Abstract
Deep-sea decapod crustaceans (Crustacea: Decapoda) collected during nine research cruises to the Clarion-Clipperton Zone (CCZ) in the NE Pacific Ocean and the Peru Basin in the SE Pacific Ocean were studied comprehensively using an integrative taxonomic approach. The abyssal seafloors of both areas are rich in economically interesting polymetallic nodules. All specimens were morphologically identified and genetically analysed using a fragment of the mitochondrial cytochrome c oxidase subunit I (COI). Eight species were collected, comprising three anomurans, three carideans, one dendrobranchiate, and one brachyuran, from water depths ranging between 4089 and 4511 m. COI sequences for representatives of the genera Parapagurus Smith, 1879, Ethusina SI Smith, 1884, and Bathystylodactylus Hanamura & Takeda, 1996 are provided for the first time. The molecular barcodes of the species provided herein will be valuable for the full taxonomic assignment of sequences produced in future metabarcoding and eDNA monitoring work. The new records extend the geographical distributional ranges or fill geographical gaps of the species reported, although none of the species is endemic to polymetallic nodule areas. This study is part of a taxonomic series aiming to describe the biodiversity of areas targeted for future deep-sea mining.
Similar content being viewed by others
Introduction
In large parts of the world’s oceans, nodules rich in valuable metals such as Ni, Cu, Co and Mn (Hein et al. 2013; Miller et al. 2018) are present at abyssal depths. These areas have been identified as being of economic interest and are being targeted for potential mining, despite the hitherto limited information on their intrinsic biodiversity (Amon et al. 2016). The largest known deposits of polymetallic nodules occur in the Clarion-Clipperton Zone (CCZ), spanning an area of about 6 million km2 in size between Hawaii and Mexico. Additional important occurrences have been found in the Peru Basin (e.g. Hein et al. 2013; Miller et al. 2018), around the Cook Islands (Hein et al. 2015), and in the Central Indian Ocean (Mukhopadhyay et al. 2008). Nodule mining comes with significant environmental concerns, such as habitat loss, fragmentation or modification after the removal of nodules and associated organisms, and the generation of sediment plumes that may bury organisms or clog their feeding apparatuses (Ramirez-Llodra et al. 2011; Vanreusel et al. 2016; Van Dover et al. 2017; Niner et al. 2018; Stratmann et al. 2018). Studies have suggested that abyssal fields appear to support higher densities of both sessile and mobile epifauna in locations with high nodule coverage compared to those lacking nodules (Amon et al. 2016; Vanreusel et al. 2016; Simon-Lledó et al. 2019a, 2019b, 2019c, 2020). The deposits of the CCZ as well as those of the Peru Basin and the Indian Ocean lie in waters beyond National Jurisdictions, and thus fall under the legal mandate of the International Seabed Authority, ISA (Wedding et al. 2013). The ISA has issued, so far, 16 contract areas for the exploration for polymetallic nodules in the CCZ. Prior to exploitation, a benthic biological baseline study must be undertaken by the contractor for each exploration area, and effective environmental impact assessments and management, monitoring and contingency plans responding to possible environmental impacts arising from mining should be developed (Wedding et al. 2015). Furthermore, the ISA assigned a series of Areas of Particular Environmental Interest (APEIs) outside these contract areas, where exploitation is prohibited. The thirteen APEIs areas were installed to preserve and protect source populations, which may then recolonize future impacted areas (Lodge et al. 2014). Accurate documentation of species diversity and geographic distribution are essential for marine ecosystems’ management, which, despite recent scientific studies (i.e. Kersken et al. 2018, 2019; Jakiel et al. 2019; Christodoulou et al. 2020; Malyutina et al. 2020; Gooday et al. 2020), still remains poorly documented across the CCZ.
Decapods are one of the most diverse group of crustaceans numbering over 15,000 living species (De Grave et al. 2009), and are a significant faunal component in an impressive variety of habitats, although the majority of decapods are marine species with ranges spanning intertidal to hadal depths (Jamieson et al. 2009). Because of their ecological and morphological diversity, and coupled with their economic importance and size, they are the most thoroughly studied of all crustaceans. Nevertheless, studies describing the abyssal decapod fauna and specifically that of polymetallic areas of the East Pacific are limited, including a few historical studies resulting from the great expeditions of the late nineteenth and early twentieth centuries such as “HMS Challenger” (Spence Bate 1888) and “Albatross” (Faxon 1893, 1895). Only a handful of recent studies exist, all reporting a number of image-based putative species (Amon et al. 2017; Simon-Lledó et al. 2019a). As such, the diversity of the deep-sea decapod fauna in the CCZ and Peru Basin remain only fragmentarily known. Thus, the aim of this study was to provide morphology-based species identifications of decapods together with molecular species assignments using COI sequences in an integrative approach, thereby compiling a reference library for future genetic work.
Material and methods
Study areas
The study areas (Fig. 1) are located within the Clarion and Clipperton Zone (CCZ) in the northeast equatorial Pacific Ocean and at the DISCOL Experimental Area (DEA) in the Peru Basin, an area in which the German project DISCOL (DISturbance and reCOLonisation experiment) was performed in the late 1980s (Thiel and Schriever 1990; Thiel et al. 2001). Water depths vary from 4050 m to 4933 m (Hein et al. 2013). In the CCZ, Decapod samples were collected from the UKSRL and OMS contract areas (UK Seabed Resources Ltd, UK; Ocean Mineral Singapore Ltd), the BGR contract area (German Federal Institute for Geoscience and Natural Resources, Germany) and the GSR contract area (Global Sea Mineral Resources NV, Belgium), as well as from one APEI (APEI#6) during nine scientific cruises between 2013 and 2019: ABYSSLINE I (R/V Melville), ABYSSLINE II (R/V Thompson), MANGAN 2013 (R/V Kilo Moana), JPIO-EcoResponse/SO239 (R/V Sonne), MIDAS JC120 (R/V James Cook), MANGAN 2016 (R/V Kilo Moana), MANGAN 2018 (R/V Sonne), and JPIO-Mining Impact/SO268-1, SO268-2 (R/V Sonne). Furthermore, the DEA area in the Peru Basin was revisited in 2015 in the framework of the JPIO Pilot Action “Ecological Aspects of Deep-Sea Mining”. Decapod samples were collected from the DEA during cruise SO241-2 using the R/V Sonne.
Compilation of study areas in the Clarion-Clipperton Zone (CCZ) and in the DISCOL Experimental Area (DEA, Peru Basin). Insets represent detailed maps of sampling locations in the GSR#1, GSR#2, OMS, BGR and UKSRL exploration contract areas for polymetallic nodules. Copyright for shapefiles (for CCZ area): © International Seabed Authority 2009–2019; copyright for raster file (bathymetry): © Natural Earth 2009–2020
Specimen sampling and processing
Anomura and Brachyura were collected with a remotely operated vehicle (ROV 6000, Kiel, GEOMAR) using either the ROV’s suction sampler or the manipulator arm by direct picking. Following their collection, specimens were preserved in pre-cooled 96% EtOH. For all specimens the ethanol was decanted after 24 hours and replaced with new 96% EtOH to guarantee sufficient ethanol concentration for preservation of high-quality DNA, and subsequently stored at -20°C. Caridea and Dendrobranchiata were collected using baited traps, trawling and a Brenke-type epibenthic sledge (EBS), following standard deployment procedures (Brenke 2005). The cod ends of the suprabenthic- and epibenthic-net were sieved through a 500 μm- and 300 μm-mesh with cold (+10°C) sea water and immediately transferred to pre-cooled (-20°C) 96% EtOH. In the laboratory at Senckenberg am Meer, Germany, an integrative molecular-morphological approach was implemented for the identification of the decapod specimens. Voucher specimens are stored at Senckenberg am Meer, DZMB, Wilhelmshaven, Germany and in the Oxford University Museum of Natural History, Oxford, UK. Carapace length (cl) was measured from the posterior margin of the orbit to the posterior margin of the carapace for the Caridea and Dendrobranchiata, and from the tip of the frontal teeth to the posterior margin of the carapace for the Brachyura. Postorbital carapace length (pcl) and shield length (sl) was measured from the tip of the rostrum to the midpoint of the posterior margin of the carapace or shield in Munidopsidae and Parapaguridae, respectively.
Barcoding data collection
DNA extraction, amplification, and sequencing
For the mtDNA COI analyses, genomic DNA was extracted from abdominal or pereiopod tissue. DNA extractions were carried out using 30 μl Chelex (InstaGene Matrix, Bio-Rad) according to the protocol of Estoup et al. (1996) and directly used as DNA template for PCR. All DNA samples were stored at −20°C. A fragment of 658bp of the mitochondrial cytochrome c oxidase subunit (COI) was amplified by polymerase chain reaction (PCR). Amplifications were performed using AccuStart PCR SuperMix (ThermoFisher Scientific) in a 25-μL volume containing 12.5 μL AccuStart PCR SuperMix, 9.5 μL ddH2O, 0.5 μL of each primer (10 pmol μL−1) and 2 μL of DNA template. For the COI amplification the forward primer jgLCO1490 and the reverse primer jgHCO2198 (Geller et al. 2013), tailed with M13F and M13R-pUC, respectively were used. The amplification conditions consisted of an initial denaturation step of 3 min at 94°C, 35 cycles of 30 s at 94°C, 60 s at 47°C and 1 min at 72°C, followed by a final extension step of 5 min at 72°C. All PCR products were purified using ExoSap-IT (ThermoFisher Scientific). Amplified fragments were sequenced in both directions at Macrogen Europe Laboratory (Amsterdam, The Netherlands).
