Abstract
Eudendrium capillaroides Schuchert, 2008 has not been reported since its original description. The new and abundant material found in Ceuta, southern coast of the Strait of Gibraltar, allows us to refine the morphological diagnosis of this species. Eudendrium capillaroides is characterized by small monosiphonic colonies, a dense ring at the base of the hydranth, and it seems to have a preference for growing on other hydroids. Male gonophores on atrophied polyps, two-chambered (occasionally one chamber), and female gonophores on reduced polyps in the initial stages of development but hydrants later completely atrophied. Nematocysts are heterotrichous microbasic euryteles of two size classes, a larger size densely distributed in a band on the hydrant body basally and a smaller size abundant mainly on the tentacles. Furthermore, we carried out molecular analyses to assess the status of E. capillaroides and its most similar congener E. capillare within the genus Eudendrium. The use of few morphological characters or incomplete descriptions may thus lead to an incorrect wide distribution of a nominal species that actually represents a species complex. This can particularly be the case in less conspicuous species, such as many hydroids, where the degree of diversity might be underestimated. The accurate description of tiny, inconspicuous and/or cryptic species is important in order to better estimate global marine diversity as well as to understand marine communities and the relationships between their components.
Similar content being viewed by others
Introduction
The genus Eudendrium Ehrenberg, 1834 is a monophyletic taxon with a worldwide distribution that includes more than 70 nominal species with only three new species described in the last 13 years (Bouillon et al. 2006; Schuchert 2022). However, studies based on genetic markers indicated that there could be a greater number of species, hidden under similar morphology (Moura et al. 2008, 2012; Lindner et al. 2011). Usually easily identified as belonging to this genus, hydranths are relatively large and distinguished from all other families of the athecates by a wide, trumpet-shaped, or spherical hypostome (Schuchert 2008). Eudendrium species are dioecious, since simultaneous hermaphrodites occur rarely (e.g., E. simplex), and their life cycle is characterized by the absence of a medusa stage (Bouillon et al. 2004). The Eudendriidae is one of the athecate families in which identification at species level is most difficult (Totton 1930; Mammen 1963; Millard 1975; Marinopoulos 1992; Vervoort 2006) and, in addition, the inappropriate use of some characters, such as colony size, led to a confused state in the Eudendriidae taxonomy (Marques et al. 2000a).
Up to 18 nominal species of Eudendriidae have been reported in the Mediterranean Sea (Marques et al. 2000a; Bouillon et al. 2004; Schuchert 2008; González-Duarte 2011, 2016; Megina et al. 2016), whose validity has been discussed by different authors for more than a century. Motz-Kossowska (1905) carried out the first review of Mediterranean eudendriid species, while Picard (1951) was the first to include the use of nematocysts as a useful taxonomic criterion of the group. In several works, Picard (1951; 1955; 1958) distinguished eleven eudendriid Mediterranean species. Bavestrello and Piraino (1991) increased the number of Eudendriidae to 13 (12 belonging to the genus Eudendrium and 1 to Myrionema). Marinopoulos (1992) listed 12 species of Eudendrium, considering Myrionema amboinense as a species of the genus Eudendrium. In their revision of the Mediterranean species of Eudendrium, Marques et al. (2000b) added two species: Eudendrium elsaeoswaldae Stechow, 1921, which had not been considered as a valid Mediterranean species after Picard (1958), and the new species Eudendrium moulouyensis Marques, Peña-Cantero & Vervoort, 2000a. These authors reaffirmed Eudendrium cunninghami Kirkpatrick, 1910 as a synonymy of E. carneum Clarke, 1882, considering a total of 13 valid species for the Mediterranean Sea. Bouillon et al. (2004) compiled the data known for Mediterranean hydroids and listed a total of 15 species of Eudendrium. They essentially used the criteria proposed by Marques et al. (2000b) with regard to E. arbusculum Wright, 1859 and considered it as a doubtful record for the Mediterranean. These authors also considered E. tenellum Allman, 1877 as a doubtful species. To date, E. moulouyensis is the last species described for the Mediterranean Sea (Chafarinas Is., South Alboran Sea), being unique among Mediterranean species in hosting symbiotic zooxanthellae.
Schuchert (2008) reviewed the European species of the family Eudendriidae and listed 19 valid species of Eudendrium. For this author, Eudendrium fragile Motz-Kossowska, 1905 and E. islandicum Schuchert, 2000 are synonyms of E. album Nutting, 1896; E. elsaeoswaldae and E. insigne Hincks, 1861 are considered as synonyms of E. ramosum (Linnaeus, 1758); and E. planum and E. stratum Bonnevie, 1898 are recognized as synonyms of E. rameum (Pallas, 1766). In addition, Schuchert (2008) described two new species occurring in the northeastern Atlantic: E. capillaroides from NW France and Eudendrium unispirum from the British Isles and Svalbard.
Regarding the Strait of Gibraltar, the natural boundary between the Atlantic Ocean and Mediterranean Sea, Medel (1996), reported three species of Eudendrium: E. armatum, Tichomiroff, 1887, E. capillare Alder, 1856, and E. racemosum (Cavolini, 1785) (Medel-Soteras, pers. com.). Among the 22 hydroid species collected from bathyal environments of the Gulf of Cadiz used to evaluate the potential of the mitochondrial 16S rRNA gene as a taxonomic tool, Moura et al. (2008) included newly sequenced colonies of three species of Eudendrium, two of them identified just at the genus level plus E. rameum (Pallas, 1766). More recent articles on hydroid fauna in this area have explored the differences between the hydroid assemblages of the Strait of Gibraltar and nearby zones considering both natural (González-Duarte et al. 2013b, 2014, 2016) and artificial habitats, with special consideration to non-indigenous species (González-Duarte et al. 2013a; Megina et al. 2013, 2016). These authors sampled up to 116 hydroid species and reported 12 Eudendrium species (one of them identified just at the genus level). Among these species, tiny specimens belonging to the genus Eudendrium were found growing on Eudendrium racemosum and initially identified as a possible new species. However, this material was eventually assigned to Eudendrium capillaroides. In 2019, during an intensive benthic survey program carried out on both sides of the Strait of Gibraltar and nearby zones, the species Eudendrium capillaroides was again identified from material collected in North Africa (southern coast of the Strait of Gibraltar, Mediterranean Sea). These findings represent the first records since its original description in the North Atlantic (Britanny, France) (Schuchert 2008). The abundant newly collected material described here allows us to complete the original description with unknown or poorly known additional morphological (optical and scanning electron microscopy [SEM]), molecular (16S), and biological features (association with biological substrata). Finally, a phylogenetic hypothesis based on the mitochondrial 16S marker allows us to discuss the relationships of this tiny athecate hydroid with the species of the genus Eudendrium.
