Planktonic associations between medusae (classes Scyphozoa and Hydrozoa) and epifaunal crustaceans
- Published
- Accepted
- Received
- Academic Editor
- Antonina Dos Santos
- Subject Areas
- Ecology, Marine Biology, Zoology
- Keywords
- Hydrozoa, Scyphozoa, Crustacea, Association, Commensal, Epifauna, Marine, Jellyfish, Medusa
- Copyright
- © 2021 Muffett and Miglietta
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. Planktonic associations between medusae (classes Scyphozoa and Hydrozoa) and epifaunal crustaceans. PeerJ 9:e11281 https://doi.org/10.7717/peerj.11281
Abstract
Jellyfish are known to carry various epibionts, including many of the subphylum Crustacea. However, the associations between gelatinous zooplankton and other invertebrates have been chronically overlooked. Crustacea, a massive clade of economically, ecologically, and culturally important species, includes many taxa that utilize gelatinous zooplankton for food, transport, and protection as both adults and juveniles. Here we compile 211 instances of epifaunal crustaceans recorded on Hydromedusae and Scyphomedusae from a century of literature. These include 78 identified crustacean species in 65 genera across nine orders found upon 37 Hydromedusa species and 48 Scyphomedusae. The crustacean life stage, location, nature of the association with the medusa, years, months, and depths are compiled to form a comprehensive view of the current state of the literature. Additionally, this review highlights areas where the current literature is lacking, particularly noting our poor understanding of the relationships between juvenile crabs of commercially valuable species and medusae.
Background
An increased focus on ocean climate research in the past 20 years has made clear the fragility of the world’s oceans and the organisms that live within them. The rate at which species are disappearing, undergoing climate-related range fluctuations, and experiencing developmental and behavioral changes is unlike anything seen in the time of record (Walther et al., 2002; Guinotte & Fabry, 2008; Comeaux, Allison & Bianchi, 2012). Attempts to model changes in populations, species, and ecosystems have laid bare the degree to which dynamics among many marine invertebrates remain unknown and poorly understood (Uye, 2008; Brodeur, Ruzicka & Steele, 2011; Henschke et al., 2014). This problem is especially apparent in jellyfish of the phylum Cnidaria, which are chronically understudied and poorly categorized (Riascos et al., 2013; Gambill & Peck, 2014; Sweetman et al., 2016; Gómez Daglio & Dawson, 2017). Long considered a pure pest, the last decade has demonstrated an increasing number of ways in which jellyfish are critical components of the ecosystems they reside in (Cardona et al., 2012; Hays, Doyle & Houghton, 2018). While they are best known for the vertebrates that depend on them for nutrition, including turtles and birds, they provide a host of ecosystem services unrelated to a “prey” designation. Reef and non-reef fish juveniles readily congregate around large scyphozoans, some hiding within the bell or between tentacles when disturbed (Brodeur, 1998; D’Ambra et al., 2014; Tilves et al., 2018). Large jellyfish can reach sizes that allow them to support independent encrusting organisms, like barnacles and brittle stars (Ohtsuka et al., 2010; Álvarez-Tello, López-Martínez & Rodríguez-Romero, 2013; Yusa et al., 2015).
While research has expanded around services jellyfish provide (Riascos et al., 2018), much of this research focuses on benefit and harm to vertebrates (Brodeur, 1998; Cardona et al., 2012; Mir-Arguimbau, Sabatés & Tilves, 2019). However, the relationships between scyphomedusae, hydromedusae and other invertebrates are currently poorly characterized. A prime invertebrate group to analyze through this lens is Crustacea. Crustaceans are some of the most visible and well-studied marine invertebrates. They are present in every region and are integral components of food webs, including species of high commercial value and known ecological significance (Boudreau & Worm, 2012). Ecological processes that impact them are thus relevant to humans. However, studies focusing on epifaunal crustaceans and jellyfish interactions have been scarce, incomplete, and taxonomically imprecise. Moreover, such studies are often narrowly focused accounts of interactions with single individuals (Weymouth, 1910; Reddiah, 1968; Yusa et al., 2015). Some early communications discuss these interactions as common knowledge that has, however, failed to be recorded in the scientific literature (Jachowski, 1963). This review provides a list of documented crustacean epibionts on medusae of the orders Scyphozoa and Hydrozoa. This work aims to assess the breadth and depth of jellyfish-crustacean interaction and develop a resource for further studies.
Methodology
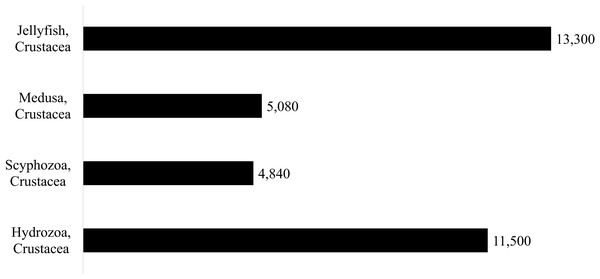
Four independent sets of searches were conducted in Google Scholar using keywords, as described in Fig. 1. All four searches were conducted in early November 2019 and were revisited in January 2021 to include all results through the end of 2019. Searches were performed in English, and as such, only papers published in or with an available translation to English were included. The number of papers yielded by each of the four searches is shown in Fig. 1, ranges from 4,840 articles (for keywords Crustacea, Scyphozoa) to 13,300 (for keywords Crustacea, Jellyfish) (See Fig. 1 for details). Only papers in which the primary focus was associations between medusae (Hydrozoa and Scyphozoa) and crustaceans were further selected.
Figure 1: Summary of Google Search Results.
The number of results reported by Google Scholar Advanced Search where both “Crustacea” and one of the four medusa describer terms was included (“Hydrozoa”, “Scyphozoa”, “medusa”, or “jellyfish”) and at least one of the following terms was included (Association, Associated, Symbiotic, Symbiosis, Commensal, Epifaunal, Harboring, Parasitic, Parasitoid, Epibiont or Epibiotic).The four searches performed returned many invariable results. All titles and abstracts were checked for relevance. Results from 161 papers were obtained initially and then narrowed to 81, after excluding repeat papers mistakenly included multiple times and papers on cubomedusae, ctenophores, ascidians, and non-crustacean epibionts. Also, results from six relevant literature reviews were included (Vader, 1972; Pagès, 2000; Towanda & Thuesen, 2006; Ohtsuka et al., 2011; Schiariti et al., 2012; Wakabayashi, Tanaka & Phillips, 2019). These reviews account for 40 interactions from 29 sources (Table 1). The inclusion of the literature reviews was deemed essential to include results from earlier sources and non-English sources not available on Google Scholar. Results from literature reviews that had no information on the nature of the interaction between the medusa and crustaceans (such as taxa identification, location, etc.) were eliminated. Records were also analyzed for taxon validity using the World Register of Marine Species (WoRMS). Seven papers within the database that referred to invalid taxa with no valid synonymized name in WoRMS were removed. Results from 97 unique sources (68 articles from the Google Scholar search and 29 from literature reviews) were kept. From these 97 sources, 211 distinct interactions were extracted. Details provided by each paper were recorded in Table 1.
| Host Species | Epibiont | Notes | Life Stage and Sex | Location on Medusa | Location | Collection | Limited | Month/Year | Depth | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Scyphozoa | ||||||||||
| Coronatae | ||||||||||
| Nausithoe rubra Vanhöffen, 1902 | Prohyperia shihi Gasca, 2005 | Not visibly parasitizing host, female and male pair | F, M | EX | Gulf of California | ROV | L | 2012 Feb | 907 m | Gasca, 2013 |
| Rhizostomeae | ||||||||||
| Acromitoides purpurus Mayer, 1910 | Charybdis feriata Linnaeus, 1758 | Never more than one per medusa | ? | ? | Various bays, Philippines | HC | N | 2014–2015, Feb–Apr | NS | Boco & Metillo, 2018 |
| Acromitoides purpurus Mayer, 1910 | Paramacrochiron sp. | Present 44–100% of medusae depending on location and medusa color morph | ? | ? | Various bays, Philippines | HC | N | 2014–2015, Feb–Apr | NS | Boco & Metillo, 2018 |