Alignment, genetic divergence
The obtained COI sequences were compared with the GenBank nucleotide database using BLASTN (Altschul et al. 1990). Forward and reverse sequences for each individual were assembled and edited using Geneious v.9.1.7 (www.geneious.com; Kearse et al. 2012). The edited COI sequences and over 500 bp in length were aligned using MAFFT v7.308 under G-INS-I algorithm (Katoh et al. 2002), while alignments were further manually edited. The sequence of B. echinus was omitted from the alignment because of its short length (315 bp). Sequence data are available in GenBank (Table 1). The sequences, trace files, collection data and photos for each specimen are listed in the datasets CCZ-DEA_Decapoda (https://doi.org/10.5883/DS-CCZ2) in BOLD (Ratnasingham and Hebert 2007).
Results
Taxonomic and systematic remarks
Order Decapoda Latreille, 1802
Suborder Dendrobranchiata Spence Bate, 1888
Family Solenoceridae Wood-Mason in Wood-Mason & Alcock, 1891
Hymenopenaeus doris (Faxon, 1893)
(Fig. 2)
Hymenopenaeus doris (Faxon, 1893): female (cl 24.9 mm), AB01_TR01_2, lateral view. Scale 1.0 cm (photo by Inga Mohrbeck)
Haliporus doris Faxon 1893: 214 [type locality: off Cabo Velas, Costa Rica, 10°14′N 96°28′W, 4082 m, Albatross stn. 3414; S of Punta Maldonado, Guerrero, Mexico, 14°46′N 98°40′W, 3436 m, Albatross stn. 3415]. — Faxon 1895: 191; Plate 49, Figs. 1a–c.
Hymenopenaeus doris. — Burkenroad 1938: 60. — Crosnier and Forest 1973: Fig. 83d. — Perez-Farfante 1977: 283; Figs. 9, 17, 18a, 19-20. — Hanamura 1983: 55; Fig. 2. — Hendrickx 2001: 97. — Hendrickx 2008: 999; Fig. 1. — Hendrickx 2013: 442.
Aliporis doris. — del Solar 1972: 7.
Material examined. Clarion Clipperton Zone: ABYSSLINE1 cruise, R/V Melville, stn. TR01 (baited trap), 13°52.933N 116°26.931W (UKSRL contract area), 4203 m, 08.10.2013, 2 females, cl 30.2 mm (AB01_TR01_1) and 24.9 mm (AB01_TR01_2); ABYSSLINE2 cruise, R/V Thompson, stn. EB04 (epibenthic sled), 12°07.83N 117°18.67W (OMS contract area), 4111 m, 25.02.2015, 1 female, cl 6.1 mm (AB02_EB04_1); MANGAN13 cruise, R/V Kilo Moana, stn. 18EBS (epibenthic sled), 11°49.754N 117°0.371W (BGR contract area), 4133 m, 14.03.2013, 1 male, cl 20.0 mm (MA13_18_35).
Distribution. Eastern Pacific Ocean. Previously known from Mexico (Gulf of California, south of Punta Maldonado), Costa Rica (off Cabo Velas, Cocos Island), Peru (Guanape) and Dowd Tablemount in the Northeast Pacific; at depths of 549–4082 m (Faxon 1893; Burkenroad 1938; del Solar 1972; Perez-Farfante 1977; Hanamura 1983; Hendrickx 2001, 2008, 2013). The present specimens reported from the UKSRL, OMS and BGR contract areas at depths of 4078-4203 m represent new records for the CCZ, slightly extending the known geographical range of the species to the west and its bathymetric distribution by more than 120 m.
Remarks. The genus Hymenopenaeus Smith, 1882 is currently composed of 17 species, with the vast majority occuring in the Indian and Pacific Oceans (Perez-Farfante 1977; Crosnier 1995; Crosnier and Dall 2004). In the East Pacific where the CCZ is located, only H. doris and H. nereus (Faxon, 1893) are known to occur (Hendrickx 2008). Hymenopenaeus nereus has been reported from the CCZ and specifically from the UKSRL and BGR contract areas (as H. cf. nereus: Amon et al. 2017, as H. nereus: Hendrickx and Wicksten 2016; Harbour et al. 2020), while this is the first time H. doris is reported from this area. The females of Hymenopenaeus doris can be distinguished from those of H. nereus by having a strong setose triangular protrusion (cusp) between the bases of the fifth pereiopods rather than a low non-dentate ridge and two triangular lamellae (projections) flanking the transverse diaphragm between the fourth pereiopods (Faxon 1893, 1895; Burkenroad 1938; Crosnier and Forest 1973; Perez-Farfante 1977). The males of both species can be distinguished by the shape of their petasma and the form of their appendices masculina and interna (Hanamura 1983; Hendrickx 2008). The morphology of the largest female collected agrees well with the descriptions and illustrations provided by Faxon (1895), Crosnier and Forest (1973) and Perez-Farfante (1977). Although the cusp on the 14th sternite (between P5 bases) is not as dentate as illustrated by Crosnier and Forest (1973), a clear and strong cusp is present. The two smaller females differ from the typical form of H. doris by having a much smaller triangular cusp and less distinct triangular lamellae. The characteristics of the male specimen collected agree well with the description and illustrations provided by Hanamura (1983) picturing a younger individual than the one described by Hendrickx (2008).
This is the first time COI sequences (OM892117, OM892118, OM892125, OM892127) have been made available for this species. All collected specimens shared the same haplotype and differ significantly from other Hymenopenaeus species (p-distance: < 10.91%–17.14%) present in Genbank (Fig. 3).
Suborder Pleocyemata Burkenroad, 1963
Infraorder Anomura MacLeay, 1838
Superfamily Galatheoidea Samouelle, 1819
Family Munidopsidae Ortmann, 1898
Munidopsis kensmithi Jones & Macpherson, 2007
(Fig. 4)
Munidopsis kensmithi Jones & Macpherson, 2007: a male, pcl 30.96 mm (SO268-2_46) in situ; 1 female, pcl 33.47 mm (SO268-1_101); b in situ (up); c dorsal and ventral view (down). Scale 1.0 cm (photo by Nicol Mahnken) d example of Munidopsis cf. kensmithi image taken using OFOS
Munidopsis kensmithi Jones and Macpherson 2007: 485; Figs 5–6 [type locality: off California, 34°50′N 123°00′W, 4100 m]. — Dong et al. 2017: 394; Figs. 3b-c, 4c.
Material examined. Clarion Clipperton Zone: SO268-1 cruise, R/V Sonne, stn. 64 (dive ROV09), 14°06.76746'N 125°52.25934'W (GSR contract area), 4507 m, 17.03.2019, 1 male, pcl 34.93 mm (SO268-1_75); SO268-1 cruise, R/V Sonne, stn. 64 (dive ROV09), 14°06.7677'N 125°52.26018'W (GSR contract area), 4507 m, 17.03.2019, 1 female, pcl 35.31 mm (SO268-1_76). SO268-1 cruise, R/V Sonne, stn. 77 (dive ROV12), 14°07.10280'N 125°52.0356'W (GSR contract area), 4511 m, 20.03.2019, 1 female, pcl 33.47 mm (SO268-1_101). SO268-2 cruise, R/V Sonne, stn. 134 (dive ROV19), 14°06.78372'N 125°52.2565'W (GSR contract area), 4508 m, 17.04.2019, 1 female pcl 39.45 mm (SO268-2_32). SO268-2 cruise, R/V Sonne, stn. 137 (dive ROV20), 14°06.68514'N 125°52.30224'W (GSR contract area), 4496 m, 18.04.2019, 1 male, pcl 34.78 mm (SO268-2_43); SO268-2 cruise, R/V Sonne, stn. 137 (dive ROV20), 14°06.67074'N 125°52.2912'W (GSR contract area), 4497 m, 18.04.2019, 1 male, pcl 30.11 mm (SO268-2_44); SO268-2 cruise, R/V Sonne, stn. 137 (dive ROV20), 14°06.65148'N 125°52.27782'W (GSR contract area), 4496 m, 18.04.2019, 1 male, pcl 26.16 mm (SO268-2_45); SO268-2 cruise, R/V Sonne, stn. 137 (dive ROV20), 14°06.63096'N 125°52.26186'W (GSR contract area), 4496 m 18.04.2019, 1 male, pcl 30.96 mm (SO268-2_46). SO268-2 cruise, R/V Sonne, stn. 174 (dive ROV28), 11°50.61690'N 117°03.49254'W (BGR contract area), 4137 m, 09.05.2019, 1 female, pcl 39.33 mm (SO268-2_131).
Distribution. Tropical Pacific Ocean. Previously known from off California (eastern Pacific Ocean) at 4100 m (Jones and Macpherson 2007) and Lamont Guyot, east Mariana Trench (tropical west Pacific Ocean) (Dong et al. 2017); at depths of 4100 and 4833 m respectively. The present study records the species in the CCZ, eastern Pacific Ocean for the first time, at 4137-4511 m.