Material and methods
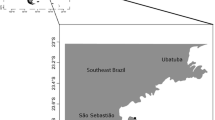
Hydroid colonies were collected in Ceuta (southern coast of the Strait of Gibraltar; South-western Mediterranean) during summer 2007 and 2019 to investigate the structure of hydrozoan communities in three different stations (Fig. 1). These places were characterized by well-preserved and highly diverse precoralligenous and coralligenous communities on rocky cliffs with a steep profile (González-Duarte et al. 2013b). Map was created using Quantum Geographic information System software (QGIS 2018).
The samplings were carried out by SCUBA diving along two transects at each station, at five bathymetric levels from 0- to 30-m depth (0/ − 5 m, − 5/ − 10 m, − 10/ − 15 m, − 15/ − 20 m, and − 20/ − 30 m). After collection, the specimens were preserved either in ethanol 90% or 4% formaldehyde solution. The male and female gonophores, as well as gastrozooid polyps, were mounted for examination by SEM. Critical point drying was carried out as described by Cohen (1979), using a Leica EM-CPC-300 critical point dryer. Dry samples were mounted using double-sided carbon conductive tape and then covered with a 5–10-nm gold–palladium film using a Leica ACE600 sputter coater for 120 s. Samples were studied at 5 kV and low magnification using a Hitachi S5200 microscope. Histological sections were stained with Ramón y Cajal’s Triple Stain (Gabe 1968). The examination and measurement of nematocysts were performed using the software NIS-Element Advance Research v.3.10 and optical and stereo microscopes (Nikon SMZ745 and Nikon eclipse 90i, equipped with Nikon DXM1200F camera). All measurements of the specimens are based on preserved material.
Total genomic DNA was extracted from an ethanol-preserved specimen using the E.Z.N.A. DNA kit (Omega Bio-Tek) following the manufacturer’s instructions. The partial 16S mitochondrial region was amplified using the primers SHA (ACGGAATGAACTCAAATCATGT) and SHB (TCGACTGTTTACCAAAAACATA) (Cunningham and Buss 1993; Moura et al. 2011). The PCR used 1 U of MyTaq Red DNA Polymerase (Bioline), 10 μM of each primer, approximately 30 ng of genomic DNA, and was brought to a final volume of 25 μL with H2O for molecular biology (PanReac-AppliChem). 16S PCR was carried out using the following cycle profile: initial denaturation at 95 °C for 1 min, 35 cycles of denaturation at 95 °C for 15 s, annealing at 50 °C for 15 s, and extension at 72 °C for 10 s, and a final extension at 72 °C for 5 min. The PCR product was purified using ExoSAP–IT™ PCR Product Cleanup Reagent (Thermo Fisher Scientific) following the manufacturer’s instructions and sent to Macrogen Spain for sequencing in both directions. All chromatograms were visualized and sequence pairs were matched and edited using Sequencher v4.0. The new sequence obtained in this study and those homologous from GenBank were aligned using MUSCLE implemented in MEGA6. The sequences of two Myrionema species from GenBank were selected as an out-group. The aligned matrix had 61 sequences, 59 of them from Eudendrium specimens.
Phylogenetic analysis was performed by applying maximum likelihood (ML) and Bayesian inference (BI) methods. The best nucleotide substitution model was selected using Modeltest v.2.1.10 (Darriba et al. 2012), according to Akaike information criterion (AIC) and hierarchical likelihood ratio test (hLRT) values. The maximum likelihood method was carried out in MEGA6 (Tamura et al. 2013) using the NNI (nearest neighbor interchange) heuristic method and 1000 bootstrap replications. The selected nucleotide substitution model was GTR + G + I. The Bayesian inference was carried out with MrBayes v3.1.2 (Huelsenbeck and Ronquist 2001; Ronquist and Huelsenbeck 2003), using the substitution model GTR + G (lset nst = 6 rates = gamma), 107 generations, and discarding 25% of the initial trees. According to Hillis and Bull (1993), for ML bootstraps, those values < 70% should be considered as low, between 70 and 94% moderate, and ≥ 95% high. According to Alfaro et al. (2003), for Bayesian posterior probabilities, those values < 0.95 should be considered as low and ≥ 0.95 as high. The stationarity of the chains and the convergence of the two runs were monitored for each parameter by Tracer (v.1.7.1) (Rambaut et al. 2018), determining whether the effective sample size (ESS) of all parameters was greater than 200, as recommended.
The newly collected material of E. capillaroides has been deposited in the Museu de Zoologia in Barcelona (MZB).
Results
Systematics
Class Hydrozoa Owen, 1843
Order Anthoathecata Cornelius, 1992
Suborder Filifera Kühn, 1913
Family Eudendriidae L. Agassiz, 1862
Genus Eudendrium Ehrenberg, 1834
Eudendrium capillaroides Schuchert, 2008
Eudendrium capillaroides. a Colony silhouette; scale bar 10 mm; b typical hydranth, note ring of nematocysts on body; scale bar 100 μm; c small and large eurytele nematocysts, undischarged and discharged microbasic euryteles; scale bar 5 μm; d male blastostyle with terminal buttons of nematocyst; e female gonophores on reduced polyps; f more advanced female blastostyle on completely atrophied polyps. Scale bar 100 μm (d–f)
Eudendrium capillaroides. a Colonies growing on E. racemosum; scale bar 1 mm. b Ring of nematocysts at the base of the hydrant, scale bar 100 μm. c and d Large microbasic eurytele nematocysts at the base of the hydrant; c discharged nematocysts; d undischarged nematocysts. e, f smaller microbasic euryteles nematocyst; e discharged nematocysts; f undischarged nematocysts. Scale bar 5 μm (c–f)
Eudendrium capillaroides. Scanning Electron Micrographs. a Gastrozooid polyp; b ring of nematocysts; c male gonophores; d detail of terminal nematocyst button of male gonophores; e initial stages of female blastostyle development on reduced polyp; f female gonophores in older stages with simple spadix
Examined material MZB 2022–1510, “Ceuta Sur,” several hydrocauli up to 14 mm. Female colonies on E. racemosum, 20/30 m in depth, 25/07/2007. MZB 2022–1511, “Ceuta Norte,” several short hydrocauli up to 8 mm. Female colonies on E. racemosum, 20/30 m in depth, 24/07/2007. MZB 2022–1512, “Ceuta Sur,” few hydrocauli up to 8 mm. Male colonies on E. racemosum, 15/20 m in depth, 25/07/2007. MZB 2022–1513, “Ceuta Sur,” several hydrocauli up to 10 mm. Male colonies on E. racemosum, 20/30 m in depth, 25/07/2007. MZB 2022–1514, “Ceuta Norte 2,” several hydrocauli up to 10 mm. Female colonies on E. racemosum, 15/20 m in depth, 06/08/2019. MZB 2022–1515, “Ceuta Norte 2,” several hydrocauli up to 10 mm. Male colonies on E. racemosum, 15/20 m in depth, 06/08/2019.