| Acromitus flagellatus Maas, 1903 | Latreutes anoplonyx Kemp, 1914 | N/A | ? | ? | Indonesia | ? | ? | ? | ? | Hayashi, Sakagami & Toyoda, 2004 |
| Acromitus sp. | Hourstonius pusilla K.H. Barnard, 1916 | Present throughout the adult medusa population | ? | SUM, O | Chilka Lake, India | ? | L | ? | ? | Chilton, 1921 via Vader, 1972 |
| Cassiopea sp. | Ancylomenes aqabai Bruce, 2008 | N/A | OF & F | O | Aqaba, Jordan | HC | L | 1976 Mar | NS | Bruce, 2008 |
| Cassiopea sp. | Ancylomenes holthuisi Bruce, 1969 | N/A | ? | O | Zanzibar harbour | SC | L | 1970 Dec | 20-25 m | Bruce, 1972 |
| Cassiopea sp. | Periclimenes pedersoni Chace, 1958 | N/A | OF & M | O | Santa Marta, Colombia | ? | N | ? | 3-40 m | Criales, 1984 |
| Cassiopea sp. | Periclimenes tonga Bruce, 1988 | N/A | OF | ? | Nuapapu Island (southside), Vava’u Group, Tonga | ? | L | 1985 Jul | ? | Bruce, 1988 |
| Cassiopea sp. | Periclimenes yucatanicus Ives, 1891 | N/A | OF & jM & F | O | Santa Marta, Colombia | ? | N | ? | 3–25 m | Criales, 1984 |
| Cassiopea sp. | Sewellochiron fidens Humes, 1969 | N/A | F, M | ? | Puerto Rico | ? | ? | 1959 | 3 m | Humes, 1969 |
| Catostylus mosaicus Quoy & Gaimard, 1824 | Acartia sp. | N/A | C & A | O | Botany Bay, Lake Illawarra, Smiths Lake, New South Wales | HC | N | 1999–2000 | NS | Browne & Kingsford, 2005 |
| Catostylus mosaicus Quoy & Gaimard, 1824 | Cymodoce gaimardii H. Milne Edwards, 1840 | Autumnal prevalence peak | ? | O, SUM, EX | Port Phillip Bay,Victoria | HC | N | 2009 Aug–2010 Sep | NS | Browne, 2015 |
| Catostylus mosaicus Quoy & Gaimard, 1824 | Cymodoce gaimardii H. Milne Edwards, 1840 | Highest prevalence in Mar | A & J | B, O | Port Phillip Bay,Victoria | HC | N | 2008 Aug– 2010 Sep | NS | Browne, Pitt & Norman, 2017 |
| Catostylus mosaicus Quoy & Gaimard, 1824 | Evadne sp. | Only one specimen | ? | O | Botany Bay, New South Wales | HC | L | 1999-2000 | NS | Browne & Kingsford, 2005 |
| Catostylus mosaicus Quoy & Gaimard, 1824 | Hyperia gaudichaudii H. Milne Edwards, 1840 | September prevalence peak, Es and Js embedded in host tissue | E & J & A | GVC, B | Port Phillip Bay,Victoria | HC | N | 2008 Aug– 2010 Sep | NS | Browne, 2015 |
| Catostylus mosaicus Quoy & Gaimard, 1824 | Ibacus sp. | A single specimen from Sydney museum collection | PL | SUB | Hawkesbury River, New South Wales | ? | L | 1925 | ? | Thomas, 1963 |
| Catostylus mosaicus Quoy & Gaimard, 1824 | Latreutes anoplonyx Kemp, 1914 | Found on medusa type specimen from Pakistan | OF & J | O | Korangi Creek, Pakistan | HC | L | 1995 | NS | Tahera & Kazmi, 2006 |
| Catostylus mosaicus Quoy & Gaimard, 1824 | Lucifer sp. | N/A | ? | O | Botany Bay, Lake Illawarra, New South Wales | HC | L | 1999–2000 | NS | Browne & Kingsford, 2005 |
| Catostylus mosaicus Quoy & Gaimard, 1824 | Oithona sp. | Only present on two medusae in one lake | ? | O | Lake Illawarra, New South Wales | HC | L | 1999–2000 | NS | Browne & Kingsford, 2005 |
| Catostylus mosaicus Quoy & Gaimard, 1824 | Oncaea sp. | N/A | ? | O | Botany Bay, Smiths Lake, New South Wales | HC | L | 1999–2000 | NS | Browne & Kingsford, 2005 |
| Catostylus mosaicus Quoy & Gaimard, 1824 | Oncaea venusta Philippi, 1843 | N/A | ? | O | Botany Bay, Lake Illawarra, New South Wales | HC | L | 1999–2000 | NS | Browne & Kingsford, 2005 |
| Catostylus mosaicus Quoy & Gaimard, 1824 | Paramacrochiron maximum Thompson I.C. & Scott A., 1903 | Present in hundreds per medusa at all phases of development and size class | A & J & OF | O | Botany Bay, Lake Illawarra, New South Wales | HC | N | 1999–2000 | NS | Browne & Kingsford, 2005 |
| Catostylus mosaicus Quoy & Gaimard, 1824 | Pseudodiaptomus sp. | N/A | A | O | Botany Bay, Lake Illawarra, New South Wales | HC | N | 1999–2000 | NS | Browne & Kingsford, 2005 |
| Catostylus mosaicus Quoy & Gaimard, 1824 | Temora sp. | N/A | A | O | Botany Bay, Lake Illawarra, Smiths Lake, New South Wales | HC | N | 1999–2000 | NS | Browne & Kingsford, 2005 |
| Catostylus mosaicus Quoy & Gaimard, 1824 | Tortanus barbatus Brady, 1883 | N/A | C & A | O | Botany Bay, Lake Illawarra, New South Wales | HC | N | 1999–2000 | NS | Browne & Kingsford, 2005 |
| Catostylus sp. | Charybdis feriata Linnaeus, 1758 | Present from Apr–May | ? | O, SUM | Kolambugan, Lanao del Norte | ? | N | 2013 Dec– 2014 Jul | NS | Boco, Metillo & Papa, 2014 |
| Catostylus sp. | Paramacrochiron sp. | Present from Jan–Mar | ? | O, SUM | Kolambugan, Lanao del Norte | HC | N | 2013 Dec– 2015 Jul | NS | Boco, Metillo & Papa, 2014 |
| Cephea cephea Forskål, 1775 | Alepas pacifica Pilsbry, 1907 | Barnacles 44 mm wide present on umbrella and oral arms. Additional details absent | ? | B, O | Japanese Coast | ? | ? | ? | ? | Hiro, 1937 via Pagès, 2000 |
| Lobonema sp. | Callinectes sp. | Instar 1 cm | MG, I | ? | Gulf of Tehuantepec | ? | ? | ? | ? | Bieri unpubl. data via Towanda & Thuesen, 2006 |
| Lobonemoides robustus Stiasny, 1920 | Charybdis feriata Linnaeus, 1758 | Present in Gulf of Thailand from July to October as well | MG, J | ? | Carigara Bay, Leyte Island | HC | L | 2013 23 August | NS | Kondo et al., 2014 |
| Lychnorhiza lucerna Haeckel, 1880 | Cyrtograpsus affinis Dana, 1851 | N/A | A | SG | Rio de la Plata Estuary | TR | N | 2006 Mar | ? | Schiariti et al., 2012 |
| Lychnorhiza lucerna Haeckel, 1880 | Grapsoidea gn sp. | N/A | J | ? | Cananéia, Brazil | TR | L | 2013 Feb-2014 May | 5–15m | Gonçalves et al., 2016 |
| Lychnorhiza lucerna Haeckel, 1880 | Leander paulensis Ortmann, 1897 | N/A | M | ? | Cananéia, Brazil | TR | L | 2013-2014 | 5–15m | Gonçalves et al., 2016 |
| Lychnorhiza lucerna Haeckel, 1880 | Libinia dubia de Brito Capello, 1871 | 40% of individuals were living on medusae, all juveniles were living on medusae | M, F, OF, J | O, SUB, B | Cananéia, Brazil | TR | N | 2012 Jul | 5–15 m | Gonçalves et al., 2017 |
| Lychnorhiza lucerna Haeckel, 1880 | Libinia ferreirae de Brito Capello, 1871 | N/A | F, M, J | ? | Cananéia and Rio de Janeiro state, Macaé | TR | N | 2013–2014 | 5–15m | Gonçalves et al., 2016 |
| Lychnorhiza lucerna Haeckel, 1880 | Libinia ferreirae de Brito Capello, 1871 | N/A | ? | SUM, O | Maranhão state | HC | N | 2005–2006 Mar | ? | de Andrade Santos, Feres & Lopes, 2008 |
| Lychnorhiza lucerna Haeckel, 1880 | Libinia ferreirae de Brito Capello, 1871 | Young crabs, transport and protection | J, F, M | SG, O | State of Paraná | TR | N | 1997–2004 All yr | 8–30 m | Nogueira Júnior & Haddad, 2005 |
| Lychnorhiza lucerna Haeckel, 1880 | Libinia spinosa Guérin, 1832 | N/A | F | ? | Ubatuba | TR | N | 2013 Jul–2014 Aug | 5–15m | Gonçalves et al., 2016 |
| Lychnorhiza lucerna Haeckel, 1880 | Libinia spinosa Guérin, 1832 | Dispersion, protection and food particulate theft | ? | ? | Rio del Plata | MULTI | N | 2007 Jan-Mar | ? | Schiariti et al., 2012 |
| Lychnorhiza lucerna Haeckel, 1880 | Libinia spinosa Guérin, 1832 | Dispersion and food particulate theft, Jan-Feb | ? | ? | Punta del Este | ? | ? | Jan-Feb | ? | Vaz-Ferreira, 1972 via Schiariti et al., 2012 |
| Lychnorhiza lucerna Haeckel, 1880 | Libinia spinosa Guérin, 1832 | Transportation and food theft, no more than two crabs/medusa | ? | SG | Mar Chiquita Estuary | ? | L | ? | NS | Zamponi, 2002 via Schiariti et al., 2012 |
| Lychnorhiza lucerna Haeckel, 1880 | Periclimenes paivai Chace, 1969 | 72% of collected medusae had associate | MG, F, OF, J | SUM | Paraíba River estuary | HC | N | 2016 Apr | NS | Baeza et al., 2017 |
| Lychnorhiza lucerna Haeckel, 1880 | Periclimenes paivai Chace, 1969 | N/A | OF | SUM | Sao Paolo | TR | 2012 Sep–Oct | 5–15m | de Moraes et al., 2017 | |
| Lychnorhiza lucerna Haeckel, 1880 | Periclimenes paivai Chace, 1969 | N/A | OF, M | ? | Cananéia | TR | N | 2013–2014 | 5–15m | Gonçalves et al., 2016 |
| Lychnorhiza lucerna Haeckel, 1880 | Periclimenes sp. | Facultative commensal, feeding on mucus, large proportion ovigerous females | OF, A, J | SUM | São Paulo state | HC | N | 1999–2002, 2005 Aug + 2006 Jul | NS | Filho et al., 2008 |
| Lychnorhiza lucerna Haeckel, 1880 | Synidotea marplatensis Giambiagi, 1922 | N/A | ? | SG, O, B | Guaratuba, Paraná e Barra do Saí, Santa Catarina | TR | L | 2003–2004 Aug–Dec | 8–14 m | Nogueira Junior & Silva (2005) |
| Lychnorhiza malayensis Stiasny, 1920 | Paramacrochiron sewelli Reddiah, 1968 | 100 + epibionts from 5 hosts | F, M | ? | Ennore estuary near Madras | HC | L | 1964 Apr | ? | Reddiah, 1968 |