Coloration in life. Whole body is pale-white. Cornea pale pink. However, in-situ photographs show a white-brown appearance because of sedimental mud on the body surface and pereiopods (Fig. 2).
Remarks. The genus Munidopsis is the second most species-rich genus of squat lobsters, numbering 272 species (WoRMS Editorial Board, 2022; accessed at: 2022-05-11). In the Eastern Pacific the genus is represented by at least 48 species (Hendrickx and Ayón-Parente 2013; Poore 2014). Munidopsis kensmithi can be morphologically distinguished from all other Munidopsis species by the presence of numerous granules on the dorsal surface of the carapace, pleon and pereiopods; the presence of a spiniform median process on the second, third and fourth pleon segments; the narrower and slightly directed upwards rostrum; the longer distal spines of the antennular basal segment and the equal spacing between the penultimate and ultimate teeth and dactylus, respectively (Jones and Macpherson 2007). The present specimens agree well with the original description of M. kensmithi (Jones and Macpherson 2007) and represent the second record since its original description.
The COI sequences (OM892114, OM892119, OM892120, OM892123, OM892124, OM892126, OM892130) are identical or very similar (p-distance: < 0.0%–3.0%) to COI sequences from the type series of M. kensmithi (DQ677706-DQ677709). The mean intraspecific genetic divergence (p-distance) ranged from 0% to 3.0%, including the sequences provided in Jones and Macpherson (2007), while when compared with other Munidopsis species from the East Pacific Ocean (Fig. 3), the interspecific genetic divergence (p-distance) ranged from 5.3% to 13.98%.
Superfamily Paguroidea Latreille, 1802
Family Parapaguridae Smith, 1882
Probeebei mirabilis Boone, 1926
(Fig. 5)
Probeebei mirabilis Boone, 1926, male, sl 29.83 mm (SO242-2_222_1): a in situ; b dorsal view; c ventral view. Scale 1.0 cm (photos by Nicol Mahnken)
Probeebei mirabilis Boone 1926: 73; unnumbered figure [type locality: eastern Pacific, off Peru, Albatross stn. 4647, 04°33'S 87°42′30'′W, 3667 m]. — Wolff 1961: 12; Figs. 1–10. — Garth and Haig: 6.5. — Lemaitre 1998: 300; Figs. 2b–d, 3b. — Bluhm 2001: 3847. — Drazen et al. 2019: 3139; Fig. 6n.
Material examined. DISCOL Experimental Area (DEA), Peru Basin: SO242-2 cruise, R/V Sonne, stn. 216 (dive ROV19),07°07.52781'S88°27.05246'W, 4197 m, 22.09.2015, 1 male, sl 20.65 mm (SO242-2_216_1). SO242-2 cruise, R/V Sonne, stn. 222 (dive ROV21), 07°04.68467'S 88°27.46078'W, 4193 m, 24.09.2015, 1 male, sl 29.84 mm (SO242-2_222_1).
Distribution. East Pacific Ocean. Previously known from off Peru at 3869-3995 m (Garth and Haig 1971); south of Cocos Island to southwest of Galapagos at 1145-4775 m depth (Lemaitre 1998); DISCOL Experimental Area (DEA) in the Peru Basin at 4057-4220 m (Bluhm 2001; Drazen et al. 2019).
Coloration in life. Carapace and abdomen generally pale white; chelipeds and ambulatory legs pale orange. Cornea black (Fig. 5).
Remarks. Probeebei mirabilis is the sole species of the genus Probeebei, an exclusively abyssal inhabitant (Lemaitre 1998) found usually with an attached anemone on its pleon (Drazen et al. 2019; present study). Probeebei mirabilis resembles Tylaspis anomala Henderson, 1885, another parapagurid, in having a partially fused and calcified cephalothorax, unprotected by a gastropod, a unique character within parapagurids but can easily be distinguished from T. anomala and other parapagurids by the long rostrum often exceeding the ocular peduncles (Lemaitre 1998). Additionally, the abdomen of T. anomala is slightly twisted to the right and mostly membranous, while that of P. mirabilis is well-calcified (and asymmetrical in females) up to the fifth tergite and armed with spines. The present male specimens, both bearing anemones on their pleons and having symmetrical abdomens, agree well with the description provided by Wolff (1961) and Lemaitre (1998).
This is the first time COI sequences (OM892112, OM892131) are available for this species. The COI sequences are very similar with an intraspecific variability (p-distance) of 0.76%.
Parapagurus microps de Saint Laurent, 1972
(Fig. 6)
Parapagurus microps de Saint Laurent 1972: 104; Figs. 1, 13, Plate 1, Fig. 8 [type locality: Galapagos (0°04'S 117°07'W), 4250 m, Albatross stn. 4742]. — Lemaitre 1999: 322; Figs. 10 and 11.
Material examined. Clarion Clipperton Zone: SO268-2 cruise, R/V Sonne, stn. 163 (dive ROV25), 11°55.76172'N 117°01.43946'W (BGR contract area), 4089 m, 28.04.2019, 1 male, sl 9.85 mm (SO268-2_95).
Distribution. Eastern Pacific Ocean. Parapagurus microps was previously known from off the Galapagos Islands at 3812–4721 (de Saint Laurent 1972); the DISCOL Experimental Area at 4154–4261 m and off Peru at 2710–3080 m (Lemaitre 1999). The present specimens reported from the BGR contract area represent a new record of this species in the CCZ.
Coloration in life. Carapace and abdomen generally pale orange; chelipeds and ambulatory legs orange. Cornea black (Fig. 6).
Remarks. The genus Parapagurus Smith, 1879 is represented by 17 species worldwide (Lemaitre 1999). In the eastern Pacific, the following six species have been reported: Parapagurus benedicti de Saint Laurent, 1972; P. foraminosus Lemaitre, 1999; P. holthuisi Lemaitre, 1989; P. janetae Lemaitre, 1999; P. microps de Saint Laurent, 1972; P. wolffi Lemaitre, 1999. Parapagurus microps can be distinguished from its congeneric species in having the merus, carpus, and propodus of ambulatory legs armed on the mesial and lateral faces with numerous tubercles and spines, the anterodistal margin of the branchiostegite unarmed and the propodal rasp of the fourth pereopod with one irregular row of lanceolate scales. The collected specimen, baring two anemones on its pleon, agrees well with these diagnostic characters.
This is the first time a COI sequence (OM892128) is available for this genus and species.
Infraorder Brachyura Latreille, 1802
Family Ethusidae Guinot, 1977
Ethusina cf. faxonii Rathbun, 1933
(Fig. 7)
Ethusina cf. faxonii Rathbun, 1933, 1 male, cl 8.69 mm (SO268-2_137): a specimen under an ophiuroid, in situ; b in situ collection of a crab together with an ophiuroid using the ROV arm; c dorsal and ventral view. Scale 1.0 cm (photos by Nicol Mahnken)
Ethusina faxonii Rathbun 1933: 185, [type locality: S. of Gulf of Tehuantepec, 10°14'00”N 96°28'00”W, 4082 m, Albatross stn. 3414]. — Rathbun 1937: 93; Plate 26, Fig. 3; Plate 27, Fig. 3.—Garth 1960: 120. — Garth and Haig 1971: 6.9.— Hendrickx 1995: 128.
Material examined. Clarion Clipperton Zone: SO268-2 cruise, R/V Sonne, stn. 193 (dive ROV30), 11°51.79002'N 117°00.77862'W (BGR contract area), 4128 m, 13.05.2019, 1 male, 8.69 mm (SO268-2_137).
Distribution. Eastern Pacific Ocean. Ethusina faxonii was previously known from off Mexico, south of the Gulf of Tehuantepec at 4081 m (Rathbun 1933); northwest of Tres Marias Islands at 2999 m (Garth 1960); and off Peru at 3086 to 3995 m (Garth and Haig 1971). The present specimen from the BGR contract area represent the first record of this genus and probably of the species from the CCZ.
Coloration in life. Carapace and abdomen generally light brown; chelipeds and ambulatory legs pale white with orange chelae. Cornea black (Fig. 5).
Remarks. The genus Ethusina SI Smith, 1884 comprises of 33 species, with most of them occurring in the Indo-west Pacific region (WoRMS Editorial Board, 2022; accessed at: 2022-05-11), and only two species recorded with certainty from the Eastern Pacific (E. faxonii and E. smithiana; Hendrickx 1995; Castro 2005), with Castro (2005) considering the records of E. robusta and E. gracilipes to belong to an undescribed species. Ethusina faxonii can easily be distinguished from most other species of Ethusina by the sinuous shape of the frontal portion of its carapace with the concave median lobes lacking terminal teeth (Rathbun 1933). Ethusina faxonii is very similar to E. challengeri but differs in having more slender chelae, fourth and fifth pereiopods and minute orbital teeth instead of rounded tubercle-like outer orbital teeth (Rathbun 1933). Since the original description of E. faxonii, less than a handful records have come to light, most females and only one male (Garth and Haig 1971). The current male specimen agrees well with the above characters, but differs in having a narrower and less inflated carapace than the female one illustrated by Rathbun (1933; Plate 26-27). This difference can perhaps be attributed to sexual dimorphism within the species but until a detailed morphological comparison is undertaken of all available specimens, the current specimen is conservatively identified as E. cf. faxonii. This is the first time a COI sequence (OM892122) is available for the genus Ethusina.
