Xanthan gum
Contents
|
Introduction
- The most commercially successful example of a microbial exopolysaccharide is xanthan gum, which is produced by Xanthomonas species, e.g. X. campestris, X. carotae, X. malvacearum and X. phaseoli.
- These bacteria are small, motile, aerobic Gram-negative rods that produce yellow pigments.
- Many are phytopathogens, including X. campestris, the species used for commercial production of xanthan, which causes diseases of cauliflower, cabbages and rutabagas.
- The original work on xanthan was carried out at the US Department of Agriculture Northern Regional Research Laboratory in the late 1950s and commercial production was started in 1961 by Kelco.
- Approval for food use was given by the FDA in 1969 and the polysaccharide now has GRAS status. In the EU, xanthan is classified as thickener E415.
Structure of Xanthan gum
- Xanthan is a high molecular weight helical heteropolymer of 1.0–2.0 X 106 Da, composed of D-glucose, d-mannose, d-glucuronic acid (in a molar ratio of 2 :2: 1, respectively).
- D-Glucose units are β-1,4- linked and form the backbone of the molecule, which is similar to cellulose.
- The polymer branches regularly as alternate glucose units of the backbone are linked to a trisaccharide side chain, consisting of α-D-mannose, β-D- glucuronic acid and another α-D-mannose in the terminal position.

- However, there may be variations in the substituents of these side chains, which can affect various properties of the polymer.
- Pyruvate may be present on the terminal mannose unit and the internal mannose may be O-acetylated. Commercial xanthans have a degree of substitution of 30–40% for pyruvate and 60–70% for acetate.
- The differences depend on the strain of X. campestris used for production and growth media composition.
- Gums with abbreviated side chains, formed by mutants of X. campestris, exhibit very different physical properties from those produced by the wild-type bacterium.
Industrial applications of xanthan gum
- Diverse industrial applications are based on the ability of xanthan gum to dissolve in hot or cold water and yield high viscosity, even at concentrations as low as 0.05% (w/v).
- Solutions of xanthan have higher viscosity than other gums at the same concentration.
- At a polymer concentration of 1% (w/v) in 1% (w/v) potassium chloride solution, the viscosity values for xanthan gum, guar gum, carboxymethyl cellulose and alginate are 11300, 4000, 410 and 210 mPa s, respectively.
- Additional key characteristics include:
- Translucence of xanthan solutions;
- Compatibility with acids, bases and salts;
- Sability at ambient temperature; and
- Pseudoplastic rheological behaviour, i.e. xanthan solutions regain viscosity after shearing.
- Xanthan also interacts synergistically with other polymers. For example, it can form thermoreversible gels in combination with galactomannans or glucomannans, whereas neither component will gel alone.
Non-Food applications
- Approximately 60% of the xanthan produced is used in non-food applications.
- These include use as a stabilizer for paint emulsions, a carrier for fertilizers and herbicides, a thickener for textile dyes, a drilling lubricant and for tertiary recovery in the oil industry, and in clay coatings for high-quality paper.
- Xanthan also aids the flow of pastes, e.g. facilitates toothpaste flow from containers, but recovers viscosity after the removal of the shear force.
Food applications
- Food applications involve roles as a thickener, an adhesive, a binder in films and coatings, an emulsifying agent and a stabilizer.
- Xanthan can also aid rapid flavour release and provides good ‘mouthfeel’ characteristics.
- Currently, xanthan has almost a quarter of the American market for food thickeners.
- However, widespread use of xanthan is somewhat restricted due to its relatively high cost of US$20–25/kg, when compared with starch or some synthetic polymers.
- Nevertheless, its price is similar to that of other gums with comparable functionalities.
The role of xanthan gum in food
| Function | Application |
| Adhesive | Icing and glazes |
| Binding agent | Pet foods |
| Coating | Confectionery |
| Emulsifying agent | Salad dressings |
| Encapsulation | Powdered flavours |
| Film formation | Protective coating, sausage casings |
| Foam stabilizer | Beer |
| Stabilizer | Ice cream, salad dressings |
| Swelling agent | Processed meat products |
| Syneresis inhibitor | Cheeses, frozen foods |
| Thickening agent | Jams, sauces, syrups and pie fillings |
| Synergistic xanthan–galactomannans mixtures Gel formation and gel stabilization |
Ice cream Cheese and cream cheese Dessert gels Milk shakes and milk drinks Puddings and pie fillings |
| Viscosity control | Ice cream Instant soups Chocolate drinks Milk shakes |
Xanthan gum Production
- Approximately 20 000 tonnes of xanthan are produced each year.
- The production is influenced by several factors, such as the type of reactor used, mode of operation, medium composition and operational conditions.
- Oxygen supply, normally 1 volume of air per reactor volume per minute (vvm), is particularly important, but its maintenance is not straight forward.