| Mastigias papua Lesson, 1830 | Chlorotocella gracilis Balss, 1914 | Collected from ten medusae | M, F, OF | O | Tanabe Bay, Japan | ? | N | 1965 Oct | ? | Hayashi & Miyake, 1968 |
| Mastigias papua Lesson, 1830 | Latreutes anoplonyx Kemp, 1914 | Collected from ten medusae | M, F, OF | O | Tanabe Bay, Japan | ? | N | 1965 Oct | ? | Hayashi & Miyake, 1968 |
| Mastigias papua Lesson, 1830 | Latreutes mucronatus Stimpson, 1860 | Collected from ten medusae | M, F, OF | O | Tanabe Bay, Japan | ? | N | 1965 Oct | ? | Hayashi & Miyake, 1968 |
| Nemopilema nomurai Kishinouye, 1922 | Alepas pacifica Pilsbry, 1907 | Substrate | M, F, OF | B | Western Coast of Japan | HC | N | 2005–2009 | ? | Yusa et al., 2015 |
| Nemopilema nomurai Kishinouye, 1922 | Charybdis feriata Linnaeus, 1758 | 5 juveniles present on one host on the oral arms, one adult present under the bell of a second medusa. | J & M | O, SUM | Mirs Bay, Hong Kong | ? | L | 1970 Oct | ? | Trott, 1972 |
| Nemopilema nomurai Kishinouye, 1922Netrostoma setouchianum Kishinouye, 1902 | Latreutes anoplonyx Kemp, 1914 | Exhibits hiding behavior | M, F, OF | O, SUB | Miyazu and Sanriku, Japan | OBS. HC, SC | L | 2003 Nov | ? | Hayashi, Sakagami & Toyoda, 2004 |
| Netrostoma setouchianum Kishinouye, 1902 | Chlorotocella gracilis Balss, 1914 | Single specimen | ? | O | Seto Inland Sea, Japan | HC | L | 2010 Sep | NS | Ohtsuka et al., 2011 |
| Netrostoma setouchianum Kishinouye, 1902 | Latreutes mucronatus Stimpson, 1860 | Mix of sexes and ages of epibiont from two host individuals, 7 on one and 54 epibionts on the other | M, F, OF, J | O | Seto Inland Sea, Japan | HC | L | 2010 Sep | NS | Ohtsuka et al., 2011 |
| Phyllorhiza punctata von Lendenfeld, 1884 | Charybdis feriata Linnaeus, 1758 | Single specimen from August 2014 | MG | ? | Various bays, Philippines | HC | L | 2014–2015, Feb–Apr | NS | Boco & Metillo, 2018 |
| Phyllorhiza punctata von Lendenfeld, 1884 | Latreutes anoplonyx Kemp, 1914 | N/A | OF, A | B | NT Australia | HC | L | 1993 | NS | Bruce, 1995 |
| Phyllorhiza punctata von Lendenfeld, 1884 | Libinia ferreirae de Brito Capello, 1871 | Feb–Jul | ?? | SUM | Sao Paulo | ? | ? | Feb-Jul | ? | Moreira, 1961 via Schiariti et al., 2012 |
| Phyllorhiza punctata von Lendenfeld, 1884 | Paramacrochiron sp. | Two specimens from Leyte Gulf- Guiuan in April 2015 | ? | ? | Various bays, Philippines | HC | L | 2014–2015, Feb–Apr | NS | Boco & Metillo, 2018 |
| Pseudorhiza haeckeli Haacke, 1884 | Cymodoce gaimardii H. Milne Edwards, 1840 | N/A | ? | ? | Port Phillip Bay,Victoria | HC | N | 2011 Sep + 2012 Feb | NS | Browne, 2015 |
| Pseudorhiza haeckeli Haacke, 1884 | Hyperia gaudichaudii H. Milne Edwards, 1840 | Exhibit cradle positioning for filter feeding | ? | EX | Port Phillip Bay,Victoria | HC | N | 2009 Sep + 2012 Feb | NS | Browne, 2015 |
| Pseudorhiza haeckeli Haacke, 1884 | Themisto australis Stebbing, 1888 | N/A | ? | ? | Port Phillip Bay,Victoria | HC | N | 2010 Sep + 2012 Feb | NS | Browne, 2015 |
| Rhizostoma pulmo Macri, 1778 | Hyperia galba Montagu, 1813 | Peak in Oct, preference for mature medusae, consume host gonad | J, A | O | German Bight | HC + SC | ? | 1984–1985 | ? | Dittrich, 1988 |
| Rhizostoma pulmo Macri, 1778 | Iphimedia eblanae Spence Bate, 1857 | Present in the brachial cavities, mouthpart shape leads to speculation that these are semi-parasitic short-term associates | ? | GVC | Dublin Bay, Ireland | ? | N | NS | Bate, 1862 via Vader, 1972 | |
| Rhizostoma sp. | Latreutes anoplonyx Kemp, 1914 | N/A | ? | ? | Indonesia | ? | ? | ? | ? | Hayashi, Sakagami & Toyoda, 2004 |
| Rhizostoma sp. | Paramacrochiron rhizostomae Reddiah, 1968 | N/A | F, M, J | ? | Vaalai Island, Madras State | HC | L | 1967 Mar | NS | Reddiah, 1968 |
| Rhizostomatidae gn. sp. | Alepas pacifica Pilsbry, 1907 | 2 barnacles on the umbrellar margin up to 68 mm in length | ? | MA | Morrison Bay, Mergui Arch | ? | L | 1914 | NS | Annandale, 1914 via Pagès, 2000 |
| Rhopilema esculentum Kishinouye, 1891 | Charybdis feriata Linnaeus, 1758 | Juvenile transport | J | O | Sagami Bay | ? | ? | October | ? | Suzuki, 1965 via Pagès, 2000 |
| Rhopilema esculentum Kishinouye, 1891 | Latreutes anoplonyx Kemp, 1914 | N/A | ? | ? | Northeast China | ? | ? | ? | ? | Hayashi, Sakagami & Toyoda, 2004 |
| Rhopilema hispidum Vanhöffen, 1888 | Charybdis annulata Fabricius, 1798 | N/A | ?? | SUM | Palk Bay, Sri Lanka | ? | L | 1950 Jul | ? | Panikkar & Raghu Prasad, 1952 via Towanda & Thuesen, 2006 |
| Rhopilema hispidum Vanhöffen, 1888 | Charybdis feriata Linnaeus, 1758 | Present on all medusae collected in Aug | J & MG | ? | Panguil Bay | HC | N | 2014 Feb+Aug | NS | Boco & Metillo, 2018 |
| Rhopilema hispidum Vanhöffen, 1888 | Hippolytidae gn sp. | Three associates on a single medusa from Feb | ? | ? | Panguil Bay | HC | L | 2014 Feb+Aug | NS | Boco & Metillo, 2018 |
| Rhopilema hispidum Vanhöffen, 1888 | Latreutes sp. aff. anoplonyx Kemp, 1914 | N/A | ?? | MA, O | Kukup, Malaysia | ? | L | 2009 Mar + Oct | ? | Ohtsuka et al., 2010 |
| Rhopilema hispidum Vanhöffen, 1888 | Latreutes sp. aff. anoplonyx Kemp, 1914 | N/A | ?? | ? | Sichang Island, Thailand | ? | L | 2009 Oct | ? | Ohtsuka et al., 2010 |
| Rhopilema hispidum Vanhöffen, 1888 | Paramacrochiron sp. | On 67% of medusae from Aug collection | ? | ? | Panguil Bay | HC | L | 2014 Feb+Aug | NS | Boco & Metillo, 2018 |
| Rhopilema hispidum Vanhöffen, 1888 | Paramacrochiron sp. | Theorized ectoparasite, no record of actual consumption. | A & L | O | Laem Phak Bia, Thailand | HC | L | 2010 Oct | NS | Ohtsuka, Boxshall & Srinui, 2012 |
| Rhopilema nomadica Galil, Spanier & Ferguson, 1990 | Charybdis feriata Linnaeus, 1758 | Many hosts containing multipe associations, only some possess Charybdis, never more than one crab per medusa. | ? | O, SUB | Delagoa Bight, Mozambique | HC | L | 1988 Mar + 1992 Mar | NS | Berggren, 1994 |
| Rhopilema nomadica Galil, Spanier & Ferguson, 1990 | Periclimenes nomadophila Berggren, 1994 | Many hosts containing multipe associations | F, OF, M | O, SUB | Delagoa Bight, Mozambique | HC | N | 1988 Mar + 1992 Mar | NS | Berggren, 1994 |
| Rhopilema sp. | Conchoderma virgatum Spengler, 1789 | 22 barnacles on the umbrellar Margin (ex and sub) on host of 320 mm diameter | ? | MA | Tranquebar, Bengala Gulf | ? | L | ? | ? | Fernando & Ramamoorthi, 1974 via Pagès, 2000 |
| Stomolophus meleagris, Agassiz, 1860 | Charybdis feriata Linnaeus, 1758 | N/A | F & J | O | Hong Kong | ? | ? | ? | ? | Morton, 1989 via Towanda & Thuesen, 2006 |
| Stomolophus meleagris, Agassiz, 1860 | Conchoderma cf virgatum Spengler, 1789 | Mature jellyfish, scarring and lesions around attachment site | ? | B | Gulf of California | HC | L | 2010 Apr | NS | Álvarez-Tello, López-Martínez & Rodríguez-Romero, 2013 |
| Stomolophus meleagris, Agassiz, 1860 | Libinia dubia H. Milne Edwards, 1834 | All medusa harbored crabs, no more than one crab per medusa | A | SUM | Murrell’s Inlet, SC | ? | N | 1927 May | “relatively deep” | Corrington, 1927 |
| Stomolophus meleagris, Agassiz, 1860 | Libinia dubia H. Milne Edwards, 1834 | N/A | ? | SUM | Beaufort, NC | TR | N | 1927 Jul–Oct | NS | Gutsell, 1928 |
| Stomolophus meleagris, Agassiz, 1860 | Libinia dubia H. Milne Edwards, 1834 | Juvenile associations, parasitic, transient | J | W | Mississippi sound | HC | N | 1968 Jul–Oct | NS | Phillips, Burke & Keener, 1969 |
| Stomolophus meleagris, Agassiz, 1860 | Libinia dubia H. Milne Edwards, 1834 | Highly variable seasonally, high in July, low in Dec | F, M, J | O, MA | Wrightsville Beach Jetty NC | HC | N | 1983 May–Dec | NS | Rountree, 1983 |
| Stomolophus meleagris, Agassiz, 1860 | Libinia dubia H. Milne Edwards, 1834 | Feeding | ? | EXC | Onslow Bay, NC | SC | ? | ?? | ? | Shanks & Graham, 1988 via Schiariti et al., 2012 |
| Stomolophus meleagris, Agassiz, 1860 | Libinia dubia H. Milne Edwards, 1834 | N/A | ? | ? | Indian River Lagoon, Florida | HC | ? | 2003 Mar | ? | Tunberg & Reed, 2004 |
| Stomolophus meleagris, Agassiz, 1860 | Penaeus stylirostris Stimpson, 1871 | N/A | ? | ? | Malaga Bay, Colombia | HC | ? | 2015 Nov + 2017 Apr | NS | Riascos et al., 2018 |
| Thysanostoma thysanura Haeckel, 1880 | Paramacrochiron sp. | N/A | ? | ? | Sirahama | ? | ? | 1969 | ? | Humes, 1970 |
| Versuriga anadyomene Maas, 1903 | Charybdis feriata Linnaeus, 1758 | Large medusae | ? | ? | Leyte Gulf- Guiuan | HC | L | 2014–2015, Feb–Apr | NS | Boco & Metillo, 2018 |