Diagnosis Eudendrium capillaroides species forming small monosiphonic colonies (up to 14 mm). Two categories of nematocysts: small microbasic euryteles in the body of the hydranth and tentacles and slightly larger microbasic euryteles in a dense ring at the base of the hydranth. Male gonophores on atrophied polyps, two-chambered (occasionally, one chamber), and female gonophores on reduced polyps in the initial stages of development but hydrants later completely atrophied.
Description of examined material Polyp colonies minute, stem fragile, up to 14-mm high, main stems unfascicled (Figs. 2a, 3a). Hydrocaulus arising from stolonal hydrorhiza, irregularly branched up to fourth order. Perisarc delicate, brown to yellowish in the younger parts of the colony. Perisarc at base of hydrocaulus, 61–69 µm in diameter, in younger part of colony: main stem and secondary branches, 71–88 µm in diameter. Base of branches with 3–5 annulations, sometimes along main stem or in apparently regenerated zones.
Hydranths (Figs. 2b, 3b, 4a–b), whitish to brown, 272–375-µm high; hydranth body 216–300-µm high (from hydranth base to hypostome base), 175–248-µm wide (at hydranth base), with 19–27 filiform tentacles arranged in one whorl. Up to 24–30 polyps per hydrocaulus (mostly around 15). Hypostome 74–118-µm high and 171–210-µm wide. Nematocysts are heterotrichous microbasic euryteles of two sizes classes: (1) microbasic euryteles, 7.5–8 × 2.5–3 µm, fusiform and densely distributed on a band 25–35-µm high on hydrant body basally (Figs. 2c, 3c, d); (2) smaller microbasic euryteles, 5.5–6 × 2.5–3 µm abundant mainly on the tentacles (Figs. 2c, 3e, f).
Male and female gonophores in separate colonies. Blastostyles on annulated pedicels borne in all parts of the colony, sometimes on the hydrorhiza. Male gonophores (77–136 µm in diameter), 6 to 10 per group, on completely atrophied polyps (Figs. 2d, 4c–d), mostly two chambered (occasionally only one). Terminal buttons and clusters of microbasic euryteles of the larger type at the apical end of the gonophores and sometimes basally too, not lost during development. Female gonophores (Figs. 2e–f, 4e–f) on either reduced polyps still bearing tentacles or, in older stages, on completely atrophied ones, 5–8 gonophores per blastostyle, 61–110 µm in diameter, spadix simple, with a subterminal nematocyst button.
Biology Fertile colonies were found in summer until July and August (Strait of Gibraltar, present paper) or September (Schuchert 2008).
Ecology Most of the studied material from Ceuta (~ 300 hydrocauli examined) was found living on Eudendrium racemosum (Fig. 3a), except for a small colony (3-mm high, 7 hydranths) that was observed on Sertularella ellisii (Deshayes & Milne-Edwards, 1863).
Geographic and bathymetric distribution Eudendrium capillaroides is known from North-eastern Atlantic Ocean (Brittany, France) (Schuchert 2008) and South-western Mediterranean (Ceuta, Spain) (present paper) (Fig. 1), from shallow waters (15–30-m depth).
Phylogenetic analyses The BI and ML trees obtained from the 16S sequences data (Fig. 5) are quite similar in the supported clades and support the assignment of our examined material to the species Eudendrium capillaroides (Bootstrap [Bst] 99, Posterior probability [PP] 1). Although a sequence of E. maorianus seems to be the sister group of E. capillaroides, the support of the clade is poor for ML (Bst 54) but high for BI (PP 1). Another sequence attributed to E. capillaroides (KP776804, from Trou aux singes, Roscoff, France) is also very divergent to the set of sequences in which the type material (AM991306) and our present material MZB 2022–1514 are reunited (the average and range of p-distances are 5.89% and 5.79–6.23, respectively).
Bayesian analysis (BI) showing the phylogenetic relationships of Eudendrium capillaroides with other Eudendrium species. The present hypothesis is based on 16S rRNA data. Each sequenced specimen is followed by its GenBank accession number and locality indications when available. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. Supporting values of nodes as Posterior probability Bootstrap values (PP/Bst) are indicated in each node, where (–) indicates that particular node has no supporting value in ML inference method. Newly sequenced material in bold
It is worth mentioning that the NE Atlantic sequences [U.K. (Plymouth), France (Normandy), and Spain (Gipuzkoa)] of E. capillare are noticeably divergent from one of the sequences of E. capillare (KP776807, from. Victoria, Australia), and very divergent too from other sequence also attributed to E. capillare (EU305476, material collected in Japan, from tanks in SETO aquarium, and deposited in the Natural History Museum of the University of Kansas, under the registration code KUNHM2625) (Jensen, pers. com.). The uncorrected genetic distances between Eudendrium capillaroides (NE Atlantic and SW Mediterranean sequences) and its similar species E. capillare are 5.30% (NE Atlantic sequences), 8.19% (Australia), and 5.85% (aquarium tanks from Japan).
