, Isla del coco, Costa Rica. Rev Biol Trop 60(Suppl. 3):131–185
Ferreira CEL, Gonçalves JEA, Coutinho R (2006) Ship hulls and oil platforms as potential vectors to marine species introduction. J Coastal Res 39:1341–1346 http://www.jstor.org/stable/25742972
Franklin-Junior W, Matthews-Cascon H, Bezerra LEA, Meireles CAO, Soares MO (2005) Macrofauna bentônica de ambientes consolidados no Estado do Ceará - região entremarés de praias rochosas. Relatório Científico do Projeto de Zoneamento Ecológico Econômico do Litoral do Ceará – ZEE, SEMACE/FCPC/LABOMAR-UFC, Fortaleza, Brazil
Furtado-Ogawa E (1970) Contribuição ao conhecimento da fauna malacológica intertidal de substratos duros no nordeste brasileiro. Arq Ciênc Mar Fortaleza 10(2):193–196
Golding RE, Bieler R, Rawlings TA, Collins TM (2014) Deconstructing Dendropoma: a systematic revision of a world-wide worm-snail group with descriptions of new genera (Caenogastropoda: Vermetidae). Malacologia 57(1):1–97
Guimaraens MA, Coutinho R (2000) Temporal and spatial variation of Ulva spp. and water properties in the Cabo Frio upwelling region of Brazil. Aquat Bot 66:101–114. doi:10.1016/S0304-3770(99)00070-4
Hadfield MG (1989) Latitudinal effects on juvenile size and fecundity in Petaloconchus (Gastropoda). Bull Mar Sci 45:369–376
Hadfield MG, Kay EA, Gillete MU, Lloyd MC (1972) The Vermetidae (Mollusca: Gastropoda) of the Hawaiian Islands. Mar Biol 12(1):81–98. doi:10.1007/BF00347431
Honkoop PJC, Van Der Meer J (1998) Experimentally induced effects of water temperature and immersion time on reproductive output of bivalves in the Wadden Sea. J Exp Marine Biol 220:227–246. doi:10.1016/S0022-0981(97)00107-X
Hughes RN (1985) Feeding behaviour of the sessile gastropod Tripsycha tulipa (Vermetidae). J Mollus Stud 51:326–330
Jebakumar JPP, Nandhagopal G, Ragumaran S, Rajanbabu B, Ravichandran V (2015) First record of alien species Eualetes tulipa (Rousseau in Chenu, 1843) from the Royapuram fishing harbour at Chennai, India. Bioinvasions Rec 4(3):201–204. doi:10.3391/bir.2015.4.3.08
Keen AM (1971a) Two new supraspecific taxa in the Gastropoda. Veliger 13:296–296
Keen AM (1971b) Sea shells of tropical west America. Standford University Press, California
Losada F, Martín A, Feragotto W, Alamo C (1988) Interacciones biológicas en el canal de toma de la planta termoeléctrica del centro Punta Morón, Venezuela. Ecotropicos 1:55–70
López MS, Coutinho R, Ferreira CEL, Rilov G (2010) Predator prey interactions in a bioinvasion scenario: differential predation by native predators on two exotic rocky intertidal bivalves. Mar Ecol Progr Ser 403:101–112. doi:10.3354/meps08409
López MS, Lavrado HP, Coutinho R (2014) Structure of intertidal sessile communities before and after the invasion of Isognomon bicolor (C.B. Adams, 1845) (Bivalvia, Isognomonidae) in southeastern Brazil. Aquat Invasions 9(4):457–465. doi:10.3391/ai.2014.9.4.04
Martinez AS, Mendes LF, Leite TS (2012) Spatial distribution of epibenthic mollusks on a sandstone reef in the Northeast of Brazil. Braz J Biol 72(2):287–298. doi:10.1590/S1519-69842012000200009
Matthews HR (1967) Primeira contribuição ao inventário dos moluscos marinhos do nordeste brasileiro. Arq Est Biol Mar Univ Fed Ceará 7(1):67–77
Matthews HR, Rios EC (1969) Terceira contribuição ao inventário dos moluscos marinhos do nordeste brasileiro. Arq Est Biol Mar Univ Fed Ceará 9(1):27–35