| Versuriga anadyomene Maas, 1903 | Charybdis feriata Linnaeus, 1758 | N/A | ?? | SUM | Pari Island, Indonesia | ? | L | 2009 Nov | ? | Ohtsuka, Boxshall & Srinui, 2012 |
| Versuriga anadyomene Maas, 1903 | Latreutes anoplonyx Kemp, 1914 | N/A | A & J | SUM | NT Australia | HC | L | 1993 | NS | Bruce, 1995 |
| Versuriga anadyomene Maas, 1903 | Paramacrochiron sp. | Large medusae | ? | ? | Leyte Gulf- Guiuan | HC | N | 2014–2015, Feb–Apr | NS | Boco & Metillo, 2018 |
| Semaeostomeae | ||||||||||
| Aurelia aurita Linnaeus, 1758 | Hyperia galba Montagu, 1813 | N/A | A & J & OF | ? | Narragansett Marine Laboratory | HC | ? | 1955 June | NS | Bowman, Meyers & Hicks, 1963 |
| Aurelia aurita Linnaeus, 1758 | Hyperia galba Montagu, 1813 | Preference for mature medusae, infestation increases as gonads develop, peak in Oct, consume host gonad | J, A | O | German Bight | HC + SC | 1984–1985 | ? | Dittrich, 1988 | |
| Aurelia aurita Linnaeus, 1758 | Libinia dubia H. Milne Edwards, 1834 | Eating medusa tissue, residence within bell, excavation behaviors 19.9% of medusae examined 300-500 m from shore had phyllosoma, none on Aurelia near shore, likely parasitoid. | ? | EXC | Chesapeake Bay | ? | ? | 1963 Aug | ? | Jachowski, 1963 |
| Aurelia aurita Linnaeus, 1758 | Scyllarus sp. | Riding small medusae, pierced exumbrella with pereiopods | PL | EX | Bimini, Bahamas | HC | N | 1973 Oct | NS | Herrnkind, Halusky & Kanciruk, 1976 |
| Aurelia coerulea von Lendenfeld, 1884 | Ibacus ciliatus von Siebold, 1824 | February to May, 97.6% female, largely one female per host, occasionally M/F pair, 1/3 of parasites were ovigerous. | PL | EX | Yamaguchi, Japan | OBS | L | ? | ? | Wakabayashi, Tanaka & Abe, 2017 via Wakabayashi, Tanaka & Phillips, 2019 |
| Aurelia coerulea von Lendenfeld, 1884 | Oxycephalus clausi Bovallius, 1887 | No breakdown by specific host | OF, F | EX | Nagato, Yamaguchi, Japan | OBS | N | 2012-2018 | 0–5 m | Mazda et al., 2019 |
| Aurelia limbata Brandt, 1835 | Hyperia galba Montagu, 1813 | N/A | F, J | O | Okirai Bay | ? | L | 2009 Apr | ? | Ohtsuka et al., 2010 |
| Aurelia sp. | Nitokra medusaea Humes, 1953 | Engage in excavation, many epibionts on a single 5′ medusa | F, M, OF | EXC | New Hampshire coast | HC | L | 1952 | NS | Humes, 1953 |
| Chrysaora colorata Russell, 1964 | Latreutes anoplonyx Kemp, 1914 | N/A | ? | ? | Kuwait Bay | TR | ? | 1981 Sept–1982 Aug | ? | Grabe & Lees, 1995 |
| Chrysaora colorata Russell, 1964 | Metacarcinus gracilis Dana, 1852 | Dispersion, protection and feeding, Mar–Aug | MG | ? | Monterey Bay | ? | ? | 1991/1992 Mar–Aug | ? | Graham, 1989 via Schiariti et al., 2012 |
| Chrysaora colorata Russell, 1964 | Metacarcinus gracilis Dana, 1852 | Early stages of crabs on medusae | J, MG | ? | Califorina | ? | ? | ? | ? | Wrobel & Mills, 1998 via Schiariti et al., 2012 |
| Chrysaora fuscescens Brandt, 1835 | Cancer sp. | Crabs gain dispersion | ? | ? | Monterey Bay | ? | ? | ? | ? | Graham, 1994 via Schiariti et al., 2012 |
| Chrysaora fuscescens Brandt, 1835 | Hyperoche medusarum Kröyer, 1838 | Infestations occur in late summer | ? | ? | NE Pacific, Oregon and northern California | ? | ? | ? | ? | Larson, 1990 |
| Chrysaora fuscescens Brandt, 1835 | Metacarcinus gracilis Dana, 1852 | N/A | ? | ? | NE Pacific “off California” | ? | ? | ? | ? | Larson, 1990 |
| Chrysaora hysoscella Linnaeus, 1767 | Hyperia galba Montagu, 1813 | Peak in Oct, reference for mature medusae, consume host gonad | J, A | O | German Bight | HC + SC | 1984–1985 | ? | Dittrich, 1988 | |
| Chrysaora lactea Eschscholtz, 1829 | Brachyscelus cf. rapacoides Stephensen, 1925 | Parasite | L, J | W, O | Sao Sebastian Channel | TR | L | 2015 Nov | ? | Puente-Tapia et al., 2018 |
| Chrysaora lactea Eschscholtz, 1829 | Cymothoa catarinensis Thatcher, Loyola e Silva, Jost & Souza-Conceiçao, 2003 | N/A | ? | EX | Guaratuba, Paraná e Baía Norte, Florianópolis, Santa Catarina | TR | L | 2003 + 2005, Nov + May | 8–14 m | Nogueira Junior & Silva, 2005 |
| Chrysaora lactea Eschscholtz, 1829 | Periclimenes sp. | Facultative commensal, feeding on mucus, large proportion ovigerous females | OF, A, J | SUM | São Paulo state | HC | ? | 1999–2002 + 2006 Jul | NS | Filho et al., 2008 |
| Chrysaora lactea Eschscholtz, 1829 | Synidotea marplatensis Giambiagi, 1922 | N/A | ? | SUM | Guaratuba, Paraná e Barra do Saí, Santa Catarina, | TR | L | 2003–2004 Aug–Dec | 8–14 m | Nogueira Junior & Silva, 2005 |
| Chrysaora melanaster Brandt, 1835 | Hyperia galba Montagu, 1813 | N/A | J | SUM, O | Takehara City (34 18′N, 132 55′E) | ? | L | 2009 Apr + Jun | ? | Ohtsuka, Boxshall & Srinui, 2012 |
| Chrysaora pacifica Goette, 1886 | Oxycephalus clausi Bovallius, 1887 | February to May, 97.6% female, largely one female per host, occasionally M/F pair, 1/3 of parasites were ovigerous. No breakdown by specific host | OF, F | EX | Nagato, Yamaguchi, Japan | OBS | L | 2012–2018 | 0–5 m | Mazda et al., 2019 |
| Chrysaora plocamia Lesson, 1830 | Hyperia curticephala Vinogradov & Semenova, 1985 | Mean 0f 174. 4 amphipods/host, 79% female, ingested mesoglea | M, F, OF | W | Mejillones Bay | SC | N | 2005 Feb | NS | Oliva, Maffet & Laudien, 2010 |
| Chrysaora quinquecirrha Desor, 1848 | Callinectes sapidus Rathbun, 1896 | Not feeding on medusa | ?? | EX | Mississippi sound | HC | L | 1968 Aug | NS | Phillips, Burke & Keener, 1969 |
| Chrysaora quinquecirrha Desor, 1848 | Libinia dubia H. Milne Edwards, 1834 | Lower incidence rate near surface than bottom trawls, actively feeding on medusae | ?? | B, O | Mississippi sound | MULTI | N | 1968 Aug | NS | Phillips, Burke & Keener, 1969 |
| Chrysaora quinquecirrha Desor, 1848 | Pseudomacrochiron stocki Sars, 1909 | 12 specimens from 10 hosts | F, M | ? | Madras Marina | HC | N | 1967, Oct | ? | Reddiah, 1969 |
| Chrysaora sp. | Cancer sp. cf. antennarius* | N/A | J, MG | ? | Southern California Bight | HC | N | 1989 Jul–Sep | NS | Martin & Kuck, 1991 |
| Chrysaora sp. | Hyperia medusarum Müller, 1776 | N/A | F | ? | Southern California Bight | HC | L | 1989. Jul–Sep | NS | Martin & Kuck, 1991 |
| Chrysaora sp. | Metamysidopsis elongata Holmes, 1900 | N/A | M | ? | Southern California Bight | HC | L | 1989. Jul–Sep | NS | Martin & Kuck, 1991 |
| Chrysaora sp. | Mysidopsis cathengelae Gleye, 1982 | N/A | M | ? | Southern California Bight | HC | L | 1989. Jul–Sep | NS | Martin & Kuck, 1991 |
| Cyanea capillata Linnaeus, 1758 | Alepas pacifica Pilsbry, 1907 | Seven barnacles from 14.5-37 mm in length on the exumbrella and umbrellar Margin. | ? | MA, EX | Marion Bay, Tazmania | ? | L | 1985 | ? | Liu & Ren, 1985 via Pagès, 2000 |
| Cyanea capillata Linnaeus, 1758 | Hyperia galba Montagu, 1813 | Inverted positioning, plentiful in the spring | A & J & OF | MA, EX | Narragansett Marine Laboratory | HC | N | 1954 Sep –1955 Aug | NS | Bowman, Meyers & Hicks, 1963 |
| Cyanea capillata Linnaeus, 1758 | Hyperia galba Montagu, 1813 | N/A | A & J & OF | ? | Niantic River | TR | N | 1960, May + Jun | NS | Bowman, Meyers & Hicks, 1963 |
| Cyanea capillata Linnaeus, 1758 | Hyperia galba Montagu, 1813 | Peak in Oct, reference for mature medusae, consume host gonad | J, A | O | German Bight | HC + SC | 1984–1985 | ? | Dittrich, 1988 | |
| Cyanea capillata Linnaeus, 1758 | Hyperoche medusarum Kröyer, 1838 | Single specimen in May | J | ? | Niantic River | HC | L | 1960, May + Jun | NS | Bowman, Meyers & Hicks, 1963 |
| Cyanea capillata Linnaeus, 1758 | Themisto australis Stebbing, 1888 | Cradle positioning, no bell damage, all sampled epibionts submature females | JF | EX | Rye Pier (38°23′S, 144°50′E) | HC | N | 1995, Jun–Oct | NS | Condon & Norman, 1999 |
| Cyanea nozakii Kishinouye, 1891 | Alepas pacifica Pilsbry, 1907 | Relationship uncharacterized except to note epibiont presence on umbrella and oral arms | ? | B, O | Japanese Coast | ? | ? | ? | ? | Hiro, 1937 via Pagès, 2000 |
| Cyanea nozakii Kishinouye, 1891 | Alepas pacifica Pilsbry, 1907 | 3 barnacles on the umbrella up to a length of 130 mm | ? | EX | Shanghai | ? | ? | 1946 | ? | Tubb, 1946 via Pagès, 2000 |
| Cyanea nozakii Kishinouye, 1891 | Alepas pacifica Pilsbry, 1907 | Substrate | M, F, OF | B | Western Coast of Japan | HC | L | 2005–2009 | ? | Yusa et al., 2015 |
| Deepstaria enigmatica Russell, 1967 | Anuropidae gn. sp. | Two anuropids close to the oral arm base on one medusa | ? | O, SUM | Mutsu Bay | ROV | L | 2002 Apr/May | 669 m | Lindsay et al., 2004 |