Remarks The presence of a dense ring of microbasic eurytele nematocysts at the base of the hydranth, instead of being distributed “scattered in a band above the perisarc grove” as described by Schuchert (2008:730), seems to be, at this moment, a characteristic feature of the E. capillaroides material collected in Ceuta. In the comparison of the cnidome between the original description of E. capillaroides and our material, Schuchert (2008) reported two types of nematocysts (microbasic euryteles), with a length and ratio length/width similar to our material (Table 1). Perhaps our material showed slightly smaller average dimensions, especially in the width of the large eurytele nematocysts, and length/width ratios are conversely slightly different (large nematocysts: 2.2 vs. 2.64 and tentacular nematocysts: 2.4 vs. 2.1, for Brittany and Ceuta materials, respectively). Furthermore, Schuchert (2008) did not mention the nematocyst buttons on the female gonophores, which are conspicuous in our material. At this moment, and according to our available molecular results (a single indel differentiates Ceuta and Brittany sequenced colonies), we will be conservative in this paper, although it is desirable that more data on the constancy or variability range of the abovementioned morphological differences in nematocyst measurements and the presence of cnidae bottoms on female gonophores could be provided in the near future. In addition, only 16S molecular information for E. capillaroides is available, and other markers used such as Cox1 or 28S, are poorly sequenced in species of the genus Eudendrium. However, these morphological and molecular differences can be considered as population features.
In the Mediterranean Sea, only E. racemosum presents a band of nematocysts at the base of the hydranth. However, the external morphology, colony and polyp sizes (E. racemosum is the main substrate of E. capillaroides), different types and sizes of nematocysts, the male and female gonophores, and the genetic results are significant differences between the two species.
A ring of nematocyts at the base of the hydranth is a feature shared by nine other species of Eudendrium, although these species can be distinguished from each other on the basis of additional characters. Eudendrium annulatum Norman, 1864; E. arbuscula Wright, 1859; and E. ritchiei Millard, 1975 are polysiphonic species. The colonies, hydrants, and nematocysts of E. annulatum are larger than those of E. capillaroides (Schuchert 2008; Table S1). Also, the belt of nematocysts is absent in some specimens. Eudendrium arbuscula was initially characterized by the band of nematocysts on the hydranth body and the terminal nematocyst button of male sporosacs (Wright 1859; Hincks 1868; Marques et al. 2000a; Schuchert 2008). However, E. arbuscula presents polysiphonic colonies with numerous terminal branches. Unfortunately, Wright did not measure the nematocysts, but the data provided by Marinopoulos (1992) and Schuchert (2008) recorded nematocysts larger than those of E. capillaroides (Table S1). Eudendrium ritchiei shows a strongly annulated perisarc, in addition to the presence of a different type of nematocyst on the hypostome and hydranth body (Table S1). Furthermore, young female gonophores have a bifurcating spadix surrounding the single egg (Millard 1975). Schuchert (1996) recorded some unfasciculated colonies from New Zealand with a ring of large euryteles at the base of the hydranth larger than in E. capillaroides.
Eudendrium generale von Lendenfeld, 1885; E. jaederholmi Puce, Cerrano & Bavestrello, 2002; and Eudendrium scotti Puce, Cerrano & Bavestrello, 2002, also characterized by a belt of nematocysts at the base of the hydrant, are Antarctic species (Puce et al. 2002), and it is quite improbable to find functional and successful populations in the Mediterranean. Although E. generale was also recorded in Southern Australian waters (Watson 1994) and New Zealand (Cairns et al. 2009), some other conspicuous characteristics distinguish it from E. capillaroides such as annulations at the base of the branches, female gonophores, and large nematocysts at the base of the hydrant (Table S1). On the other hand, E. jaederholmi has the primary stems and branches of the colony with annulations at their base as well as long nematophores and female gonophores on partially atrophied hydranths. In addition, the nematocysts on the ring are larger than those of E. capillaroides (Puce et al. 2002, Table S1). Finally, E. scotti presents gonophores developed on non-reduced hydranths and large nematocysts disposed in two bands, one on the hypostome and one at the base of the hydranth (Puce et al. 2002, Table S1).
Eudendrium album Nuttig, 1896 has large macrobasic euryteles, the male and female gonophores develop on non-reduced hydranths and the male gonophores can have three chambers and a distal button without concentration of nematocysts (Schuchert 2008, Table S1).
Eudendrium californicum Torrey, 1902 is one of the most conspicuous species of Eudendriidae (see Table S1) due to a deep circular groove near the base of the hydranth. The perisarc of the stem and branches presents tightly annulated rings throughout (Torrey 1902; Marques et al. 2000a) and the nematocysts of the ring are atrichous isorhizas (Weill 1934).
Eudendrium nambuccense Watson, 1985 has one type of nematocyst of one size class sensu Watson (1985). The male and female gonophores are very different from those of E. capillaroides, and besides, this species presents a completely disjointed distribution (Table S1).
Discussion
Eudendrium capillaroides is presented here as a common species in Ceuta’s waters and has been recorded over several years from 2007 to 2019. However, although the European hydroid fauna, and especially that of the Mediterranean Sea, is considered one of the better known in the world (González-Duarte et al. 2016), E. capillaroides had not been recorded since its original description in Brittany (France) in two localities relatively close to each other: The Bay of Morlaix and Camaret (Schuchert 2008) until now. In a sampling program to study coastal benthic hydroid communities in the Gulf of Cádiz, Strait of Gibraltar, and Alboran Sea (González-Duarte 2011; González-Duarte et al. 2013b, 2014), Ceuta was the only sampling point where E. capillaroides was found, and not at all depths or sampling stations. In addition, the sampling stations where this species was found were very close to each other. Among metazoans, perhaps hydroids are unique because many species have a cosmopolitan distribution with the absence of local speciation (Gili and Hughes 1995). In hydroids, an anfiatlantic distribution seems to be more common in species that do not release medusae (Cornelius 1992; Haydar 2012), such as Eudendrium species. Therefore, it is likely that E. capillaroides is present in other Mediterranean or European zones and, because of its small size and similarity to other species such as E. capillare, it may have been identified, if detected, as belonging to other species. Eudendrium capillaroides is an example of how tiny or cryptic species can be easily overlooked and the diversity of a group can be underestimated. Especially in less conspicuous species, such as many hydroids, the diversity might be underestimated (Haydar 2012).
The most significant and evident features of E. capillaroides are its small colony and polyp sizes. There is enough evidence to establish that fully developed and mature colonies do not exceed 20 mm at different depths and different locations. These facts link E. capillaroides, from an ecological point of view, with the so-called E. capillare species group (Vervoort 2006), which shows colony sizes not exceeding 40 mm and polyp sizes usually not exceeding 500 µm. This is a fixed and significant character undoubtedly linked to a different ecological niche within their habitats. Eudendrium capillaroides is a common species in Ceuta waters with a specialized ecological role, exclusively epibiotic over other hydroids, and a marked preference for Eudendrium racemosum as substrate.