Infraorder Caridea Dana, 1852
Family Acanthephyridae Spence Bate, 1888
Acanthephyra trispinosa Kemp, 1939
(Fig. 8)
Acanthephyra trispinosa Kemp, 1939: female, cl 20.01 mm (SO239_133_1252), lateral view. Scale 1.0 cm (photo by Nicol Mahnken)
Acanthephyra trispinosa Kemp 1939: 577 [type locality: west coast of Central America from 7°N to 4°S, extending westwards to 116°W].— Chace 1986: 9; Figs. 3m, 4y, 5y, 7l, 10h. — Vereshchaka 1990: 139. — Guzman 2003: 3.
Material examined. Clarion Clipperton Zone: SO239 cruise, R/V Sonne, stn. 133EBS, 13°51.31002'N 123°13.72998'W (GSR contract area), 4507 m, 10.04.2015, 1 female, cl 20.01 mm (SO239_133_1252).
Distribution. Eastern Pacific Ocean. Reported from west coast of Central America between 7°N and 4°S (off Peru), westward to 116°W (Chace 1986); off Chile, from Sala y Gomez and Nazca seamounts (21°41'–25°40'S 81°19'–86°34'W) at 160–2280 m (Vereshchaka 1990) and 18°25'– 21°04'S 70°40'–71°3'W at 374–540 m (Guzman 2003). The present specimens reported from the GSR contract area represent a new record for the CCZ, and significantly extend the geographical range of the species to the north (13°N) and west (123°W) as well as its bathymetric distribution to abyssal (4507 m) depths.
Remarks. The genus Acanthephyra A. Milne-Edwards, 1881, contains 27 species (De Grave and Fransen 2011) of which 12 have been recorded in the eastern Pacific. Acanthephyra trispinosa can be distinguished from all species in the genus by the presence of a dorsal posteromedian tooth on the third to sixth somites, with that of the third being the largest; telson with three pairs of dorsolateral cuspidate setae; small branchiostegal tooth supported by a short-rounded ridge; sixth somite length about twice its posterior width; and the rostrum being shorter than the carapace, extending to or slightly beyond the scaphocerite (Kemp 1939; Chace 1986). The present specimen agrees well with these diagnostic characters, despite having a broken rostrum tip. The rostrum bears 7 dorsal and five ventral teeth, as also mentioned by Guzman (2003).
This is the first time a COI sequence (OM892121) is available for this species. The haplotype of the collected specimen different significantly from other Acanthephyra species present in Genbank (p-distance: < 12.35%–17.61%).
Family Crangonidae Haworth, 1825
Parapontophilus occidentalis (Faxon, 1893)
(Fig. 9)
Parapontophilus occidentalis (Faxon, 1893): female, cl 18.35 mm (MA16_28_1), lateral view. Scale 1.0 cm
Pontophilus occidentalis Faxon 1893: 200 [type locality: off Ecuador, 01°07.0’N 80°21.0’W, 2831 m; Gulf of Panama, 02°34.0’N 92°06.0’W, 2448 m; 04°56.0’N 80°52.30’W, 3243 m; 05°43.0’N 85°50.0’W, 1760 m; 06°10.0’N 83°06.0’W, 2648 m; 06°21.0’N 80°41.0’W, 3281 m; 07°05.30’N 79°40.0’W 2286 m; off Costa Rica, 10°14.0’N 96°28.0’W, 4018 m; off Guatemala, 14°46.0’N 98°40.0’W, 3190 m].— Zarenkov 1976: 14; Fig. 6.
Pontophilus gracilis occidentalis.— Wicksten and Hendrickx 2003: 69.
Parapontophilus occidentalis. — Wicksten 1977: 963. — Komai 2008: 287; Figs. 8, 20d. — Hendrickx 2012: 329; Fig. 5a. — Moscoso 2012: 60. — Hendrickx 2015: 373; Figs. 2, 3, 4.
Material examined. Clarion Clipperton Zone: ABYSSLINE II cruise, R/V Thompson, stn. EB04 (EBS), 12°07.83'N 117°18.67'W (OMS contract area), 4111 m, 25.02.2015, 1 female, cl 10.13 mm (AB02_EB04_2); ABYSSLINE II cruise, R/V Thompson, stn. EB07 (EBS), 12°27.08'N 116°38.80'W (UKSRL contract area), 4145 m, 02.03.2015, 1 female, cl 15.14 mm (AB02_EB07_1); MANGAN16 cruise, R/V Kilo Moana, stn. 28EBS, 11°49.654'N 117°00.299'W (BGR contract area), 4143 m, 01.05.2016, 1 ovigerous female, cl 18.35 mm (MA16_28_1); MANGAN18 cruise, R/V Sonne, stn. 59EBS, 11°50.055'N 116°59.530'W (BGR contract area), 4128 m, 23.04.2018, 1 ovigerous female, cl 18.27 mm (MA18_59_1).
Distribution. Eastern Pacific Ocean. Previously known from off Ecuador to Costa Rica, between 01°– 14°N and 79°–92°W at depths between 1760 and 4018 m (Faxon 1893; Komai 2008); off Chile at 1910–2120 m and off Peru at 1850–2100 m (Zarenkov 1976); off San Clemente Island, California at 1810 m (Wicksten 1977); Gulf of California at 837–840 m (Hendrickx 2012); off Peru at 3475 m (Moscoso 2012). These records extend the known geographical range of the species to the northwest (116°–117°W) and its bathymetric distribution to 4145 m.
Remarks. The genus Parapontophilus Christoffersen, 1988, currently includes 19 species (De Grave and Fransen 2011; Kim and Chan 2020). Parapontophilus occidentalis is the only member of the genus recorded for the eastern Pacific. Diagnostic characters of P. occidentalis include the presence of cardiac and epibranchial teeth on the carapace; rostrum failling to reach distal margins of corneas; corneas bearing two pairs of small lateral teeth; postorbital ridge on carapace conspicuous; non-pigmented partly faceted corneal surface; subequal anterior and posterior epigastric teeth on carapace; and the dactyli of fourth and fifth pereiopod being more than half the propodi length (Faxon 1893; Komai 2008; Hendrickx 2015). The present specimens have faceted corneas as observed by Hendrickx (2015), which are not present in the syntypes (Faxon 1893; Komai 2008). Otherwise, the specimens agree well with the description of P. occidentalis provided by Faxon (1893) and Komai (2008).
This is the first time COI sequences (OM892113, OM892115, OM892116, OM892129) are available for this species.
Family Stylodactylidae Spence Bate, 1888
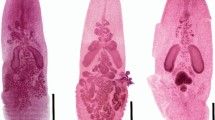
Bathystylodactylus echinus Wicksten & Martin, 2004
(Fig. 10)
Bathystylodactylus echinus Wicksten & Martin, 2004: female, pocl 42.0 mm (OUMNH-ZC.2018.01.041), lateral view. Scale 1.0 cm (photo by National Oceanography Centre Southampton)
Bathystylodactylus echinus Wicksten and Martin 2004: 377; Figs.1, 2, 3, 4, 5 [type locality: Basin of Magdalena Bay, Baja California, Mexico (24°35'N 113°25'W), 3563-3621 m].
Material examined. Clarion Clipperton Zone: MIDAS JC120 cruise, R/V James Cook, 17°15.08'N 123°03.73'W (APEI-6), 4160 m, 28.04.2015, 1 female, pocl 42.0 mm (OUMNH-ZC.2018.01.041).
Distribution. Eastern Pacific Ocean. Previously known with certainty only from its type locality, off Magdalena Bay, Baja California, Mexico at depths of 3563-3621 m (Wicksten and Martin 2004). Simon-Lledó et al. (2019a) reported Bathystylodactylus cf. echinus from the Area of Particular Environmental Interest 6 (APEI-6), in the CCZ during an AUV survey. A specimen was collected and was identified in the current study, confirming the presence of this species in the proposed conservation zone APEI-6, in the CCZ. Amon et al. (2017) reported the presence of Bathystylodactylus sp. from the UKSRL contract area and although highly likable to be B. echinus since no specimen was collected its presence cannot be confirmed yet.
Remarks. Three species of Bathystylodactylus Hanamura & Takeda, 1996 are currently known: B. bathyalis (Cleva, 1994) from the West Pacific (Coral Sea), B. inflatus Hanamura & Takeda, 1996 from off Taiwan, West Pacific and B. echinus from off Magdalena Bay (Baja California), East Pacific (Cleva 1994; Hanamura and Takeda 1996; Wicksten and Martin 2004). Bathystylodactylus echinus can be distinguished from the other two species by its curved rostrum; presence of a sharp tooth on the ventral margin of the third abdominal pleuron; lack of a wide elevation near the posterodorsal margin of the carapace; less sinuous shape of the suprabranchial carina; and fewer spinules on the carapace posterior to the rostrum (Wicksten and Martin 2004). The specimen collected agrees well with the diagnosis of B. echinus provided by Wicksten and Martin (2004).
This is the first time a COI sequence (OM951239) is available for this species, and indeed the genus.