| Deepstaria enigmatica Russell, 1967 | Anuropus sp. | Parasitic | ? | SUM | San Diego Trough | ROV | L | 1966 Oct | 723 m | Barham & Pickwell, 1969 |
| Diplulmaris malayensis Stiasny, 1935 | Alepas pacifica Pilsbry, 1907 | 15 barnacles found on 10 hosts, mostly attached to the subumbrellar margins. 1 to 3 epibionts per host. 11 were oriented towards the GVC opening and oral arms of the host. Hypothesized consumption of gonadal tissue by this epibiont | ? | MA | 34 29.4′N, 138 32.6′E | TR | N | 1981 Jun | NS | Pagès, 2000 |
| Pelagia noctiluca Forsskål, 1775 | Alepas pacifica Pilsbry, 1907 | Over 100 barnacles on the umbrellar and oral arm regions of an unknown number of medusae | ? | B, O | Japanese Coast | ? | ? | ? | ? | Hiro, 1937 via Pagès, 2000 |
| Pelagia noctiluca Forsskål, 1775 | Alepas pacifica Pilsbry, 1907 | N/A | ? | SUM | 39N, 52W | ? | ? | ? | ? | Madin unpubl data via Pagès, 2000 |
| Pelagia noctiluca Forsskål, 1775 | Alepas pacifica Pilsbry, 1907 | One barnacle 20 mm long, present on an oral arm | ? | O | Misaki, Japan | ? | L | ? | ? | Utinomi, 1958 via Pagès, 2000 |
| Pelagia noctiluca Forsskål, 1775 | Anelasma sp. | Medusae up to 60 mm in diameter, unknown epibiont number, size and position. | ? | ? | Kuroshio, Japan | ? | ? | ? | ? | Kishinouye, 1902 via Pagès, 2000 |
| Pelagia noctiluca Forsskål, 1775 | Oxycephalus clausi Bovallius, 1887 | February to May, 97.6% female, largely one female per host, occasionally M/F pair, 1/3 of parasites were ovigerous. No breakdown by specific host | OF, F | EX | Nagato, Yamaguchi, Japan | OBS | L | 2012–2018 | 0–5 m | Mazda et al., 2019 |
| Pelagia noctiluca Forsskål, 1775 | Thamneus rostratus Bovallius, 1887 | Relatively rare species | A & J | SUM | Gulf of California | SC | L | 2003 Mar | 10 m | Gasca & Haddock, 2004 |
| Pelagia panopyra Péron & Lesueur, 1810 | Ibacus sp. | Each medusa had a phyllosoma larva firmly attached to the bell surface. The larvae were difficult to remove without injuring them, considered parasitoid relationship | PL | EX | Sydney Harbor | ? | L | 1960 May | ? | Thomas, 1963 |
| Phacellophora camtschatica Brandt, 1835 | Alepas pacifica Pilsbry, 1907 | 2 5–5.1 cm long barnacles on a 50 mm | ? | ? | Tasman sea | ? | L | 1968 | ? | Utinomi, 1968 via Pagès, 2000 |
| Phacellophora camtschatica Brandt, 1835 | Hyperia medusarum Müller, 1776 | Parasitoid, May to Sept, 100s of amphipods, 100% of hosts had infestation in July | M & F & J | O | Puget Sound | HC | N | 1994-2003 May-Oct | NS | Towanda & Thuesen, 2006 |
| Phacellophora camtschatica Brandt, 1835 | Metacarcinus gracilis Dana, 1852 | Association appears in May, once bell widths of hosts begin to exceed 3 cm, peaks in June/July, few after mid-Oct | MG & I | B, O | Puget Sound | HC | N | 1994–2003 May–Oct | NS | Towanda & Thuesen, 2006 |
| Poralia rufescens Vanhöffen, 1902 | Lanceola clausii Bovallius, 1885 | N/A | F, M, J | SUM | Suruga Bay | ROV | L | 2002 Apr | 867–1,697 m | Hughes & Lindsay, 2017 |
| Poralia rufescens Vanhöffen, 1902 | Lysianassinae gn sp. | Attached at base of oral arms, 1–6 per medusa | ? | O, SUM | Japan Trench | ROV | N | 2002 Apr/May | 500–1000 m | Lindsay et al., 2004 |
| Poralia rufescens Vanhöffen, 1902 | Pseudocallisoma coecum Holmes, 1908 | Only juvenile specimens | J | O | Japan Trench | ROV | L | 2002 Apr–May | 576–732 m | Hughes & Lindsay, 2017 |
| Hydrozoa | ||||||||||
| Anthoathecata | ||||||||||
| Bythotiara depressa Naumov, 1960 | Scina sp. | N/A | ? | ? | Gulf of California | ROV | L | 2007 Dec | 494 m | Gasca, Hoover & Haddock, 2015 |
| Bythotiara sp. | Mimonectes sphaericus Bovallius, 1885 | N/A | ? | B | Gulf of California | ROV | L | 2006 May | 690 m | Gasca, Hoover & Haddock, 2015 |
| Leuckartiara octona Fleming, 1823 | Hyperia medusarum Müller, 1776 | N/A | JM | ? | Gulf of California | SC | L | 2006 Sep | <30 m | Gasca, Hoover & Haddock, 2015 |
| Leuckartiara zacae Bigelow, 1940 | Hyperia medusarum Müller, 1776 | N/A | F, J | ? | Monterey California | SC | L | 2004 May | 10 m | Gasca, Suárez-Morales & Haddock, 2007 |
| Leuckartiara zacae Bigelow, 1940 | Lestrigonus schizogeneios Stebbing, 1888 | N/A | JF | ? | Monterey California | SC | L | 2004 May | 5–15m | Gasca, Suárez-Morales & Haddock, 2007 |
| Neoturris sp. | Hyperia medusarum Müller, 1776 | N/A | OF, J | ? | Monterey California | ROV | L | 2004 May | 237 m | Gasca, Suárez-Morales & Haddock, 2007 |
| Leptothecata | ||||||||||
| Aequorea coerulescens Brandt, 1835 | Brachyscelidae gn sp. | N/A | J | ? | Gulf of California | SC | L | 2003 Mar | 10 m | Gasca & Haddock, 2004 |
| Aequorea coerulescens Brandt, 1835 | Brachyscelus crusculum Spence Bate, 1861 | N/A | JM, A & OF | EX | Gulf of California | SC | L | 2003 Mar | 10–15 m | Gasca & Haddock, 2004 |
| Aequorea coerulescens Brandt, 1835 | Ibacus ciliatus von Siebold, 1824 | N/A | PL | ? | Yamaguchi, Japan | ? | ? | ? | ? | Wakabayashi, Tanaka & Abe, 2017 via Wakabayashi, Tanaka & Phillips, 2019 |
| Aequorea coerulescens Brandt, 1835 | Oxycephalus clausi Bovallius, 1887 | February to May, 97.6% female, largely one female per host, occasionally M/F pair, 1/3 of parasites were ovigerous. No account breakdown by specific host | OF, F | EX | Nagato, Yamaguchi, Japan | OBS | N | 2012–2018 | 0–5 m | Mazda et al., 2019 |
| Aequorea coerulescens Brandt, 1835 | Sapphirina nigromaculata Claus, 1863 | N/A | ? | MA | Gulf of California | SC | L | 2003 Mar | 10 m | Gasca & Haddock, 2004 |
| Aequorea coerulescens Brandt, 1835 | Thamneus rostratus Bovallius, 1887 | Relatively rare amphipod species | J | B | Gulf of California | SC | L | 2003 Mar | 10 m | Gasca & Haddock, 2004 |
| Aequorea eurodina* Péron & Lesueur, 1810 | Hyperia gaudichaudii H. Milne Edwards, 1840 | 2 attached to one medusa | ? | ? | Port Phillip Bay, Australia | HC | L | 2009 Sep + 2012 Feb | NS | Browne, 2015 |
| Aequorea macrodactyla Brandt, 1835 | Ibacus novemdentatus Gibbes, 1850 | N/A | PL | ? | Nagasaki, Japan | ? | ? | ? | ? | Shojima, 1973 via Wakabayashi, Tanaka & Phillips, 2019 |
| Aequorea victoria Murbach & Shearer, 1902 | Ibacus ciliatus von Siebold, 1824 | Riding small medusae, pierced exumbrella with pereiopods, attached to a salp as well, parasitoid relationship hypothesized | PL | EX | Japan | OBS | L | ? | ? | Wakabayashi, Tanaka & Phillips, 2019 |
| Chromatonema erythrogonon, Bigelow, 1909 | Hyperoche medusarum Kröyer, 1838 | N/A | OF | ? | Gulf of California | ROV | L | 2003 Mar | 1,100 m | Gasca & Haddock, 2004 |
| Clytia hemisphaerica Linnaeus, 1767 | Eduarctus martensii Pfeffer, 1881 | N/A | PL | ? | Yamaguchi, Japan | ? | ? | ? | ? | Wakabayashi, Tanaka & Abe, 2017 via Wakabayashi, Tanaka & Phillips, 2019 |
| Clytia sp. | Metopa borealis G. O. Sars, 1883 | Association from Oct to March, epibionts passed between medusae | ? | B, O | West Scotland | ? | N | Oct–Mar | ? | Elmhirst, 1925 via Vader, 1972 |
| Eutonina indicans Romanes, 1876 | Tryphana malmii Boeck, 1871 | N/A | ? | ? | Gulf of California | ROV | L | 2006 May | 202 m | Gasca, Hoover & Haddock, 2015 |
| Mitrocoma cellularia Agassiz, 1862 | Hyperoche medusarum Kröyer, 1838 | N/A | OF, J | W | Monterey California | SC | L | 2004 May | 10 m | Gasca, Suárez-Morales & Haddock, 2007 |
| Mitrocoma cellularia Agassiz, 1862 | Tryphana malmii Boeck, 1871 | N/A | JF | Monterey California | SC | L | 2004 May | 5-15m | Gasca, Suárez-Morales & Haddock, 2007 | |
| Tima bairdii Johnston, 1833 | Metopa alderi Spence Bate, 1857 | Speculates year-round relationship, mobile on medusa, did not feed on host tissue, fed on mucus | J & A & OF | SUM, O, B, T | Bergen | ? | N | 1970 Apr | ? | Vader, 1972 |
| Tima formosa Agassiz, 1862 | Hyperoche medusarum Kröyer, 1838 | N/A | JF | ? | Narragansett Marine Laboratory | HC | L | 1954 Sep– 1957 Aug | NS | Bowman, Meyers & Hicks, 1963 |
| Tima sp. | Iulopis mirabilis Bovallius, 1887 | N/A | J & A | ? | Gulf of California | SC | L | 2006 Sep | <30 m | Gasca, Hoover & Haddock, 2015 |
| Limnomedusae | ||||||||||
| Liriope tetraphylla Chamisso & Eysenhardt, 1821 | Simorhynchotus antennarius Claus, 1871 | N/A | 0F | ? | Gulf of California | SC | L | 2006 Jun | <30 m | Gasca, Hoover & Haddock, 2015 |
| Liriope tetraphylla Chamisso & Eysenhardt, 1821 | Ibacus ciliatus von Siebold, 1824 | N/A | PL | ? | Nagasaki, Japan | ? | ? | ? | ? | Shojima, 1973 via Wakabayashi, Tanaka & Phillips, 2019 |