Cryptic species are those difficult or impossible to distinguish based on external morphological characters (Moura et al. 2008, 2011; Postaire et al. 2016a, 2017a, b). Defining species boundaries is the main problem raised by cryptic species, due to possible intraspecific polymorphisms (Moura et al. 2008, 2011; Schuchert 2014; Postaire et al. 2015, 2016a, b). In addition, cryptic speciation seems to be common in benthic invertebrates with a planktonic stage in their life cycle (Lindner et al. 2011; Postaire et al. 2016b, 2017a). Because of their similar morphology, many species are not recorded or identified as belonging to other species and, diversity in many groups may currently be underestimated. This phenomenon has already been described for hydrozoans (Moura et al. 2008, 2011; Folino-Rorem et al. 2009; Miglietta et al. 2009; Lindner et al. 2011; Postaire et al. 2016b).
Eudendrium teissieri Cabioch, 1970 is an unavailable name (see ICZN art. 13.1) and is currently considered a synonym of E. capillaroides. As commented, Schuchert (2008), Castric and Michel (1982), and Castric et al. (1987) included the former name in their faunistic guides of the Brittany (France), and a sketch of the species showed complementary nematocysts relatively small and concentrated in a band on the hydrant body. According to this fact, we could conclude that the material collected by Schuchert (2008) in the Bay of Morlaix (Brittany), E. teissieri and the present material are the same species. In agreement with our material, specimens collected by Schuchert (2008) also behaved as a specialized epibiotic of other hydroids, in Nemertesia antennina in the latter case. Schuchert (2008) pointed out that E. capillariodes is very similar to E. capillare Alder, 1856. However, the presence of two different types of nematocysts and a dense ring of microbasic euryteles at the base of the hydranth in the former make both species easily distinguishable.
Differing in only one indel in the 16S sequence, our examined material from Ceuta agrees well with the sequences of the type material. However, the existence of another quite divergent sequence attributed to C. capillaroides from Roscoff (KP776804) (Fig. 5) deserves further study concerning the possibility of cryptic species in the same area. In the case of the apparently most similar species, E. capillare, the tree obtained (16S data, Fig. 5) suggests a more complex situation. The type material of E. capillare is from Embleton Bay, England (NE Atlantic) (Alder 1856: 355); however, the set of sequences from European waters (NE Atlantic-W Mediterranean—KP776823, KP776824, AM991294, AM991295, AM991296, AY787884) are quite separated from those from Australia (KP776807) and aquarium tanks from Japan (EU305476). The latter material belongs to specimens from aquarium tanks, making it difficult to know with certainty its true origin. However, as shown for many other hydroids (Moura et al. 2008, 2011; Postaire et al. 2016b), E. capillare is likely to be a species complex. More research with additional sequenced materials is needed to examine if there are morphological features that would allow for agreement with molecular phylogenies.
Benthic hydroids are among the first to colonize virgin substrata (Boero and Fresi 1986; Gili and Hughes 1995; Zintzen et al. 2007; González-Duarte et al. 2018). They can be epibionts or serve as substrate for other organisms (Boero 1984; Boero and Fresi 1986; Bouillon et al. 2004). The benthic phase of these organisms is in continuous competition for substrate, and therefore, they are adapted to colonize a wide range of different substrates: cliff, caves, crevices, reefs, big rocks, artificial habitats, etc. (Boero et al. 1986; Gili and Hughes 1995; Megina et al. 2013), and can grow on algae and other invertebrates: sponges, polychaetes, crustaceans, bivalve, bryozoans, or other hydroids (Dales 1966; Boero 1984; Genzano and Rodriguez 1998; Govindarajan et al. 2005). Most of the hydroid species are substrate generalists, commonly occurring on a wide variety of hard substrates (Gill et al. 1989; Calder 1991; Ronowicz et al. 2013). However, Eudendrium capillaroides showed a preference for growing on other hydroids, mainly on E. racemosum. Although this occurs in the material from Ceuta and while waiting to find more material of this species, the high number of colonies found on E. racemosum (more than 300 hydrocauli over different years) and not on any other substrate, led us to propose that this is an essential characteristic of E. capillaroides. This information could help to find more specimens of E. capillaroides in the Atlantic and in the Mediterranean Sea.
Hydroids comprise a high number of inconspicuous or cryptic organisms and, together with the increasing loss of taxonomical expertise, this leads to them often being reported in ecological and faunistic studies in a generic way as “hydroids”, without identification at species level or simply identified at genus level (Fraschetti et al. 2001; Dethier et al. 2003; Bussotti et al. 2006; Vertino et al. 2010; Angeletti et al. 2014; among others). The real diversity of many benthic marine communities is also underestimated because rare, inconspicuous, or cryptic species are not considered (Piraino et al. 2002; Lindner et al. 2011; Haydar 2012; Dee et al. 2019). Rare species could be crucial in the description and survival of the communities (Dee et al. 2019). They are a reservoir of potential diversity, contain information about the possible future composition of the communities, and can replace the dominant species to successfully face new situations as important disturbances (Boero 1994; Chapman 1999; Piraino et al. 2002; Dee et al. 2019). An accurate description of these species that often pass unnoticed, as do many hydroids, is important in order to better estimate global marine diversity and to understand marine communities and the relationships between their components.
