Abstract
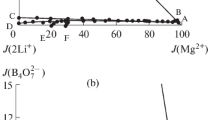
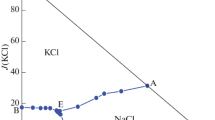
The isothermal dissolution method was used to study the stable phase equilibria in the simple quaternary system Li+//Cl–, S\({\text{O}}_{4}^{{2 - }}\), B4\({\text{O}}_{7}^{{2 - }}\)–H2O and reciprocal quaternary system Li+, Mg2+//Cl–, B4\({\text{O}}_{7}^{{2 - }}\)–H2O at 273 K. The stable phase equilibrium of simple quaternary system Li+//Cl–, S\({\text{O}}_{4}^{{2 - }}\), B4\({\text{O}}_{7}^{{2 - }}\)–H2O belongs to hydrate type I, and there is no complex salt. The phase diagram is constituted of an invariant point, three univariant solubility curves, and three solid phase crystalline regions. The stable phase equilibrium of reciprocal quaternary system Li+, Mg2+//Cl–, B4\({\text{O}}_{7}^{{2 - }}\)–H2O belongs to complex type, and the double salt of lithium carnallite (LiCl·MgCl2·7H2O) is found. There are three invariant points, seven univariant solubility curves, and five solid phase crystalline regions.









Similar content being viewed by others
REFERENCES
Sh. Tursunbadalov, Russ. J. Inorg. Chem. 65, 412 (2020). https://doi.org/10.1134/S0036023620030195
M. M. Asadov, N. A. Akhmedova, S. R. Mamedova, et al., Russ. J. Inorg. Chem. 65, 1061 (2020). https://doi.org/10.1134/S0036023620070013
A. I. Rasulov, P. A. Akhmedova, B. Yu. Gamataeva, et al., Russ. J. Inorg. Chem. 64, 135 (2019). https://doi.org/10.1134/S0036023619010169
L. Zayani and R. Rokbani, J. Therm. Anal. Calorim. 59, 885 (2000). https://doi.org/10.1023/A:1010126409399
H. T. Yang, D. W. Zeng, T. Y. Liang, et al., Ind. Eng. Chem. Res. 52, 17057 (2013). https://doi.org/10.1021/ie4027555
C. Y. Xue, B. Zhao, H. F. Guo, et al., Fluid Phase Equilib. 408, 115 (2013). https://doi.org/10.1021/je400509p
S. Q. Wang, Y. F. Guo, W. J. Liu, et al., J. Solution Chem. 44, 1545 (2015). https://doi.org/10.1007/s10953-015-0359-4
S. Tursunbadalov and L. Soliev, J. Chem. Eng. Data 61, 2209 (2016). https://doi.org/10.1021/acs.jced.5b00875
L. Z. Meng, D. Li, Y. F. Guo, et al., J. Chem. Eng. Data 56, 5060 (2011). https://doi.org/10.1021/je2006852
Y. H. Liu, Y. F. Guo, X. P. Yu, et al., J. Chem. Eng. Data 59, 1685 (2014). https://doi.org/10.1021/je500140e
D. C. Li, J. S. Yuan, and S. Q. Wang, Russ. J. Phys. Chem. A 88, 42 (2014). https://doi.org/10.1134/S0036024414010300
D. L. Gao, Y. F. Guo, X. P. Yu, et al., J. Chem. Eng. Data 60, 2594 (2015). https://doi.org/10.1021/acs.jced.5b00121
T. L. Deng and D. C. Li, Fluid Phase Equilibr. 269, 98 (2008). https://doi.org/10.1016/j.fluid.2008.05.005
R. Z. Cui, S. H. Sang, K. J. Zhang, et al., J. Chem. Eng. Data 57, 3498 (2012.). https://doi.org/10.1021/je3006387
S. H. Sang, X. P. Li, D. W. Li, et al., Russ. J. Inorg. Chem. 63, 1644 (2018). https://doi.org/10.1134/S0036023618160027
S. H. Sang, H. Y. Yu, and D. Z. Cai, Inorg. Chem. 9, 1316 (2005). https://doi.org/10.3321/j.issn:1001-4861.2005.09.008
S. H. Sang, M. L. Tang, H. A. Yin, et al., J. Chem. Eng. Data 25, 381 (2003). https://doi.org/10.3969/j.issn.1005-9954.2003.04.015
S. H. Sang, J. Peng, and L. N. Wei, Phys. Chem. (China) 25, 331 (2009). https://doi.org/10.3866/PKU.WHXB20090223
S. H. Sang, and S. D. Qu, Mineral Rock (China) 30, 102 (2010). https://doi.org/10.3969/j.issn.1001-6872.2010.04.017
S. H. Sang, H. A. Y, and S. J. Ni, Phys. Chem. 23, 1285 (2007). https://doi.org/10.3866/PKU.WHXB20070828
Y. Jing, Sea–Lake Salt Chem. Ind. 29, 24 (2000). https://doi.org/10.16570/j.cnki.issn1673-6850.2000.02.009
S. A. Mazunin, M. N. Noskov, and A. V. Elsukov, Russ. J. Inorg. Chem. 64, 257 (2019). https://doi.org/10.1134/S003602361902013X
R. Z. Cui, S. H. Sang, Y. X. Hu, et al., Acta Geol. Sin. Engl. Ed. 87, 1668 (2013). https://doi.org/10.1111/1755-6724.12167
S. Y. Dou, H. F. Guo, B. Zhao, et al., J. Chem. Eng. Data 61, 450 (2016). https://doi.org/10.1021/acs.jced.5b00639
D. C. Li, F. Li, Y. Y. Zhao, et al., J. Chem. Eng. Data 60, 82 (2015). https://doi.org/10.1021/je500738g
S. H. Sang and J. Peng, CALPHAD 34, 64 (2010). https://doi.org/10.1016/j.calphad.2009.12.001
Funding
This project was supported by the National Natural Science Foundation of China (41873071) and the National Natural Science Foundation of China-Qaidam Saline Lake, Chemical Engineering Science Research Joint Fund of Qinghai Provincial People’s Government (U1407108).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sang, S.H., Guo, X.F., Zhang, T.T. et al. Stable Phase Equilibria in Li + //Cl – , S \({\text{O}}_{4}^{{2 - }}\) , B 4 \({\text{O}}_{7}^{{2 - }}\) –H 2 O and Li + , Mg 2+ //Cl – , B 4 \({\text{O}}_{7}^{{2 - }}\) –H 2 O Quaternary Systems at 273 K . Russ. J. Inorg. Chem. 66, 374–384 (2021). https://doi.org/10.1134/S0036023621030141
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023621030141