| Liriope sp. | Scyllarus chacei Holthuis, 1960 | 30% of phyllosoma attached to at least one GZ species, primarily hydrozoa, parasitoid relationship | PL | EX | Northern Gulf of Mexico | OBS, TR |
N | 2015 Oct | 1–31 m | Greer et al., 2017 |
| Olindias sambaquiensis Müller, 1861 | Brachyscelus cf. rapacoides Stephensen, 1925 | Reduction in mouthpart of epibionts higher in females | J | ? | Sao Sebastian Channel | TR | L | 2015 Nov | ? | Puente-Tapia et al., 2018 |
| Olindias sambaquiensis Müller, 1861 | Synidotea marplatensis Giambiagi, 1922 | N/A | ? | EX | Guaratuba, Paraná e Barra do Saí, Santa Catarina, | TR | L | 2003–2004 Aug-Dec | 8–14 m | Nogueira Junior & Silva, 2005 |
| Narcomedusae | ||||||||||
| Aegina citrea Eschscholtz, 1829 | Iulopis loveni Bovallius, 1887 | N/A | F | ? | Gulf of California | ROV | L | 2007 Jan | 83 m | Gasca, Hoover & Haddock, 2015 |
| Aegina citrea Eschscholtz, 1829 | Iulopis mirabilis Bovallius, 1887 | N/A | A | ? | Gulf of California | ROV | L | 2006 Oct | 1,286–1,478 m | Gasca, Hoover & Haddock, 2015 |
| Aegina citrea Eschscholtz, 1829 | Lanceola pacifica Stebbing, 1888 | N/A | M | Monterey California | ROV | L | 2005 Apr | 1,322 m | Gasca, Suárez-Morales & Haddock, 2007 | |
| Aegina citrea Eschscholtz, 1829 | Prohyperia shihi Gasca, 2005 | N/A | ? | ? | Gulf of California | ROV | L | 2007 Aug | 554 m | Gasca, Hoover & Haddock, 2015 |
| Aegina citrea Eschscholtz, 1829 | Pseudolubbockia dilatata Sars, 1909 | Refuge and mating, mating pairs with long residence time evident on more than one occasion | M, F | SUM | Monterey California | ROV | L | 2004 May | 606–1,098 m | Gasca, Suárez-Morales & Haddock, 2007 |
| Pegantha laevis Bigelow, 1909 | Prohyperia shihi Gasca, 2005 | N/A | JF | GVC | Gulf of California | ROV | L | 2015 Mar | 926 m | Gasca & Browne, 2018 |
| Solmissus incisa Fewkes, 1886 | Brachyscelus sp. | N/A | J | ? | Gulf of California | ROV | L | 2006 May | 497 m | Gasca, Hoover & Haddock, 2015 |
| Solmissus incisa Fewkes, 1886 | Thamneus rostratus Bovallius, 1887 | N/A | ? | Monterey California | ROV | L | 2005 Apr | 243 m | Gasca, Suárez-Morales & Haddock, 2007 | |
| Solmissus incisa Fewkes, 1886 | Tryphana malmii Boeck, 1871 | N/A | F | Monterey California | ROV | L | 2004 May | 458 m | Gasca, Suárez-Morales & Haddock, 2007 | |
| Solmissus incisa Fewkes, 1886 | Tryphana malmii Boeck, 1871 | N/A | OF | ? | Gulf of California | ROV | L | 2006 May | 295 m | Gasca, Hoover & Haddock, 2015 |
| Solmissus sp. | Hyperia medusarum Müller, 1776 | N/A | JF | ? | Gulf of California | ROV | L | 2006 Sep | 498 m | Gasca, Hoover & Haddock, 2015 |
| Solmissus sp. | Hyperia sp. | N/A | ? | ? | Gulf of California | ROV | L | 2006 Sep | 396–435 m | Gasca, Hoover & Haddock, 2015 |
| Apolemia sp. | Megalanceoloides aequanime Gasca, 2017 | N/A | OF | GVC | Gulf of California | ROV | L | 2015 Mar | 2,094 m | Gasca & Browne, 2018 |
| Apolemia sp. | Mimonectes loveni Bovallius, 1885 | N/A | F | GVC | Gulf of California | ROV | L | 2015 Mar | 2,325–2,589 m | Gasca & Browne, 2018 |
| Athorybia rosacea Forsskål, 1775 | Parascelus edwardsi Claus, 1879 | Relatively rare amphipod species | ? | ? | Gulf of California | SC | L | 2003 Mar | 10 m | Gasca & Haddock, 2004 |
| Chelophyes appendiculata Eschscholtz, 1829 | Paralycaea hoylei Stebbing, 1888 | N/A | JF | Monterey California | SC | L | 2004 May | 5–15m | Gasca, Suárez-Morales & Haddock, 2007 | |
| Diphyes bojani Eschscholtz, 1825 | Lestrigonus bengalensis Giles, 1897 | N/A | F, JF | W | Cabo Frio (RJ) and the Santa Catarina Island (SC) | TR | L | 1980, 17-23 Jan | ? | de Lima & Valentin, 2001 |
| Nectadamas diomedeae Bigelow, 1911 | Mimonectes sphaericus Bovallius, 1885 | N/A | M | Monterey California | ROV | L | 2005 Apr | 1,082 m | Gasca, Suárez-Morales & Haddock, 2007 | |
| Nectadamas diomedeae Bigelow, 1911 | Mimonectes sphaericus Bovallius, 1885 | N/A | J | ? | Gulf of California | ROV | L | 2006 May | 1,344 m | Gasca, Hoover & Haddock, 2015 |
| Nectadamas diomedeae Bigelow, 1911 | Mimonectes stephenseni Pirlot, 1929 | N/A | F | Monterey California | ROV | L | 2003 May | 392 m | Gasca, Suárez-Morales & Haddock, 2007 | |
| Siphonophorae | ||||||||||
| Muggiea sp. | Scyllarus chacei Holthuis, 1960 | 30% of phyllosoma attached to at least one GZ species, primarily hydrozoa, parasitoid relationship hypothesized. | PL | EX | Northern Gulf of Mexico | OBS, TR | N | 2015 Oct | 1–31 m | Greer et al., 2017 |
| Physophora hydrostatica Forsskål, 1775 | Tryphana malmii Boeck, 1871 | N/A | ? | ? | Gulf of California | ROV | L | 2006 Jan | 116 m | Gasca, Hoover & Haddock, 2015 |
| Prayidae gn sp | Scyllaridae gn sp | Attached with pereiopods | PL | EX | Gran Canaria, Spain | OBS | L | 1999 Feb | 3 m | Ates, Lindsay & Sekiguchi, 2007 |
| Resomia ornicephala Pugh & Haddock, 2010 | Anapronoe reinhardti Stephensen, 1925 | N/A | F, JM | ? | Gulf of California | ROV | L | 2006 Sep | 254 m | Gasca, Hoover & Haddock, 2015 |
| Resomia ornicephala Pugh & Haddock, 2010 | Tryphana malmii Boeck, 1871 | N/A | OF, A, J | ? | Gulf of California | ROV | L | 2006 May | 204 m | Gasca, Hoover & Haddock, 2015 |
| Rosacea cymbiformis Delle Chiaje, 1830 | Brachyscelus crusculum Spence Bate, 1861 | N/A | JF | GVC | Gulf of California | SC | L | 2015 Mar | 15 m | Gasca & Browne, 2018 |
| Rosacea cymbiformis Delle Chiaje, 1830 | Eupronoe minuta Claus, 1879 | N/A | JF | ? | Gulf of California | ROV | L | 2006 Sep | 161 m | Gasca, Hoover & Haddock, 2015 |
| Rosacea cymbiformis Delle Chiaje, 1830 | Paraphronima gracilis Claus, 1879 | N/A | J | ? | Gulf of California | ROV | L | 2006 May | 430 m | Gasca, Hoover & Haddock, 2015 |
| Sulculeolaria quadrivalvis de Blainville, 1830 | Simorhynchotus antennarius Claus, 1871 | N/A | F | W | Cabo Frio (RJ) and the Santa Catarina Island (SC) | TR | L | 1980, 17–23 Jan | ? | de Lima & Valentin, 2001 |
| Trachymedusae | ||||||||||
| Haliscera bigelowi Kramp, 1947 | Hyperia medusarum Müller, 1776 | N/A | J | ? | Gulf of California | SC | L | 2006 Sep | <30 m | Gasca, Hoover & Haddock, 2015 |
| Haliscera bigelowi Kramp, 1947 | Scina spinosa Vosseler, 1901 | N/A | M | Monterey California | ROV | L | 2005 Apr | 394 m | Gasca, Suárez-Morales & Haddock, 2007 | |
| Haliscera sp. | Scina spinosa Vosseler, 1901 | N/A | J | ? | Gulf of California | ROV | L | 2006 Oct | 1,263 m | Gasca, Hoover & Haddock, 2015 |
| Haliscera sp. | Scina uncipes Stebbing, 1895 | N/A | A | ? | Gulf of California | ROV | L | 2006 May | 449 m | Gasca, Hoover & Haddock, 2015 |
| Pectis tatsunoko Lindsay & Pagès, 2010 | Mimonectes spandlii Stephensen & Pirlot, 1931 | N/A | JM | SUM | Suruga Bay | ROV | L | 2002 Apr | 1,967 m | Lindsay & Pagès, 2010 |
Notes:
Life Stage and Sex: F, Female; M, Male; MG, Megalopa; A, Adult; E, Egg; J, Juvenile; OF, Ovigerous female; C, Copepodid/Copepodite; I, Instar; PL, Phyllosoma larva
Location on Medusa: EX, Exumbrella; SUM, Subumbrella; O, Oral arms; B, Bell (undifferentiated); GVC, Gastrovascular cavity; SG, Subgenital pit; W, Within medusa (undif.); MA, Umbrellar margin; T, Tentacles
Collection: HC, Hand collection (Nets, buckets, bags, etc.); SC, Scuba and Blue Water Diving; ROV, Remote and Human Operated Vehicles; TR, Boat trawls; MULTI, Multiple methods used; OBS, Observational methods with imaging
Limited Observations: 5 or fewer occurrences catalogued; N, >5 medusae with this epibiont
Depth: NS, Near surface
All: ?, Data missing
Results and discussion
The final table produced by this review process includes 211 recorded interactions between hydrozoan or scyphozoan medusae and crustaceans, extracted from 97 papers (Table 1). For both cnidarians and crustaceans, order, family, genus, and species are included in Supplementary Materials. Results that lacked taxonomic identification (at least Family level) were not included. The final table (Table 1) provides sampling information, such as year and month of sampling, sampling method, and region of sampling. For crustaceans, records include the life stage involved in the interaction, sex of the epibiont, location on the hosts, and additional notes, if available. In most studies, fewer data were available on the cnidarian hosts, reducing the degree to which these interactions could be analyzed in terms of hydromedusan or scyphomedusan life stage. In the next paragraphs, we discuss the jellyfish-crustacea interactions through all of the categories included.