References
Alder J (1856) A notice of some new genera and species of British hydroid zoophytes. Ann Mag Nat Hist 2:353–362
Alfaro ME, Zoller S, Lutzoni F (2003) Bayes or Bootstrap? A simulation study comparing the performance of Bayesian Markov chain Monte Carlo sampling and bootstrapping in assessing phylogenetic confidence. Mol Biol Evol 20:255–226. https://doi.org/10.1093/molbev/msg028
Angeletti L, Taviani M, Canese S et al (2014) New deep water cnidarian sites in the southern Adriatic Sea. Mediterr Mar Sci 15:263–273. https://doi.org/10.12681/mms.558
Bavestrello G, Piraino S (1991) On two Eudendrium (Cnidaria, Hydrozoa) species from the Mediterranean Sea. Oebalia 17:197–207
Boero F (1984) The ecology of marine hydroids and effects of environmental factors: a review. Mar Ecol 5:93–118. https://doi.org/10.1111/j.1439-0485.1984.tb00310.x
Boero F (1994) Fluctuations and variations in coastal marine environments. Mar Ecol 15:3–25
Boero F, Fresi E (1986) Zonation and evolution of a rocky bottom hydroid community. Mar Ecol 7:123–150
Boero F, Balduzzi A, Bavestrello G, Caffa BC, Vietti R (1986) Population dynamics of Eudendrium glomeratum (Cnidaria: Anthomedusae) on the Portofino promontory (Ligurian Sea). Mar Biol 85:81–85
Bouillon J, Medel F, Pagés F et al (2004) Fauna of the Mediterranean Hydrozoa. Sci Mar 68:5–449. https://doi.org/10.3989/scimar.2004.68s25
Bouillon J, Gravili C, Pagès F et al (2006) An introduction to Hydrozoa. Mémoires du Muséum National d’Histoire Naturelle, Paris
Bussotti S, Terlizzi A, Fraschetti S et al (2006) Spatial and temporal variability of sessile benthos in shallow Mediterranean marine caves. Mar Ecol Prog Ser 325:109–119
Cairns SD, Gershwin LA, Brook FJ, et al (2009) Phylum cnidaria; corals, medusae, hydroids, myxozoa. In: Gordon D (ed) New Zealand inventory of biodiversity, vol 1. Kingdom animalia: radiata, lophotrochozoa, deuterostomia. Canterbury University Press, Christchurch, pp 59–101
Calder DR (1972) Some athecate hydroids from the shelf waters of Northern Canada. J Fish Res Board Canada 29:217–228. https://doi.org/10.1139/f72-040
Calder DR (1991) Associations between hydroid species assemblages and substrate types in the mangal at Twin Cays, Belize. Can J Zool 69:2067–2074. https://doi.org/10.1139/z91-288
Calder DR (2012) On a collection of hydroids (Cnidaria, Hydrozoa, Hydroidolina) from the west coast of Sweden, with a checklist of species from the region. Zootaxa 3171:1–77
Castric A, Girard A, Michel C (1987) Roches sous marines de Bretagne: flore et faune fixée, 4th edn. CNRS Laboratoire de biologie marine, Concarneau
Castric A, Michel C (1982) Flore et faune fixées sous-marines de Bretagne. CNRS Laboratoire de biologie marine, Concarneau
Chapman MG (1999) Are there adequate data to assess how well theories of rarity apply to marine invertebrates? Biodivers Conserv 8:1295–1318
Cohen AL (1979) Critical point drying-principles and procedures. Scanning Electron Microsc 2:303–322
Cornelius P (1992) Medusa loss in leptolid Hydrozoa (Cnidaria), hydroid rafting, and abbreviated life-cycles among their remote-island faunae: an interim review. Sci Mar 56:245–261
Cunningham CW, Buss LW (1993) Molecular evidence for multiple episodes of paedomorphosis in the family Hydractiniidae. Biochem Syst Ecol 21:57–69. https://doi.org/10.1016/0305-1978(93)90009-G
Dales RP (1966) Symbiosis in marine organisms. In: Henry SM (ed) Associations of microorganisms, plants and marine organisms, vol 1. Academic Press, New York, pp 299–326
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9(8):772
Dee LE, Cowles J, Isbell F et al (2019) When do ecosystem services depend on rare species? Trends Ecol Evol 34:746–758. https://doi.org/10.1016/j.tree.2019.03.010
Dethier M, McDonald K, Strathmann R (2003) Colonization and connectivity of habitat patches for coastal marine species distant from source populations. Conserv Biol 17:1024–1035
Folino-Rorem NC, Darling JA, D’Ausilio CA (2009) Genetic analysis reveals multiple cryptic invasive species of the hydrozoan genus Cordylophora. Biol Invasions 11:1869–1882. https://doi.org/10.1007/s10530-008-9365-4
Fraschetti S, Bianchi CN, Terlizzi A et al (2001) Spatial variability and human disturbance in shallow subtidal hard substrate assemblages: a regional approach. Mar Ecol Prog Ser 212:1–12
Gabe M (1968) Techniques histologiques. Masson et Cie, Paris
Galea HR (2007) Hydroids and hydromedusae (Cnidaria: Hydrozoa) from the fjords region of southern Chile. Zootaxa 1597:1–116
Galea HR, Häussermann V, Försterra G (2009) New additions to the hydroids (Cnidaria: Hydrozoa) from the fjords region of southern Chile. Zootaxa 28:1–28
Genzano G, Rodriguez GM (1998) Association between hydroid species and their substrates from the intertidal zone of Mar del Plata Argentine. Miscel lània Zoològica 21:21–29
Gili J, Hughes RG (1995) The ecology of marine benthic hydroids. Oceanogr Mar Biol Annu Rev 33:351–426
Gill J, Murill J, Ros J (1989) The distribution pattern of benthic Cnidarians in the Western Mediterranean. Sci Mar 53:19–35
González-Duarte MM (2011) Variaciones de las comunidades de hidrozoos en la transición entre el Atlántico y el Mediterráneo. PhD thesis, University of Cádiz, Cádiz
González-Duarte MM, Megina C, Bethencourt M (2013) Sertularia marginata (Cnidaria: Hydrozoa) in the Mediterranean: an alien species in expansion? Mediterr Mar Sci 14:384–389. https://doi.org/10.12681/mms.445
González-Duarte MM, Megina C, Piraino S, Cervera JL (2013b) Hydroid assemblages across the Atlantic-Mediterranean boundary: is the Strait of Gibraltar a marine ecotone? Mar Ecol 34:33–40. https://doi.org/10.1111/maec.12022
González-Duarte MM, Megina C, Piraino S (2014) Looking for long-term changes in hydroid assemblages (Cnidaria, Hydrozoa) in Alboran Sea (South-Western Mediterranean): a proposal of a monitoring point for the global warming. Helgol Mar Res 68:511–521. https://doi.org/10.1007/s10152-014-0406-3
