Abstract
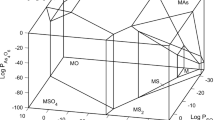
The two-dimensional predominance area (Kellogg) diagram (PAD) surfaces bounding the three-dimensional Cu-As-S-O system were constructed at 900 K. The computations employed gases As4O6(g), O2(g), and SO2(g) as the independent coordinate species to generate diagrams of As4O6(g) − O2(g),O2(g) − SO2(g), and As4O6(g) − SO2(g), while the third gas (As4O6, O2, or SO2) was fixed. The results indicate that there is no shared boundary between enargite (Cu3AsS4) and copper sulfate (CuSO4); however, at \( {\mathrm{P}}_{{\mathrm{O}}_2}<{10}^{-6} \)atm, \( {\mathrm{P}}_{{\mathrm{As}}_4{\mathrm{O}}_6}<{10}^{-10} \) atm, and \( {\mathrm{P}}_{{\mathrm{SO}}_2}>{10}^{-4} \) atm, CuSO4 is the stable phase at 900 K. The chemical driving force for oxidation of intermediate phases between enargite and copper sulfate is substantial except for basic sulfate (CuO · CuSO4).



Similar content being viewed by others
References
Safarzadeh MS, Moats MS, Miller JD (2014a) Recent trends in the processing of enargite concentrates. Miner Process Extr Metall Rev 35(5):283–367
Safarzadeh MS, Moats MS, Miller JD (2014b) An update to recent trends in the processing of enargite concentrates. Miner Process Extr Metall Rev 35(6):390–422
Safarzadeh MS, Miller JD (2016) The pyrometallurgy of enargite: a literature update. Int J Miner Process 157:103–110
Yoshimura Z (1962) The fundamental investigation of desarsenifying roasting of copper concentrate and its industrial practice. J Min Metall Inst Japan 78:447–453
Secco AC, Riveros GA, Luraschi AA (1988) Thermal decomposition of enargite and phase relations in the system copper-arsenic-sulfur. In: Diaz C, Landolt C, Luraschi A (eds) Copper 87, Pyrometallurgy of Copper, vol 4. Universidad de Chile, Santiago, Chile, pp 225–238
Mihajlović I, Štrbac N, Živković Ž, Kovacević R, Stehernik M (2007) A potential method for arsenic removal from copper concentrates. Miner Eng 20:26–33
Denbigh KG (1981) The principles of chemical equilibrium, 4th edn. Cambridge University Press, New York, NY, pp 182–199
Lynch DC (1988) A review of the physical chemistry of arsenic as it pertains to primary metalsproduction. In: RG, Hendrix JL, Queneau PB (eds) Arsenic metallurgy: fundamentals and applicationsReddy, Warrendale, PA, TMS-AIME, pp 3–32
Rao YK (1985) Stoichiometry and thermodynamics of metallurgical processes. Cambridge University Press, Cambridge, UK
Chase MW Jr (1988) NIST-JANAF thermochemical tables. American Institute of Physics for the National Institute of Standards and Technology, American Chemical Society, Washington, D.C., New York
Craig JR, Barton PB (1973) Thermochemical approximations for sulfosalts. Econ Geol 68:493–506
Seal RR, Robie RA, Hemingway BS, Evans HT (1996) Heat capacity and entropy at temperatures 5 K to 720 K and thermal expansion from the temperatures 298 K to 573 K of synthetic enargite (Cu3AsS4). J Chem Thermodyn 28:405–412
Wagman DD, Evans WH, Parker VB, Schumm RH, Halow I, Balley SM, Churney KL, Nuttal RL (1982) NBS tables of chemical thermodynamic properties: selected values for inorganic and C1 and C2 organic substances in SI units. American Chemical Society and the American Institute of Physics for the National Bureau of Standards, Washington, D.C.
Safarzadeh MS, Howard SM (2018) Thermal removal of arsenic from copper concentrates: three-dimensional isothermal predominance diagrams for the Cu-As-S-O system. J Hazard Mater 347:371–377
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Safarzadeh, M.S., Howard, S.M. Predominance Area Diagrams Bounding the Cu-As-S-O System’s 3D Predominance Diagram at 900 K (627 °C). Mining, Metallurgy & Exploration 36, 817–823 (2019). https://doi.org/10.1007/s42461-019-0069-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-019-0069-3