Discussion
The present study is part of a series of studies (i.e. Kersken et al. 2018, 2019; Christodoulou et al. 2019, 2020) that aim to document the megafaunal biodiversity in the CCZ and DEA based on a combination of morphological and genetic evidence. As a result, the presence of eight species is revealed, of which six were reported for the first time from the CCZ and DEA using integrative taxonomy. The finding of these previously unreported decapod species is the direct result of a longer-term sampling effort, in which a greater number of specimens (23 specimens, 8 species) from a larger sampling area were collected than during any previous studies in the DEA or in the CCZ, spanning over three exploration contract areas and one APEI. Furthermore, the use of different sampling equipment, such as Epibenthic Sledge (EBS), Remotely Operated Vehicle (ROV) and baited traps permitted the collection of species with different life modes. Nevertheless, decapods, due to their high mobility, remain a challenging group to sample in the abyss. The collection of specimens allowed the compilation of a DNA barcode reference library with COI sequences given for the first time for the genera Parapagurus, Ethusina, and Bathystylodactylus. The sequences acquired will allow the future assignment of unknown or damaged specimens to a taxon name, thus producing more accurate and reproducible data in monitoring studies. Furthermore, taxonomically validated and curated reference libraries based on voucher specimens such as the one herein will be a great support for biodiversity studies based on environmental DNA or metabarcoding (Vieira et al. 2021).
The accurate documentation of species diversity through integrative taxonomy together with their geographic distribution and life history is essential for the prediction and management of possible environmental impacts of mining in the CCZ (Lodge et al. 2014; Wedding et al. 2015; Glover et al. 2018). Integrative taxonomy is even more imperative in the deep sea, where most known species have only recently been identified, while the vast majority remain undiscovered (Glover et al. 2018). Modelling the ecosystem-wide impacts of deep-sea exploitation is not possible without any knowledge of the animals that live there (Glover et al. 2018).
Environmental impacts caused by mining can include removal of abyssal nodules and severe habitat disruption, generation of large sediment plumes, and immediate loss of local biodiversity in the mined sites (Vanreusel et al. 2016; Miller et al. 2018). The removal of nodule-associated and habitat-forming benthic species such as corals and sponges will affect species, among which decapods, that utilise these habitats for food or shelter (Christiansen et al. 2020). A survey carried out in the DISCOL area showed that the scavenging deep-sea fish density was lower in the first years following disturbance, but increased over time reaching to zero net difference after 26 years (Drazen et al. 2019). The scavenging community besides fish included shrimps (H. nereus, Cerataspis monstrosus, Benthiscymus sp.), and the hermit crab Probeebei mirabilis. Although no firm conclusions were drawn for the decapod scavengers, it was speculated that extensive disturbance in their habitat would have a negative effect (Drazen et al. 2019). However, decapods most likely can avoid direct mining activities and since not one of the species found herein is endemic to the CCZ or DEA area, no regional species extinctions are expected. Nevertheless, long-term habitat loss at larger spatial scales and the dispersion of sediment plumes impairing feeding well beyond the mined sites could result in local population declines. Deep-sea decapods obtain food as carnivores by preying on small benthic and benthopelagic invertebrates or by scavenging on carcasses of dead animals (i.e. whales), or as detrivores or omnivores by feeding on any nutritional source available (Wicksten 2020). Observations on Bathystylodactylus species from the Marianas Trench and the CCZ show that shrimps probably filter-feed on small particles carried by currents using their fringed first and second pereiopods (Wicksten et al. 2017). The generation of plumes could impair the ability of these shrimps to feed. The shrimps H. nereus (Drazen et al. 2019) and H. doris (personal observations), the hermit crab Probeebei mirabilis (Drazen et al. 2019) and the squat lobster M. kensmithi (personal observations) scavenge on any available dead prey. It has been argued that olfactory sensing is likely the main mechanism for deep-sea scavengers to detect bait (Sainte-Marie 1992). Although any mining-generated sediment plumes are expected to influence mainly filter- and suspension-feeders they could potentially interfere in a smaller scale with odour plumes released from food falls, resulting in lower detection rates and generally lower food availability for scavengers (Christiansen et al. 2020).
Despite the fact that the CCZ is perhaps the most persistently-studied abyssal ecosystem (Glover et al. 2018), the high proportion of new species recorded and discovered (i.e. Bonifacio et al. 2020; Christodoulou et al. 2019, 2020; Brix et al. 2020) underlines the limited information presently available on its biodiversity. Megafauna biodiversity assessments in the CCZ and DEA areas are largely dependent on seafloor image surveys (i.e. Tilot et al. 2018; Simon-Lledó et al. 2019a, 2019b, 2020; Shoening et al. 2020; Drazen et al. 2019, 2021), which, due to their minimal disturbance and cost efficiency (Beisiegel et al. 2017), are considered to be a good and environmentally-friendly exploratory tool. However, although the collection of biological samples is not a full prerequisite for biodiversity studies, a comprehensive image-based assessment essentially requires prior knowledge of the area’s species, effectively through specimen collection (Hanafi-Portier et al. 2021; Horton et al. 2021). Recent surveys in the CCZ and DEA resulted in the development of a baseline image catalogue, documenting the fauna recorded on images or videos (Simon-Lledó et al. 2020 and references within). Although this is a significant step in the right direction, most of the images are not linked to voucher specimens, leading to potential misidentifications or biodiversity underestimates. This is especially the case with the cryptic and pseudo-cryptic taxa that are prevalent in Decapoda, and likely cannot be distinguished in the often-crude imagery routinely available (Fig. 4 and compare Figs. 4 and 5 vs Fig. 2e in Simon-Lledó et al. 2019b, Fig. 4i in Drazen et al. 2021) (Hanafi-Portier et al. 2021). ROVs can produce much higher-resolution imagery, as well as collect voucher specimens, but their use is costlier than more routinely used towed cameras (i.e., OFOS, Ocean Floor Observation System) and AUVs (Autonomous Underwater Vehicles). A fully integrative taxonomic approach, linking DNA sequences to the morphological identification of voucher specimens and matched with in-situ photos (supplemented by in vitro, ship-board photos as needed) as applied to the Decapoda in this study allows for a more complete documentation of biodiversity, which in turn informs larger scale, cost-effective monitoring work by means of OFOS and/or AUVs.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Amon DJ, Ziegler AF, Dahlgren TG, Glover AG, Goineau A, Gooday AJ, Wiklund H, Smith CR (2016) Insights into the abundance and diversity of abyssal megafauna in a polymetallic-nodule region in the eastern Clarion-Clipperton Zone. Sci Rep 6:30492. https://doi.org/10.1038/screp30492
Amon DJ, Ziegler AF, Drazen JC, Grischenko AV, Leitner AB, Lindsay DJ, Voight JR, Wicksten MK, Young CM, Smith CR (2017) Megafauna of the UKSRL exploration contract area and eastern Clarion-Clipperton Zone in the Pacific Ocean: Annelida, Arthropoda, Bryozoa, Chordata, Ctenophora, Mollusca. Biodivers Data J 14:e14598. https://doi.org/10.3897/BDJ.5.e14598
Beisiegel K, Darr A, Gogina M, Zettler ML (2017) Benefits and shortcomings of non-destructive benthic imagery for monitoring hard-bottom habitats. Mar Pollut Bull 121:5–15. https://doi.org/10.1016/j.marpolbul.2017.04.009
Bluhm H (2001) Re-establishment of an abyssal megabenthic community after experimental physical disturbance of the seafloor. Deep-Sea Res Pt II 48:3841–3868. https://doi.org/10.1016/S0967-0645(01)00070-4
Bonifacio P, Martinez Arbizu P, Menot L (2020) Alpha and beta diversity patterns of polychaete assemblages across the nodule province of the Clarion-Clipperton Fracture Zone (Equatorial Pacific). Biogeosciences 17:865–886. https://doi.org/10.5194/bg-17-865-2020
Boone L (1926) A new family of Crustacea. Preliminary technical description. NY Zool Soc Bull:29:73
Brenke N (2005) An epibenthic sledge for operations on marine softbottom and bedrock. Mar Technol Soc J 39:13–24. https://doi.org/10.4031/002533205787444015
Brix S, Osborn KJ, Mollusca, Truskey SB, Schnurr SM, Brenke N, Malyutina M, Martinez Arbizu P (2020) Adult life strategy affects distribution patterns in abyssal isopods – implications for conservation in Pacific nodule areas. Biogeosciences 17:6163–6184. https://doi.org/10.5194/bg-17-6163-2020