González-Duarte MM, Megina C, De Vito D et al (2016) A unified assessment of marine Mediterranean assemblages: a lesson from benthic hydroids. Mar Ecol 37:155–163. https://doi.org/10.1111/maec.12271
González-Duarte MM, Fernández-Montblanc T, Bethencourt M, Izquierdo A (2018) Effects of substrata and environmental conditions on ecological succession on historic shipwrecks. Estuar Coast Shelf Sci 200:301–310. https://doi.org/10.1016/j.ecss.2017.11.014
Govindarajan AF, Piraino S, Gravili C, Kubota S (2005) Species identification of bivalve-inhabiting marine hydrozoans of the genus Eugymnanthea. Invertebr Biol 124:1–10. https://doi.org/10.1111/j.1744-7410.2005.1241-01.x
Haydar D (2012) What is natural? The scale of cryptogenesis in the North Atlantic Ocean. Divers Distrib 18:101–110. https://doi.org/10.1111/j.1472-4642.2011.00863.x
Hillis DM, Bull JJ (1993) An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst Biol 42:182–192. https://doi.org/10.1093/sysbio/42.2.182
Hincks T (1868) A history of the British hydroid zoophytes I. John van Voorst, London
Huelsenbeck JP, Ronquist F (2001) MrBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755. https://doi.org/10.1093/bioinformatics/17.8.754
Lindner A, Govindarajan AF, Migotto AE (2011) Cryptic species, life cycles, and the phylogeny of Clytia (Cnidaria: Hydrozoa: Campanulariidae). Zootaxa 2980:23–36
Mammen TA (1963) On a collection of hydroids from South India. J Mar Biol Assoc India 5:27–61
Marinopoulos J (1992) Contribution à l’étude du genre Eudendrium (Hydrozoa: Hydroida) de la Méditerranée: taxonomie et phylogénie. Bulletin De L’institut Océanographique (monaco) 9:53–66
Marques AC (2001) The genus Eudendrium (Hydrozoa, Anthomedusae, Eudendriidae) from Brazil. Papéis Avulsos De Zoologia 41:329–405
Marques AC, Mergner H, Höinghaus R et al (2000a) Morphological study and taxonomical notes on Eudendriidae (Cnidaria: Hydrozoa: Athecatae/Anthomedusae). Zoologische Mededelingen 74:75–118
Marques AC, Peña Cantero AL, Vervoort W (2000b) Mediterranean species of Eudendrium Ehrenberg, 1834 (Hydrozoa, Anthomedusae, Eudendriidae) with the description of a new species. J Zool 252:197–213
Medel MD (1996) Estudio taxonómico de los Hidrozoos del Estrecho de Gibraltar. PhD thesis, University of Seville, Seville
Megina C, González-Duarte MM, López-González PJ, Piraino S (2013) Harbours as marine habitats: hydroid assemblages on sea-walls compared with natural habitats. Mar Biol 160:371–381. https://doi.org/10.1007/s00227-012-2094-3
Megina C, González-Duarte MM, López-González PJ (2016) Benthic assemblages, biodiversity and invasiveness in marinas and commercial harbours: an investigation using a bioindicator group. Biofouling 32:465–475. https://doi.org/10.1080/08927014.2016.1151500
Miglietta MP, Schuchert P, Cunningham CW (2009) Reconciling genealogical and morphological species in a worldwide study of the Family Hydractiniidae (Cnidaria, Hydrozoa). Zool Scr 38:403–430. https://doi.org/10.1111/j.1463-6409.2008.00376.x
Millard NAH (1975) Monograph on the Hydroida of southern Africa. Ann S Afr Museum 68:1–513
Motz-Kossowska S (1905) Contribution à la connaissance des hydraires de la Méditerranée occidentale. I. Hydraires gymnoblastiques. Arch De Zoologie Exp Et Générale 4:39–98
Moura CCJ, Harris DDJ, Cunha MRM, Rogers AD (2008) DNA barcoding reveals cryptic diversity in marine hydroids (Cnidaria, Hydrozoa) from coastal and deep-sea environments. Zool Scr 37:93–108. https://doi.org/10.1111/j.1463-6409.2007.00312.x
Moura CJ, Cunha MR, Porteiro FM, Rogers AD (2011) The use of the DNA barcode gene 16S mRNA for the clarification of taxonomic problems within the family Sertulariidae (Cnidaria, Hydrozoa). Zool Scr 40:520–537. https://doi.org/10.1111/j.1463-6409.2011.00489.x
Moura CJ, Cunha MR, Porteiro FM et al (2012) Evolution of Nemertesia hydroids (Cnidaria: Hydrozoa, Plumulariidae) from the shallow and deep waters of the NE Atlantic and western Mediterranean. Zool Scr 41:79–96. https://doi.org/10.1111/j.1463-6409.2011.00503.x
Oliveira OMP, Ayón P, Cedeño-Posso CM, et al (2016) Census of the Cnidaria (Ceriantharia and Medusozoa) and Ctenophora from South American marine waters. Zootaza 4194(1):1–256
Peña Cantero AL (2013) Benthic hydroids from off Low Island (Southern Ocean, Antarctica). Mar Ecol 34:123–142. https://doi.org/10.1111/maec.12031
Peña Cantero AL, Vervoort W (2009) Benthic hydroids (Cnidaria: Hydrozoa) from the Bransfield Strait area (Antarctica) collected by Brazilian expeditions, with the description of a new species. Polar Biol 32:83–92. https://doi.org/10.1007/s00300-008-0506-0
Picard J (1951) Note sur les hydraires littoraux de Banyuls-Sur-Mer. Vie Et Milieu 2:338–349
Picard J (1955) Hydraires des environs de Castiglione (Algérie). Picard, J. (1955). Hydraires des environs de Castiglione (Algérie). Bullet De La Station D’aquiculture Et De Pêche De Castiglione, Nouveau Série 7:177–199
Picard J (1958) Origines et affinités de la faune d’hydropolypes (Gymnoblastes et Calyptoblastes) et d’hydroméduses (Anthoméduses et Leptoméduses) de la Méditerranée. Rapp. P.-V. Réun. Commn Int Explor Scient Mer Méditerr 14:187–199
Piraino S, Fanelli G, Boero F (2002) Variability of species’ roles in marine communities: change of paradigms for conservation priorities. Mar Biol 140:1067–1074. https://doi.org/10.1007/s00227-001-0769-2
Postaire B, Aurelle D, Bourmaud CAF, Bruggemann JH, Magalon H (2015) Isolation and characterisation of 16 microsatellite loci from a widespread tropical hydrozoan, Lytocarpia brevirostris (Busk, 1852). Conserv Genet Resour 7:505–507. https://doi.org/10.1007/s12686-014-0407-1
Postaire B, Magalon H, Bourmaud CAF, Bruggemann JH (2016a) Molecular species delimitation methods and population genetics data reveal extensive lineage diversity and cryptic species in Aglaopheniidae (Hydrozoa ). Mol Phylogenet Evol 105:36–49. https://doi.org/10.1016/j.ympev.2016.08.013