Diversity
Diversity of scyphozoan hosts
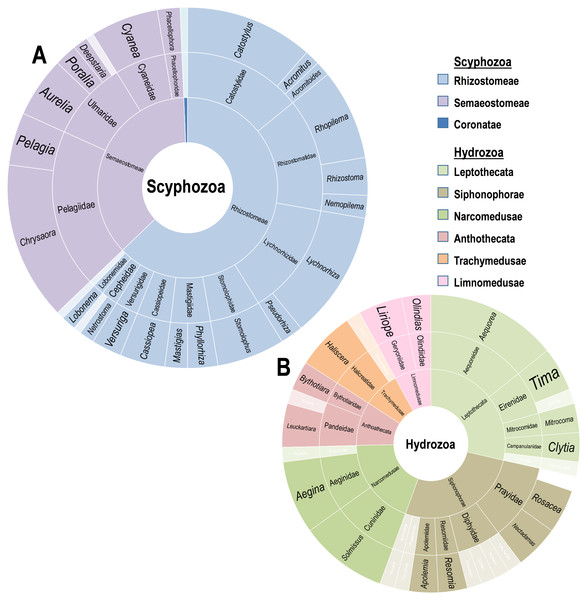
A supermajority of records (70%, or 148/211) involves Scyphomedusae, with 53 records involving just the five most common scyphozoan species: Lychnorhiza lucerna (Haeckel, 1880), Catostylus mosaicus (Quoy & Gaimard, 1824), Stomolophus meleagris (Agassiz, 1860), Cyanea capillata (Linnaeus, 1758) and Rhopilema hispidum (Vanhöffen, 1888). These records are heavily concentrated in the upper water column. Deeper water collections (ROV/HOV) were dominated by hydromedusae (69%, or 27/39), while records involving the upper water column (0–30 m) were more common and dominated by scyphomedusae (78%, or 83/106). Sixty-seven records included no specific sampling depth. These records were generally more than 50 years old. Although they are likely near-surface sampling records and mainly report known shallow-water species, they cannot be verified as such because of the lack of explicit information. Most of these (87%, or 58/67) are records of scyphomedusae. Overall, the diversity of scyphomedusae was low, with only 39 species from 27 genera represented in records (Fig. 2A). The genus Chrysaora had the largest contingent of accounts, with 21 individual records of associations across at least seven Chrysaora species. This genus has been reported to interact with 16 different epifaunal crustaceans. The genera Chrysaora, Lychnorhiza, and Catostylus accounted for a third of scyphozoan records. These records originate mainly from the upper water levels of various locations (i.e., the east coast of the United States, the southeast of Brazil, the southern Australian coast, and the western Philippines, Japan and Pakistan).
Figure 2: Diversity of Scyphozoa and Hydrozoa species.
Rings from innermost to outermost are order, family, genus in the classes (A) Scyphozoa and (B) Hydrozoa as distributed by number of accounts including a host in that group. Families and genera with single reports are whitened.Diversity of hydrozoan hosts
Twenty-six genera, and six Hydrozoan orders were reported interacting with Crustacea in 63 records (Fig. 2B). The order Leptothecata included the greatest number of records (18), with 17 records of Siphonophorae and 12 of Narcomedusae. The diversity of Hydrozoa was significantly limited by region, with 45 of the 63 records (71%) from the Gulf of California. Additionally, those from the Gulf were acquired from primarily deep water ROV missions. The medusae recorded belonged to 28 known species, with twelve records unable to provide higher resolution than genus and a single Prayid siphonophore only identified to the family level. Rosecea cymbiformis (Delle Chiaje, 1830) (4), Aegina citrea (Eschscholtz, 1829) (5), and Aequorea coerulescens (Brandt, 1835) (6) were the three most common species.
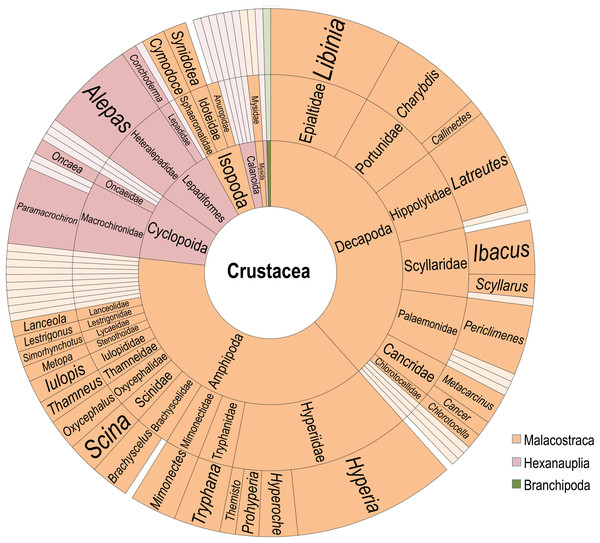
Diversity of crustacean epibionts
The crustaceans included Hexanauplia (reported in 37 discrete observations), Malacostraca (173), and a single representative of Branchiopoda (Evadne sp.) (Fig. 3). Recorded Hexanauplia consisted of mainly specialist groups known to be obligate epibionts and had overall low species resolution, with 13 of the 23 documented associations lacking a species name. The Macrochironidae, a group of known scyphozoan parasites, makes up 12 of the copepod epibiont records. Outside of this family, no additional Hexanauplia epibiont was recorded more than twice. The single reported case of a medusa with Evadne sp. occurred in a broad analysis of items found on a Catostylus medusae (Browne & Kingsford, 2005). As this was not replicated throughout medusae within the study, or in other studies, it is unlikely this is a common or genuine association.
Figure 3: Diversity of Crustacean epibionts.
From innermost ring to outermost ring: Subphylum, Order, Family, Genus. Color coded by classes Malacostraca (orange), Hexanauplia (pink), and Brachipoda (green). Families and genera reported only once are whitened.The bulk of the associations involve crustaceans of the class Malacostraca. These 173 records include amphipods and decapods in equal proportion (47%, or 81/173 each), isopods (5%, or 9/173), and mysids (1%, or 2/173). The amphipods are dominated by the parasitic family Hyperidae, recorded in 32 separate encounters. Members of the family of Hyperidae are present across 22 identified scyphozoan and hydrozoan species, making them the most widely distributed family. Hyperia galba (Montagu, 1813) is present in nine records from both surface and deep-water samples, making it the single most plentiful within the amphipods. Outside of the family Hyperidae, Tryphana malmii (Boeck, 1871) is recorded six times in association with deep-sea jellyfish. Most amphipod species recorded were recorded on multiple host species.