Burkenroad MD (1938) The Templeton Crocker expedition. XIII. Penaeidae from the region of lower California and Clarion Island, with descriptions of four new species. Zoologica, New York 23:55–91
Burkenroad MD (1963) The evolution of the Eucarida (Crustacea, Eumalacostraca), in relation to the fossil record. Tulane stud geol paleontol 2:3–17
Castro P (2005) Crabs of the subfamily Ethusinae Guinot, 1977 (Crustacea, Decapoda, Brachyura, Dorippidae) of the Indo-West Pacific region. Zoosystema 27:499–600
Chace FA (1986) The caridean shrimps (Crustacea: Decapoda) of the Albatross philippine expedition, 1907-1910, Part 4: Families Oplophoridae and Nematocarcinidae. Smithson Contr Zool 432:1–82
Christiansen Β, Denda Α, Christiansen S (2020) Potential effects of deep seabed mining on pelagic and benthopelagic biota. Mar Policy 114:103442. https://doi.org/10.1016/j.marpol.2019.02.014
Christodoulou M, O’Hara TD, Hugall AF, Martinez Arbizu P (2019) Dark ophiuroid biodiversity in a prospective abyssal mine field. Curr Biol 29:3909–3912. https://doi.org/10.1016/j.cub.2019.09.012
Christodoulou M, O’Hara T, Hugall AF, Khodami S, Rodrigues CF, Hilario A, Vink A, Martinez Arbizu P (2020) Unexpected high abyssal ophiuroid diversity in polymetallic nodule fields of the northeast Pacific Ocean and implications for conservation. Biogeosciences 17:1845–1876. https://doi.org/10.5194/bg-17-1845-2020
Cleva R (1994) Some Australian Stylodactylidae (Crustacea: Decapoda) with descriptions of two new species. The Beagle, Records of the Museum and Art Galleries of the Northern Territory 11:53–64
Crosnier A (1995) Crevettes Pénéides récoltées en mer Rouge et dans le golfe d'Aden par le navire ”METEOR” en 1987. Senck marit 25: 187–196
Crosnier A, Dall W (2004) Redescription of Hymenopenaeus obliquirostris (Crustacea, Decapoda, Penaeoidea, Solenoceridae) and description of two new species of Hymenopenaeus from the Indo-West Pacific. Zootaxa 600:1–26. https://doi.org/10.11646/zootaxa.600.1.1
Crosnier A, Forest J (1973) Les crevettes profondes de l’ Atlantique oriental tropical. Faune tropicale XIX, ORSTOM, Paris
Dana JD (1852) Conspectus of the Crustacea of the Exploring Expedition under Capt. C. Wilkes, U.S.N. Paguridea, continued, Megalopidea and Macroura. Amer J Sci Arts Second Series 14:116–125
De Grave S, Fransen CHJM (2011) Carideorum Catalogus: The recent species of the dendrobranchiate, stenopodidean, procarididean and caridean shrimps (Crustacea: Decapoda). Zool Med Leiden 85:195–589
De Grave S, Pentcheff ND, Ahyong ST, Chan T-Y, Crandall KA, Dworschak PC, Felder DL, Feldmann RM, Fransen CHJM, Goulding LYD, Lemaitre R, Low MEY, Martin JW, Ng PKL, Schweitzer CE, Tan SH, Tshudy D, Wetzer R (2009) A classification of living and fossil genera of decapod crustaceans. Raffles Bull Zool 21:1–109
del Solar EMC (1972) Addenda al catalogo de crustaceos del Peru. Inf Inst Mar Perú 38:1–21
Dong D, Li X, Lu B, Wang C (2017) Three squat lobsters (Crustacea: Decapoda: Anomura) from tropical West Pacific seamounts, with description of a new species of Uroptychus Henderson, 1888. Zootaxa 4311:389–398 https://doi.org/10.11646/zootaxa.4311.3.4
Drazen JC, Leitner A, Morningstar S, Marcon Y, Greinert J, Purser A (2019) Observations of deep-sea fishes and mobile scavengers from the abyssal DISCOL experimental mining area. Biogeosciences 16:3133–3146. https://doi.org/10.5194/bg-16-3133-2019
Drazen JC, Leitner A, Jones DOB, Simon-Lledó E (2021) Regional variation in communities of demersal fishes and scavengers across the CCZ and Pacific Ocean. Front Mar Sci 8:630616. https://doi.org/10.3389/fmars.2021.630616
Estoup A, Largiader CR, Perrot E, Chourrout D (1996) Rapid one-tube DNA extraction for reliable PCR detection of fish polymorphic markers and transgenes. Mol Mar Biol Biotechnol 5:295–298
Faxon W (1893) Reports on the dredging operations off the west coast of Central America to the Galapagos, to the west coast of Mexico, and in the Gulf of California, in charge of Alexander Agassiz, carried on by the U.S. Fish Commission steamer”Albatross”, during 1891, Lieut. Commander Z.L. Tanner, U.S.N., commanding. VI. Preliminary descriptions of new species of Crustacea. Bull Mus Comp Zool 24:149–220
Faxon W (1895) Reports on an exploration off the west coasts of Mexico, Central and South America, and off the Galapagos Islands, in charge of Alexander Agassiz, by the U.S. Fish Commission Steamer “Albatross”, during 1891, Lieut.-Commander Z.L. Tanner, U.S.N., commanding. XV. The Stalk-eyed Crustacea. Mem Mus Comp Zoology Harv Coll 18:1–280
Garth JS (1960) Distribution and affinities of the brachyuran Crustacea. Syst Zool 9(3/4):105–123
Garth JS, Haig J (1971) Decapod Crustacea (Anomura and Brachyura) of the Peru-Chile Trench. Anton Bruun Report 6:3–20
Geller J, Meyer C, Parker M, Hawk H (2013) Redesign of PCR primers for mitochondrial cytochrome c oxidase subunit I for marine invertebrates and application in all-taxa biotic surveys. Mol Ecol Resour 13. https://doi.org/10.1111/1755-0998.12138
Glover A, Wiklund H, Chen C, Dahlgren T (2018) Point of View: Managing a sustainable deep-sea ‘blue economy’ requires knowledge of what actually lives there. eLife 7:e41319
Gooday AJ, Durden JM, Holzmann M, Pawlowski J, Smith CR (2020) Xenophyophores (Rhizaria, Foraminifera), including four new species and two new genera, from the western Clarion-Clipperton Zone (abyssal equatorial Pacific). Eur J Protistol 75:125715. https://doi.org/10.1016/j.ejop.2020.125715
Guzman (2003) Oplophoridae (Decapoda, Caridea) in Southeastern Pacific Ocean. Revision of Chilean species. Gayana 68:70–75. https://doi.org/10.4067/S0717-65382004000100007
Hanafi-Portier M, Samadi S, Corbari L, Chan T-Y, Chen W-J, Chen J-N, Lee M-Y, Mah C, Saucède T, Borremans C, Olu K (2021) When imagery and physical sampling work together: Toward an integrative methodology of deep-Sea image-based megafauna identification. Front Mar Sci 8:749078. https://doi.org/10.3389/fmars.2021.749078
Hanamura Y (1983) Pelagic shrimps (Penaeidea and Caridea) from Baja California and its adjacent region with description of a new species. Bull biogeogr Soc Japan 38:51–85
Hanamura Y, Takeda M (1996) Establishment of a new genus Bathystylodactylus (Crustacea: Decapoda: Stylodactylidae), with description of a new species from northwestern Pacific. Zool Sci 13:929–934
Harbour RP, Leitner AB, Ruehlemann C, Vink A, Sweetman AK (2020) Benthic and demersal scavenger biodiversity in the Eastern end of the Clarion-Clipperton Zone – An area marked for polymetallic nodule mining. Front Mar Sci 7:458. https://doi.org/10.3389/fmars.2020.00458
Haworth AH (1825) A new binary arrangement of the macrurous Crustacea. Philosophical Magazine and Journal 65(323):183–184
Hein J, Mizell K, Koschinsky A, Conrad T (2013) Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: comparison with land-based resources. Ore Geol Rev 51:1–14. https://doi.org/10.1016/j.oregeorev.2012.12.001
Hein JR, Spinardi F, Okamoto N, Mizell K, Thorburn D, Tawake A (2015) Critical metals in manganese nodules from the Cook Islands EEZ, abundances and distributions. Ore Geol Rev 68:97–116. https://doi.org/10.1016/j.oregeorev.2014.12.011
Hendrickx ME (1995) Checklist of brachyuran crabs (Crustacea: Decapoda) from the Eastern Tropical Pacific. Bull Inst Roy Sci Nat Belgique 65:125–150
Hendrickx ME (2001) Occurrence of a continental slope decapod crustacean community along the edge of the minimum oxygen zone in the southeastern Gulf of California, Mexico. Belg J Zool 131:95–109
Hendrickx ME (2008) New records of and notes on decapod crustaceans in the east Pacific. Crustaceana 81:999–1006. https://doi.org/10.1163/156854008X354957
Hendrickx ME (2012) Los Glyphocrangonidae y Crangonidae (Crustacea: Decapoda: Caridea) recolectados durante los cruceros TALUD en el Pacífico mexicano. In: El proyecto TALUD. Instituto Nacional de Ecología, Secretaría del Medio Ambiente y Recursos Naturales (SEMARNAT), Mexico
Hendrickx ME (2013) Pelagic shrimps collected during the TALUD I-VII cruises aboard the R/V “El Puma” in the SE Gulf of California, Mexico. Crustaceana 86:437–448. https://doi.org/10.1163/15685403-00003171
Hendrickx ME (2015) Insights on the biology and ecology of the deep-water shrimp Parapontophilus occidentalis (Faxon, 1893) (Crustacea: Caridea: Crangonidae) in the eastern Pacific with notes on its morphology. Zootaxa 4007:370–388. https://doi.org/10.11646/zootaxa.4007.3.4
Hendrickx ME, Ayón-Parente M (2013) A new species of Munidopsis (Crustacea: Anomura: Galatheoidea: Munidopsidae) from the Gulf of California, western Mexico. Crustaceana 86:1306–1317
Hendrickx ME, Wicksten MK (2016) New records of decapod crustaceans in the eastern Pacific. Crustaceana 89:603–610. https://doi.org/10.1163/15685403-00003541
Horton T, Marsh L, Bett BJ, Gates AR, Jones DOB, Benoist NMA, Pfeifer S, Simon-Lledó E, Durden JM, Vandepitte L, Appeltans W (2021) Recommendations for the standardisation of open taxonomic nomenclature for image-based identifications. Front Mar Sci 8:620702. https://doi.org/10.3389/fmars.2021.620702