- As xanthan is synthesized during the fermentation, the viscosity of the medium increases, which impedes mixing and leads to reduced oxygen transfer rates.
- Fermenter design, agitation speed and the air flow rate are key factors.
- Fed-batch fermentation systems are mostly used, but xanthan can also be produced in continuous processes, often under nitrogen limitation with dilution rates of 0.025–0.05/h.
- This offers the advantage of high yields and lower operating costs.
- Standardization of physiological conditions also results in a more uniform product.
- However, continuous operations can suffer from aeration and microbial contamination problems.
- X. campestris can utilize several carbon sources, including starch, starch hydrolysates, corn syrup, molasses, glucose and sucrose.
- Other acceptable and cheaper substrates are whey, cereal grain hydrolysates and dry milled corn starch.
- The characteristics of the xanthan produced, particularly their molecular weight and rheological properties, are influenced by the composition of the substrates employed.
Production process of xanthan gum
- Initially, the bacterium is grown in a rich propagation medium to build up the inoculum within a pilotscale fermenter.
- This culture in then used to inoculate mechanically agitated industrial-scale fermenters of 50–200m3 capacity.
The production media normally contain:
- A carbon source, commonly d-glucose, sucrose, starch or hydrolysed starch at 30–40 g/L.
- A nitrogen source: casein or soya bean hydrolysate, ammonium salts, peptone, corn steep liquor, yeast extract or urea. The best product yields are attained with a carbon:nitrogen ratio of about 10 :1.
- MgCl2 and other trace salts.
- K2HPO4 as a buffer.
In fed-batch mode
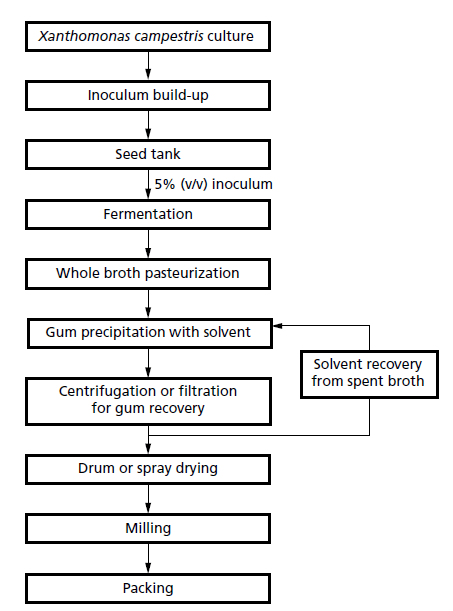
- In fed-batch mode, the fermentation is usually maintained at 28–30°C and pH 7.0; if the pH is allowed to fall gum production decreases rapidly.
- The bacterium starts producing xanthan during the exponential phase at rates in relation to the growth rate and production continues into the stationary phase.
- These fermentations are normally completed within 3 days.
- A final concentration of 25 g/L is the minimum normally required for the process to be economically viable, but most industrial fermentations achieve up to 50 g/L.
- At the end of the fermentation, the broth is heated to 100–110°C for 10 min to kill the bacteria and improve the rheological properties of the xanthan.
- This is followed by a series of purification steps that are defined by the final use of the polymer.
- For some applications it is necessary to remove cells by filtration or centrifugation.
- Xanthan is then precipitated with ethanol, or isopropanol (especially when preparing food-grade product), and then separated by centrifugation.
- More than 50% of the production costs are incurred by these downstream processing steps and it is essential that the solvent is recovered.
- The product is dewatered, drum- or spray-dried, milled, sieved and finally packaged as a dispersible granulated powder.
Reference and sources
- https://www.sciencedirect.com/topics/engineering/inoculum-development
- https://www.researchgate.net/publication/225453233_Solutions_of_xanthan_gumguar_gum_mixtures_Shear_rheology_porous_media_flow_and_solids_transport_in_annular_flow
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2665694/
- https://www.frontiersin.org/articles/10.3389/fmars.2020.567126/full
- https://www.researchgate.net/publication/223678634_Xylitol_production_in_a_bubble_column_bioreactor_Influence_of_the_aeration_rate_and_immobilized_system_concentration
- https://www.researchgate.net/publication/241765470_Reactors_in_Process_Engineering
- https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/konjac-mannan
Also Read:
- Gel electrophoresis: types, principles, instrumentation and applications
- Electrophoresis: Overview, Principles and Types
- Proteomics: Introduction, Methods, Types and Application
- Nitrogen Cycle
- Vector: properties, types and characteristics
- DNA Replication in eukaryotes: Initiation, Elongation and Termination
- Spectroscopy: Introduction, Principles, Types and Applications
- Transcription in prokaryotes: Initiation, Elongation and Termination
- Measurements of microbial growth
- Whole-Genome Shotgun Sequencing: overview, steps and achievements
- Plasmid: Properties, Types, Replication and Organization
- Milk: Composition, Processing, Pasteurization, Pathogens and Spoilage