Postaire B, Magalon H, Bourmaud CAF, Gravier-Bonnet N, Bruggemann JH (2016b) Phylogenetic relationships within Aglaopheniidae (Cnidaria, Hydrozoa) reveal unexpected generic diversity. Zool Scr 45:103–114. https://doi.org/10.1111/zsc.12135
Postaire B, Gélin P, Bruggemann JH, Magalon H (2017a) One species for one island? Unexpected diversity and weak connectivity in a widely distributed tropical hydrozoan. Heredity 118:385–394. https://doi.org/10.1038/hdy.2016.126
Postaire B, Gélin P, Bruggemann JH, Pratlong M, Magalon H (2017b) Population differentiation or species formation across the Indian and the Pacific Oceans? An example from the brooding marine hydrozoan Macrorhynchia phoenicea. Ecol Evol 7:8170–8186. https://doi.org/10.1002/ece3.3236
Puce S, Cerrano C, Bavestrello G (2002) Eudendrium (Cnidaria, Anthomedusae) from the Antarctic Ocean with description of two new species. Polar Biol 6:366–373. https://doi.org/10.1007/s00300-001-0353-8
QGIS Development Team (2018) QGIS geographic information system. Open source geospatial foundation project. https://qgis.org
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst Biol 67:901–904. https://doi.org/10.1093/sysbio/syy032
Ronowicz M, Włodarska-Kowalczuk M, Kukliński P (2013) Depth- and substrate-related patterns of species richness and distribution of hydroids (Cnidaria, Hydrozoa) in Arctic coastal waters (Svalbard). Mar Ecol 34:165–176. https://doi.org/10.1111/maec.12034
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19(12):1572–1574
Schuchert P (1996) The marine fauna of New Zealand: athecate hydroids and their medusae (Cnidaria: Hydrozoa). NZ Oceanogr Inst Mem 106:1–159
Schuchert P (2008) The European athecate hydroids and their medusae (Hydrozoa, Cnidaria): Filifera Part 4. Rev Suisse Zool 115:677–757
Schuchert P (2014) High genetic diversity in the hydroid Plumularia setacea: A multitude of cryptic species or extensive population subdivision? Mol Phylogenet Evol 76:1–9. https://doi.org/10.1016/j.ympev.2014.02.020
Schuchert P (2022) World Hydrozoa Database. Eudendrium Ehrenberg, 1834. Accessed through: World Register of Marine Species at https://www.marinespecies.org/aphia.php?p=taxdetails&id=117093
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol 30:2725–2729. https://doi.org/10.1093/molbev/mst197
Torrey HB (1902) The Hydroida of the Pacific coast of North America. Univ California Publ Zool 1(1):1–104
Totton AK (1930) Coelenterata. Part V.- Hydroida. Br Museum (Natural Hist Br Antart (“Terra Nova”) Exped 1910. Nat Hist Rep Zool 5:131–252
Vertino A, Savini A, Rosso A et al (2010) Benthic habitat characterization and distribution from two representative sites of the deep-water SML Coral Province (Mediterranean). Deep Res Part II Top Stud Oceanogr 57:380–396. https://doi.org/10.1016/j.dsr2.2009.08.023
Vervoort W (2006) Leptolida (Cnidaria: Hydrozoa) collected during the CANCAP and Mauritania-II expeditions of the National Museum of Natural History, Leiden, The Netherlands [Anthoathecata, various families of Leptothecata and addenda]. Zool Mededelingen 80:181–318
Watson JE (1985) The genus Eudendrium (Hydrozoa: Hydroida) from Australia. Proc R Soc Victoria 97:179–221
Watson JE (1994) Shallow water hydroids from eastern Bass Strait. Victorian Naturalist 111:65–69
Weill R (1934) Contribution à l’ étude des cnidaires et de leurs nématocystes I II Travaux de la Station. Zool de Wimereux 10 11:1–701
Wright TS (1859) Observations on British zoophytes. Edinburgh New Philos J 10:105–114
Zagal CJ, Underwood AJ, Chapman MG (2013) Distribution of hydroids along fronds of the kelp Ecklonia radiata. Hydrobiologia 720:89–99. https://doi.org/10.1007/s10750-013-1627-1
Zintzen V, Norro A, Massin C, Mallefet J (2007) Temporal variation of Tubularia indivisa (Cnidaria, Tubulariidae) and associated epizoites on artificial habitat communities in the North Sea. Mar Biol 153:405–420. https://doi.org/10.1007/s00227-007-0819-5
Acknowledgements
We would like to thank Doris De Vito and Juan Sempere-Valverde, who helped us with sampling, and S. Piraino, who also reviewed a draft of the manuscript. We would also like to thank Dr. Kirsten Jensen, curator of the Invertebrate Zoology, Biodiversity Institute of the University of Kansas, who generously provided us with information about the voucher material KUNHM2625 of E. capillare. We would like to thank the Marine Biodiversity reviewers who have contributed to the improvement of this article.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study has been supported by the project P05-RNM-369 (Consejería de Innovación, Ciencia y Empresa de la Junta de Andalucía), two Spanish Ministry grants: Ministry of Education and Science PCI2005-A7-0347 and Ministry of Foreign Affairs and Cooperation A/5481/06 and A/8688/07, and MITECO with the ESMARES2-INFRA project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Ethics approval
No animal testing was performed during this study.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities. The study is compliant with CBD and Nagoya protocols.
Data availability
Specimens are deposited at the Museu de Zoologia in Barcelona under numbers MZB 2022–1510 to MZB 2022–1515. Genetic data are deposited in GenBank (www.ncbi.nlm.nih.gov/genbank) under the accession number ON366371.
Author contribution
MMGD and CM conceived and designed the manuscript. MMGD and CM collected samples and provided DNA sequences. MMGD sorted the samples and provide morphological data. PJLG Conducted molecular analysis and SEM photos. All authors drafted and critically reviewed the manuscript.
Additional information
Communicated by D. Maggioni
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
González-Duarte, M.M., Megina, C. & López-González, P.J. How tiny species can be overlooked: the finding of Eudendrium capillaroides (Cnidaria, Hydrozoa) in the Strait of Gibraltar. Mar. Biodivers. 53, 28 (2023). https://doi.org/10.1007/s12526-023-01337-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-023-01337-0