Jakiel A, Palero F, Błażewicz M (2019) Deep ocean seascape and Pseudotanaidae (Crustacea: Tanaidacea) diversity at the Clarion-Clipperton Fracture Zone. Sci Rep 9:17305. https://doi.org/10.1038/s41598-019-51434-z
Jamieson AJ, Fujii T, Mayor DJ, Solan M, Priede IG (2009) Hadal trenches: the ecology of the deepest places on Earth. Trends Ecol Evolut 25:190–197. https://doi.org/10.1016/j.tree.2009.09.009
Jones WJ, Macpherson E (2007) Molecular phylogeny of the East Pacific squat lobsters of the genus Munidopsis (Decapoda: Galatheidae) with the descriptions of seven new species. J Crust Biol 27:477–501. https://doi.org/10.1651/S-2791.1
Katoh K, Misawa K, Kuma KI Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30:3059–3066. https://doi.org/10.1093/nar/gkf436
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Mentjies P, Drummond A (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Kemp S (1939) XLVII. On Acanthephyra purpurea and its allies (Crustacea Decapoda: Hoplophoridæ). Ann Mag Nat Hist 4:568–579. https://doi.org/10.1080/00222933908655403
Kersken D, Janussen D, Martínez Arbizu P (2018) Deep-sea glass sponges (Hexactinellida) from polymetallic nodule fields in the Clarion-Clipperton Fracture Zone (CCFZ), northeastern Pacific: Part I – Amphidiscophora. Mar Biodiv 48:545–573. https://doi.org/10.1007/s12526-017-0727-y
Kersken D, Janussen D, Martínez Arbizu P (2019) Deep-sea glass sponges (Hexactinellida) from polymetallic nodule fields in the Clarion-Clipperton Fracture Zone (CCFZ), northeastern Pacific: Part II—Hexasterophora. Mar Biodiv 49:947–987. https://doi.org/10.1007/s12526-018-0880-y
Kim JN, Chan T-Y (2020) Crangonid shrimps (Crustacea: Decapoda: Caridea) from Papua New Guinea. In: Corbari L, Ahyong ST, Chan T-Y (eds). Deep-Sea Crustaceans from Papua New Guinea. Tropical Deep-Sea Benthos 31. Mém Mus natl Hist nat 213:207–238
Komai T (2008) A world-wide review of species of the deep-water crangonid genus Parapontophilus Christoffersen, 1988 (Crustacea, Decapoda, Caridea), with descriptions of ten new species. Zoosystema 30:261–332
Latreille PA (1802) Histoire naturelle, générale et particulière des Crustacés et des Insectes. Ouvrage faisant suite à l'histoire naturelle générale et particulière, composée par Leclerc de Buffon, et rédigée par C.S. Sonnini, membre de plusieurs Sociétés savantes. Dufart, vol. 3, Paris
Lemaitre R (1989) Revision of the genus Parapagurus (Anomura: Paguroidea: Parapaguridae), including redescriptions of the western Atlantic species. Zoologische Verhandelingen 253:1–106
Lemaitre R (1998) Revisiting Tylaspis anomala Henderson, 1885 (Parapaguridae), with comments on its relationships and évolution. Zoosystema 20:289–305
Lemaitre R (1999) Crustacea Decapoda: A review of the species of the genus Parapagurus Smith, 1879 (Parapaguridae) from the Pacific and Indian Oceans. In: Crosnier A (ed) Résultats des Campagnes MUSORSTOM 20. Mémoires du Muséum national d'Histoire naturelle. Série A, Zoologie. 180:303–378
Lodge M, Johnson D, Le Gurun G, Wengler M, Weaver P, Gunn V (2014) Seabed mining: International Seabed Authority environmental management plan for the Clarion–Clipperton Zone. A partnership approach. Mar Policy 49:66–72. https://doi.org/10.1016/j.marpol.2014.04.006
MacLeay WS (1838) Illustrations of the Annulosa of South Africa. On the brachyurous decapod Crustacea. Brought from the Cape by Dr. Smith. In: Smith, A. (ed.), Illustrations of the Zoology of South Africa; consisting chiefly of Figures and Descriptions of the Objects of Natural History Collected during an Expedition into the Interior of South Africa, in the Years 1834, 1835, and 1836; fitted out by “The Cape of Good Hope Association for Exploring Central Africa”. Published under the Authority of the Lords Commissioners of Her Majesty's Treasury, London, pp. i–iv + 53–71, pls. 2, 3
Malyutina MV, Kihara TC, Brix S (2020) A new genus of Munnopsidae Lilljeborg, 1864 (Crustacea, Isopoda), with de- scriptions of two abyssal new species from the Clarion Clipperton Fracture Zone, north-eastern tropical Pacific. Mar Biodivers 50:42. https://doi.org/10.1007/s12526-020-01061-z
Miller KA, Thompson KF, Johnston P, Santillo D (2018) An overview of seabed mining including the current state of development, environmental impacts, and knowledge gaps. Front Mar Sci 4:418. https://doi.org/10.3389/fmars.2017.00418
Moscoso V (2012) Catálogo de crustáceos decápodos y estomatópodos del Perú. Bol Inst Mar Perú 27:1–207
Mukhopadhyay R, Ghosh AK, Iyer SD (2008) The Indian Ocean nodule field: Geology and resource potential. In: Hale M (ed) Handbook of exploration and environmental geochemistry, volume 10. Elsevier, Amsterdam, the Netherlands, pp 1–292.
Niner HJ, Ardron JA, Escobar EG, Gianni M, Jaeckel A, Jones DOB, Levin LA, Smith CR, Thiele T, Turner PJ, Van Dover CL, Watling L, Gjerde KM (2018) Deep-sea mining with no net loss of biodiversity–An impossible aim. Front Mar Sci 5:53. https://doi.org/10.3389/fmars.2018.00053
Ortmann, A.E. (1898) Crustacea, Malacostraca. In: Gerstäcker, A. & Ortmann, A.E. (eds.), Die Klassen und Ordnungen der Arthropoden wissenschaftlich dargestellt in Wort und Bild. C.F. Winter'sche Verlagshandlung, Leipzig, 5 (2), pp. 1057–1168, pls. 109–116
Perez-Farfante I (1977) American solenocerid shrimps of genera Hymenopenaeus, Haliporoides, Pleoticus, Hadropenaeus new genus, and Mesopenaeus new genus. Fish Bull 75:261–346
Poore GCB (2014) Three new American species of Munidopsis (Crustacea: Anomura: Munidopsidae). Nauplius 22:53–62. https://doi.org/10.1590/s0104-64972014000100006
Ramirez-Llodra E, Tyler PA, Baker MC, Bergstad OA, Clark MR, Escobar E, Levin LA, Menot L, Rowden AA, Smith CR, Van Dover CL (2011) Man and the last great wilderness: Human impact on the deep sea. PLoS ONE 6:e22588. https://doi.org/10.1371/journal.pone.0022588
Rathbun MJ (1933) Preliminary descriptions of nine new species of Oxystomatous and allied crabs. Proc Biol Soc Wash 46:183–186
Rathbun MJ (1937) The Oxystomatous and allied crabs of America. Bull US Natl Mus 166:1–278
Ratnasingham S, Hebert P (2007) Bold: the barcode of life data system (http://www.barcodinglife.org). Mol Ecol Notes 7:355–364. https://doi.org/10.1111/j.1471-8286.2007.01678.x
Saint Laurent M de (1972) Sur la famille des Parapaguridae Smith, 1882. Description de Typhlopagurus foresti gen. nov., sp. nov., et de quinze espèces ou sous-espèces nouvelles de Parapagurus Smith (Crustacea, Decapoda). Bijd Dierkunde 42(2):97–123
Sainte-Marie B (1992) Foraging of scavenging deep-Sea lysianassoid amphipods. In: Rowe GT, Pariente V (eds) Deep-sea food chains and the global carbon cycle. NATO ASI Series, 360, Springer, Dordrecht, pp 105–124. https://doi.org/10.1007/978-94-011-2452-2_7
Samouelle G (1819) The entomologists' useful compendium; or an introduction to the knowledge of British Insects, comprising the best means of obtaining and preserving them, and a description of the apparatus generally used; together with the genera of Linné, and modern methods of arranging the Classes Crustacea, Myriapoda, spiders, mites and insects, from their affinities and structure, according to the views of Dr. Leach. Also an explanation of the terms used in entomology; a calendar of the times of appearance and usual situations of near 3,000 species of British Insects; with instructions for collecting and fitting up objects for the microscope. Thomas Boys, London, 496, 12
Shoening T, Purser A, Langenkämper D, Suck I, Taylor J, Cuvelier D, Lins L, Simon-Lledó E, Marcon Y, Jones DOB, Nattkemper T, Köser K, Zurowietz M, Greinert J, Gomes-Pereira J (2020) Megafauna community assessment of polymetallic-nodule fields with cameras: platform and methodology comparison. Biogeosciences 17:3115–3133. https://doi.org/10.5194/bg-17-3115-2020
Simon-Lledó E, Bett BJ, Huvenne VAI, Schoening T, Benoist NMA, Jeffreys RM, Durden JM, Jones DOB (2019a) Megafaunal variation in the abyssal landscape of the Clarion Clipperton Zone. Prog Oceanogr 170:119–133. https://doi.org/10.1016/j.pocean.2018.11.003
Simon-Lledó E, Bett BJ, Huvenne VAI, Köse K, Schoening T, Greinert J, Jones DOB (2019b) Biological effects 26 years after simulated deep-sea mining. Sci Rep 9:8040. https://doi.org/10.1038/s41598-019-44492-w
Simon-Lledó E, Bett BJ, Huvenne VAI, Schoening T, Benoist NMA, Jones DOB (2019c) Ecology of a polymetallic nodule occurrence gradient: Implications for deep-sea mining. Limnol Oceanogr 64:1883–1894. https://doi.org/10.1002/lno.11157
Simon-Lledó E, Pomee C, Ahokava A, Drazen JC, Leitner AB, Flynn A, Parianos J, Jones DOB (2020) Multi-scale variations in invertebrate and fish megafauna in the mid-eastern Clarion Clipperton Zone. Prog Oceanogr 187:102405. https://doi.org/10.1016/j.pocean.2020.102405
Smith SI (1879) The stalk-eyed crustaceans of the Atlantic coast of North America north of Cape Cod. Trans Conn Acad Arts Sci 5(2):27–138, pls. 8–12. https://doi.org/10.5962/bhl.title.10046
Smith SI (1884) Crustacea of the Albatross dredgings off the east coast of the United States in 1883. In: Report of the Commissioner for 1882. Part X. United States Commission of Fish and Fisheries, Washington D.C., pp. 345–426, pls. 1–10.