Matthews HR, Rios EC (1974) Quarta contribuição ao inventário dos moluscos marinhos do nordeste brasileiro. Arq Ciên Mar, Fortaleza 14(1):47–56
Matthews-Cascon H, Matthews HR, Kotzian CB (1989) Os gêneros Fasciolaria Lamarck, 1799 e Leucozonia Gray, 1847 no nordeste brasileiro (Mollusca: Gastropoda: Fasciolariidae). Mem Inst Oswaldo Cruz 84(4):357–364. doi:10.1590/S0074-02761989000800064
Miloslavich P, Penchaszadeh PE (1992) Reproductive biology of Vermetus sp. and Dendropoma corrodens (Orbigny, 1842): two vermetid gastropods from the southern Caribbean. Veliger 35(1):78–88
Miloslavich P (1996) Nurse-egg feeding prosobranchs: a comparative biochemical and electrophoretic analysis of eggs and hatchlings. Am Malacol Bull 13(1–2):37–46
Miloslavich P, Klein E, Penchaszadeh PE (2010) Gametogenic cycle of the tropical vermetids Eualetes tulipa and Dendropoma corrodens (Mollusca: Caenogastropoda: Vermetidae). J Mar Biol Assoc UK 90:509–518. doi:10.1017/S0025315409991287
Miranda PTC, Freire GSS, Matthews-Cascon H (2013) Macrofauna marinha introduzida na costa do estado do Ceará, Nordeste do Brasil. Arq Ciên Mar, Fortaleza 46(2):86–91
Monteiro DO (2003) Macrofauna bentônica da faixa entre marés em dois quebra mares da região Portuária de Fortaleza-Ceará. Dissertation, Universidade Federal do Ceará, Fortaleza, Brazil
Molnar JL, Gamboa RL, Revenga C, Spalding MD (2008) Assessing the global threat of invasive species to marine biodiversity. Front Ecol Environ 6:485–492. doi:10.1890/070064
Mota SS (2006) Aspecto geral dos animais incrustantes e sua fauna associada no pilar do Porto do Pecém. Dissertation, Universidade Federal do Ceará, Fortaleza, Brazil
Oliveira MIM (1971) Contribuição ao estudo da malacofauna intertidal de recifes de arenito no nordeste brasileiro. Arq Ciên Mar, Fortaleza 11(2):83–86
Osman R, Shirley T (2007) Proceedings and final report of the Gulf of Mexico and Caribbean Marine Invasive Species Workshop, Harte Research Institute for Gulf of Mexico Studies and Smithsonian Renvironmental Research Center, Corpus Christi, Texas
Penchaszadeh P, León CA, Alvarez H et al (2000) The coastline of Venezuela. In: Sheppard C (ed) Seas at the millennium. An environmental evaluation. University of Warwick, UK, Volume I: Europe, The Americas and West Africa, pp 643–661
Phillips N, Shima J (2009) Reproduction of the vermetid gastropod Dendropoma maximum (Sowerby, 1825) in Moorea, French Polynesia. J Mollusc Stud 76:133–137. doi:10.1093/mollus/eyp049
Rabay SG, Barroso CX, Matthews-Cascon H (2012) Mollusks of recruitment panels placed on Terminal Portuário do Pecém, Ceará, NE Brazil. In: XI Internacional Congress on Medical and Applied Malacology, 2012, Rio de Janeiro. XI International Congress on Medical and Applied Malacology Crossing boundaries: Integrative Approaches to Malacology Abstract Book. Rio de Janeiro, Brazil
Rawlings TA, MacInnis MJ, Bieler R, Boore JL, Collins TM (2010) Sessile snails, dynamic genomes: gene rearrangements within the mitochondrial genome of a family of caenogastropod mollusks. BMC Genomics 11:440. doi:10.1186/1471-2164-11-440
Rogers R, Correal GO, Oliveira TC et al (2014) Coral health rapid assessment in marginal reef sites. Mar Biol Res 10:612–624. doi:10.1080/17451000.2013.841944
Schiaparelli S, Cattaneo-Vietti R (1999) Functional morphology of Vermetid feeding-tubes. Lethaia 32:41–46. doi:10.1111/j.1502-3931.1999.tb00579.x