Spence Bate C (1888) Report on the Crustacea Macrura collected by the Challenger during the years 1873–76. Report on the Scientific Results of the Voyage of H.M.S. “Challenger” during the years 1873–76. Zoology 24:1–942
Stratmann T, Lins L, Purser A, Marcon Y, Rodrigues CF, Ravara A, Cunha MR, Simon-Lledó E, Jones DOB, Sweetman AK, Köser K, van Oevelen D (2018) Abyssal plain faunal carbon flows remain depressed 26 years after a simulated deep-sea mining disturbance. Biogeosciences 15:4131–4145. https://doi.org/10.5194/bg-15-4131-2018
Thiel H, Schriever G (1990) Deep-Sea Mining, Environmental Impact and the DISCOL Project. Ambio 19:245–250
Thiel H, Schriever G, Ahnert A, Bluhm H, Borowski C, Vopel K (2001) The large-scale environmental impact experiment DISCOL: reflection and foresight. Deep-Sea Res II 48:3869–3882. https://doi.org/10.1016/S0967-0645(01)00071-6
Tilot V, Ormond R, Moreno Navas J, Catalá TS (2018) The benthic megafaunal assemblages of the CCZ (Eastern Pacific) and an approach to their management in the face of threatened anthropogenic impacts. Front Mar Sci 5. https://doi.org/10.3389/fmars.2018.00007
Van Dover CL, Ardron JA, Escobar E, Gianni M, Gjerde KM, Jaeckel A, Jones DOB, Levin LA, Niner HJ, Pendleton L, Smith CR, Thiele T, Turner PJ, Watling L, Weaver PPE (2017) Biodiversity loss from deep-sea mining. Nat Geosci 10:464. https://doi.org/10.1038/ngeo2983
Vanreusel A, Hilario A, Ribeiro PA, Menot L, Martinez Arbizu P (2016) Threatened by mining, polymetallic nodules are required to preserve abyssal epifauna. Sci Rep 6:26808. https://doi.org/10.1038/srep26808
Vereshchaka AL (1990) Pelagic decapods from seamounts of the Nazca and Sala-y-Gomez Ridges. Tr. Inst. Okeanol. im. P. P. Shirshova, Akad. Nauk SSSR 124:129–155
Vieira PE, Lavrador AS, Parente MI, Parretti P, Costa AC, Costa FO, Duarte S (2021) Gaps in DNA sequence libraries for Macaronesian marine macroinvertebrates imply decades till completion and robust monitoring. Divers Distrib 27:2003–2015. https://doi.org/10.1111/ddi.13305
Wedding LM, Friedlander AM, Kittinger JN, Watling L, Gaines SD, Bennett M, Hardy SM, Smith CR (2013) From principles to practice: a spatial approach to systematic conservation planning in the deep sea. Proc R Soc Lond Biol 280:1684–1684. https://doi.org/10.1098/rspb.2013.1684
Wedding LM, Reiter SM, Smith CR, Gierde KM, Kittinger JN, Friedlander AM, Gaines SD, Clark MR, Thurnherr AM, Hardy SM, Crowder LB (2015) Managing mining of the deep seabed: Contracts are being granted, but protections are lagging. Science 349:144–145. https://doi.org/10.1126/science.aac6647
Wicksten MK (1977) Range extensions of four species of crangonid shrimps from California and Baja California, with a key to the genera (Natantia: Crangonidae). Proc Biol Soc Wash 90:963–967
Wicksten MK (2020) Lower slope and abyssal benthic decapods of the Eastern Pacific. In: Hendrickx ME (ed) Deep-sea pycnogonids and crustaceans of the Americas. Springer, Cham, pp 395–420. https://doi.org/10.1007/978-3-030-58410-8_17
Wicksten MK, Hendrickx ME (2003) An updated checklist of benthic marine and brackish water shrimps (Decapoda: Penaeoidea, Stenopodidea, Caridea) from the Eastern Tropical Pacific. Contributions to the Study of East Pacific Crustaceans 2:49–76
Wicksten MK, Martin JW (2004) A new species of caridean shrimp of the family Stylodactylidae from the eastern Pacific Ocean. Proc Biol Soc Wash II 7:377–384
Wicksten M, De Grave S, France S, Kelley C (2017) Presumed filter-feeding in a deep-seabenthic shrimp (Decapoda, Caridea, Stylodactylidae), with records of the deepestoccurrence of carideans. ZooKeys 646:17–23. https://doi.org/10.3897/zookeys.646.10969
Wolff T (1961) Description of a remarkable deep-sea hermit crab, with notes on the evolution of the Paguridea. Galathea Rep 4:11–32
Wood-Mason J, Alcock A (1891) Natural history notes from H.M. Indian marine survey steamer ”Investigator”, Commander R.F. Hoskyn, R.N., commanding, II(1). On the results of deep-sea dredging during the season 1890–1891. Ann Mag Nat Hist (6)8:268–286.
Zarenkov NA (1976) On the fauna of decapods of the waters adjacent to South America. Biol Morya 5:8–18
Acknowledgements
The authors would like to thank Carsten Rühlemann (Federal Institute for Geosciences and Natural Resources, BGR, Hannover) for making the material from the BGR cruises MANGAN 2013, MANGAN 2016 and MANGAN 2018 available. The authors will also like to thank Drs Ana Hilario, Katja Uhlenkott, Sven Rossel and Tanja Straatman for their help in processing the samples on board the cruise SO268. The authors are very grateful to Prof. Peter Keen Lin Ng and Dr Rafael Lemaitre for their valuable comments on the animal's identifications. We are finally grateful to the reviewers for their helpful comments and recommendations on improving the quality of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The cruises SO239, SO242 and SO268 were financed by the German Ministry of Education and Science (BMBF) as a contribution to the European action JPI-Oceans “Ecological Aspects of Deep-Sea Mining”, projects Miningimpact and Miningimpact-2 (under contract 03F0707E and 03F0812E). The ABYSSLINE cruises were funded by UK Seabed Resources Ltd. The JC120 cruise was funded by the European Union Seventh Framework Programme (FP7/2007-2013) under the MIDAS (Managing Impacts of Deep-seA reSource exploitation) project, grant agreement 603418.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Sampling and field studies
Permits for sampling and observational field studies are not applicable.
Data availability
DNA sequences, trace files, collection data and taxonomic remarks are available in the datasets CCZ-DEA_Decapoda in BOLD. Furthermore, the DNA sequences and their accompany collection data are also available in GenBank.
Author contribution
PMA and AV designed the sampling. PMA, AV and MC carried out the sampling and processed the specimens on board. MC performed the genetic lab work. MC and SDG conducted the morphological identifications. MC took the lead in writing the manuscript in collaboration with all authors.
Additional information
Communicated by T. Horton
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is a contribution to the Topical Collection Biodiversity in Abyssal Polymetallic Nodule Areas
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Christodoulou, M., De Grave, S., Vink, Α. et al. Taxonomic assessment of deep-sea decapod crustaceans collected from polymetallic nodule fields of the East Pacific Ocean using an integrative approach. Mar. Biodivers. 52, 61 (2022). https://doi.org/10.1007/s12526-022-01284-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-022-01284-2