Schiaparelli S, Guidetti P, Cattaneo-Vietti R (2002) Can mineralogical features affect the distribution patterns of sessile gastropods? The Vermetidae case in the Mediterranean Sea. J Mar Biol Assoc UK 83:43–52. doi:10.1017/S0025315403008622
Shima JS, Phillips NE, Osenberg CW (2013) Consistent deleterious effects of vermetid gastropods on coral performance. J Exp Mar Biol Ecol 439:1–6. doi:10.1016/j.jembe.2012.10.012
Schluker A (2003) State of Hawaii aquatic invasive species (AIS) management plan. The Department of Land and Natural Resources, Division of Aquatic Resources. The Nature Conservancy of Hawaii, Honolulu. http://www.anstaskforce.gov/State%20Plans/More/HAWAII%20mgt%20PLAN%2003.pdf. Accessed 20 March 2016
Strathmann MF, Strathmann RR (2006) A vermetid with complex intracapsular cannibalism of nurse eggs and sibling larvae and a high potential for invasion. Pac Sci 60(1):97–108. doi:10.1353/psc.2005.0062
Thatje S, Hall S (2016) The effect of temperature on the evolution of per offspring investment in a globally distributed family of marine invertebrates (Crustacea: Decapoda: Lithodidae). Mar Biol 163:48. doi:10.1007/s00227-015-2776-8
Thorson G (1950) Reproductive and larval ecology of marine bottom invertebrates. Biol Rev 25:1–45
Valentin J, Andre D, Jacob S (1987) Hydrobiology in the Cabo Frio (Brazil) upwelling: two dimensional structure and variability during a wind cycle. Cont Shelf Res 7:77–88. doi:10.1016/0278-4343(87)90065-3
Vega AJ, González A (2002) Moluscos del PacíficoVeraguense. Tecnociencia 4:1–45
Weinberger VP, Miloslavich P, Machordom P (2010) Distribution pattern, reproductive traits, and molecular analysis of two coexisting vermetid gastropods of the genus Petaloconchus: a Caribbean endemic and a potential invasive species. Mar Biol 157(7):1625–1639. doi:10.1007/s00227-010-1435-3
Acknowledgements
We are grateful to the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro-FAPERJ and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-CAPES for providing post-doc fellowships (PSO and FTST); the Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq for providing the productivity fellowship (RC) and the Instituto Chico Mendes de Conservação da Biodiversidade for research licence SISBIO no 44575–3. We thank the Instituto de Estudos do Mar Almirante Paulo Moreira-IEAPM for field and laboratory support. We are also grateful to Cristina de Almeida Rocha-Barreira (CMPHRM – A), Helena Matthews Cascon and Cristiane Xerez Barroso (CMPHRM – B), Sérgio Mendonça Almeida and Luiz Ricardo L. Simone (MZSP), Luís Felipe Skinner (UERJ), Sula Salani Mota (MNRJ), Alina Rocha Pires Barboza and Tatiana Leite (LABECE, UFRN) for kindly assisting with the examined material in this study; to Daniel Souza dos Santos for the artwork in Fig. 1, to Gabriela Perna for kindly reviewing the English version; to Rudiger Bieler, an anonymous reviewer and the associated editor Victoriano Urgorri for improving this paper with useful comments. Rudmar Krumreick and Caroline Ruas (Centro de Microscopia Eletrônica do Sul, CEME-SUL, Universidade Federal do Rio Grande, FURG) are thanked for the SEM images.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by V. Urgorri
Rights and permissions
About this article
Cite this article
Spotorno-Oliveira, P., Coutinho, R. & de Souza Tâmega, F.T. Recent introduction of non-indigenous vermetid species (Mollusca, Vermetidae) to the Brazilian coast.. Mar Biodiv 48, 1931–1941 (2018). https://doi.org/10.1007/s12526-017-0702-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-017-0702-7