Decapod associations (81 records) are separated among twelve families, Epialidae (17), Portunidae (14), Palaemonidae (12), Hippolytidae (14), Scyllaridae (11) Cancridae (6), Chlorotocellidae (2), Scyllaridae (1), Luciferidae (1), Penaeidae (1), Varunidae (1), and Grapsoidea (1). No decapod was found in association with hydrozoans or in deep-sea records. The representatives of Epialtidae are comprised exclusively of multiple species of the genus Libinia. The Portunidae records are mainly composed of the commercially valuable Charybdis feriata (Linnaeus, 1758) (11 records), Charybdis annulata (Fabricius, 1798) (1) and two Callinectes, Calinectes sapidus (Rathbun, 1896) and an unidentified Callinectes specimen (1). Periclimenes paivai (Chace, 1969) is the most common Palaemonidae, representing three of the twelve records, with six additional Periclimenes species, two Ancylomenes species and one Leander paulensis (Ortmann, 1897). All Hippolytidae associations were between a specimen of Latreutes anoplonyx (Kemp, 1914) or Latreutes mucronatus (Stimpson, 1860) and one of an array of different scyphomedusae in Asia, Australia, and the Arabian Sea-Persian Gulf corridor. The families Scyllaridae and Scyllarinae include seven Ibacus, three Scyllarus, and Eduarctus martensii (Pfeffer, 1881). These associations were all exclusively larval. The majority (4) of Cancridae records involve Metacarcinus gracilis (Dana, 1952) with two unknown Cancer species. These crabs were found on Chrysaora medusae and one Phacellophora camtschatica (Brandt, 1835). Two Chlorotocella gracilis (Balss, 1914) (Chlorotocellidae) were found on Japanese rhizostomes, both in somewhat limited encounters. The last three accounts include a Cyrtograpsus affinis (Dana, 1851) (Family: Varunidae), Lucifer sp. (Family: Luciferidae), and a juvenile Grapsoidea of unknown genus and species. The account of Lucifer sp. was of a record of one specimen on a medusa in New South Wales, and is not likely a common or genuine association (Browne & Kingsford, 2005). Cyrtograpsus affinis and the juvenile of the family Grapsoidea were also one-off reports found in single medusae (Schiariti et al., 2012; Gonçalves et al., 2016).
Associations that involved mysids or isopods were far fewer than those involving decapods and amphipods. The isopod records include only four species, including the deep-sea parasite Anuropus associated with Deepstaria enigmatica (Russell, 1967). Besides the in situ accounts of the Deepstaria scyphomedusae with an attached Anuropus, three Isopoda species were found in association with upper water column medusae. These are Cymodoce gaimardii (H. Milne Edwards, 1840) and Synidotea marplatensis (Giambiagi, 1922), each recorded three times, and Cymothoa catarinensis (Thatcher et al., 2003), found once in association with Chrysaora lactea (Eschscholtz, 1829). Within the order Mysida, the two species Mysidopsis cathengelae (Gleye, 1982) and Metamysidopsis elongata (Holmes, 1900) were recorded on Chrysaora during a bloom in the Southern California Bight (Martin & Kuck, 1991).
Three species of cirripeds were recorded 15 times in association with jellyfish, Alepas pacifica (Pilsbry, 1907) accounting for twelve of such records, Conchoderma virgatum (Spengler, 1789) accounting for two, and a single report of an unidentified Anelasma epibiont on a Pelagia noctiluca (Forsskål, 1775) from 1902. Alepas pacifica has been found on seven separate host species, all scyphozoans. The vast majority of these records came from a single literature review included within an extensive paper from Vader (1972). None of these species were found in deep-sea records.
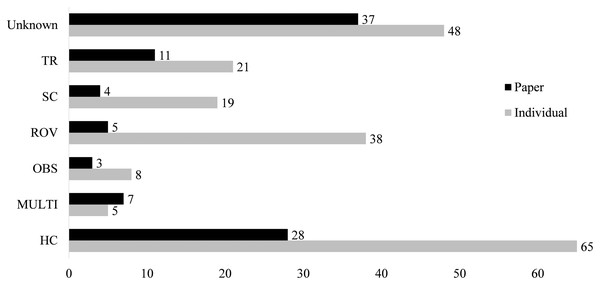
Field collections
Only 58 papers included some explicit method of capture of the jellyfish and its epibiont (Fig. 4). Between 1862 and 1962, only seven of the twenty records reported a method of capture. From 1963 to 1989, this increased to 64%, with 25 of 39 records including the collection method. Since 1990, there have been only seven failures to report collection methods out of 140 accounts. The most common method of collection, used in 31 of the papers, is “by hand”, defined as using handheld dip nets, buckets, plastic bags, and, in limited cases, collection of carcasses from beaches. Trawling was first used in 1968 and has remained in use until recently, reported in 17 of the 33 associations after 2010. Although 38 records were obtained through deep water methods (HOV and ROV), these were used scarcely before 1999. Some studies employed multiple methods, with divers and ROV, or dip net and trawl capture, such that it was unclear which associations were found by each collection method. These were listed as “multi-method” and include four papers.
Figure 4: Collections information for both number of papers using a collection method and number of associations reported from this collection type.
Types are blue water diving (BWD), collection by hand (HC), multiple methods (MULTI), ring net (RN), scuba diving (SC), trawling (TR), in situ observation (OBS) or unknown (Unknown). Associations from papers in which multiple methods were used, but specific methods are known for each association are categorized under the known method. Many papers are comprised of multiple associations, as such, the “Individual” columns include each association separately, “Paper” columns report by paper.The larger proportion of scyphozoan hosts to hydrozoan hosts may be a sampling artifact. The vast majority of the papers discussed here were only analyzing interactions in the top 30 m of the water column. A fair number, especially earlier texts, involve serendipitous encounters at the water’s edge or within sight of the surface (Bowman, Meyers & Hicks, 1963; Jachowski, 1963; Vader, 1972; Martin & Kuck, 1991). The larger, more visible nature of surface water scyphozoans of the rhizostomes and semaeostomes makes them an easier collection target than deep water species. Note that only a single scyphozoan of the order Coronatae, which has no large shallow representatives, was recorded as well. Many elements of the sampling methods impact the scope of this data, and the preeminence of hand collection and papers written on chance occurrences, as opposed to prolonged study, result in a picture that heavily weights organisms more frequently seen or interacted with by humans.
The oldest records of jellyfish-crustacean interaction involved hand collection with buckets and nets, often from shore. These include first accounts of hyperiid amphipod-jellyfish associations from the Chesapeake Bay (Bowman, Meyers & Hicks, 1963). Buckets and nets have remained mainstays, with hand collection accounting for 34 of the 108 post-2000 records and 32 of the 55 pre-2000 records. Buckets and plastic bags are likely preferable to nets, as they may reduce chances of epibiont detachment and medusa damage.
Trawling (by ring nets, otter nets, and bottom trawls), while reported in twelve papers, has been a prominent capture method in South America for the last two decades. However, trawling provides an additional threat, as epibionts may detach, get caught in the bell of a medusa, or move to a different location within the carcass. Given the damage sustained by gelatinous bodies during trawls, and the inability to capture more delicate associations, this is the methodology that seems most likely to provide low-quality relationship information. A focus on a lower number of medusae examined in more detail, may provide more useful information on the ecology of the interaction between jellyfish and their epibionts. Notably, Greer et al. (2017) uses a combination of in situ imaging (with an automatic ISIIS imaging system) and trawls. Trawls were used to verify the identity of organisms seen in the captured images. Such a protocol should be considered for future quantitative and qualitative work.
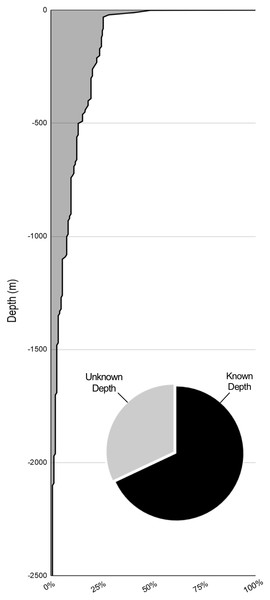
A total of 66% of the records (136/211) are from known surface encounters. 18% of the records (38/211) involve deep water accounts using either an ROV/HOV. These records are distributed unevenly across depths with few records below the mesopelagic zone (Fig. 5). Most of these records fail to provide epibiont location on the jellyfish but provide the only available information on deep water scyphomedusa and hydromedusa hosts. Most of the deep water records are from the Gulf of California. While this sampling method is useful, the high cost and difficulty of use of ROV and HOV equipment make it unrealistic for the vast majority of researchers. The limited number of deep-water accounts and the novelty of many of the findings on each dive can be attributed mainly to these limitations (Gasca & Haddock, 2004; Gasca, Suárez-Morales & Haddock, 2007; Gasca, Hoover & Haddock, 2015).
Figure 5: Percent of sampling by depth.
The depths of samples with known depths. 68% of samplings had known depth data (pie chart). 74.4% of sampling was done above 30 m. Where depth ranges were given (i.e., 8 to 30 m) the deeper value was used.Given the fragility of scyphozoan and hydrozoan medusae, as well as the delicacy of the interaction with their epibionts, the most precise picture of the jellyfish-crustacean associations has been achieved from dip net, plastic bag, bucket, or other by-hand collection methods. These are not only a cost-effective strategy requiring little additional equipment, they also maintain maximum integrity of the organisms. Hand collection, however, is restricted to analyzing associations that are close to the surface. Trawl sampling provides a reliable way to collect many medusae offshore but sacrifices sample integrity. ROV is an imperfect sampling method, often failing to record epibiont positioning, but allows for the only viewing, documentation, and collection of deep water associations, thereby being uniquely important, especially for hydromedusa research. Moreover, the majority of the records document all symbionts on the target host species, often with little data beyond a name or tentative classification for the epibiont. This lack of closer examination leads to an inability to correctly categorize the nature of the relationship, including positioning, feeding behaviors, and duration of the interaction.
In conclusion, the overall best sampling results come from observation-first methodologies such as collection by-hand while snorkeling and diving, as in Mazda et al. (2019), ROV/HOV in situ underwater photography, as employed by Gasca, Hoover & Haddock (2015), or imaging and supplemental trawling as in Greer et al. (2017). Obtaining underwater pictures of medusae and epibiont is crucial to the understanding of the associate placement in relation to host and its behavior. It is also more informative than post hoc in-lab examinations and analysis of trawl contents, because the stress of collection and sampling may impact the epibiont position within the host (Hayashi, Sakagami & Toyoda, 2004). As waterproof video equipment becomes less expensive, options like a simple GoPro may provide clear enough imaging to allow novel in situ observations. Adding an underwater imaging component to sampling may also enable collectors to revisit the ecological context of the association.
Life stages
Age classes and sex, where available, are reported in Table 1. 63% of all records (133/211) reported an age class for the crustacean. 65% of the interactions with a listed age class (65%, or 86/133) reported crustacean juveniles, eggs, larval stages, copepodites, megalopae, or other immature forms. For a minority of records (37%, or 73/211), no information on the crustaceans’ age class and sex was available. When individuals were described as “male” or “female” without any qualifier attached, they were catalogued and treated as adult specimens (Table 1). Megalopae were noted only nine times out of the 106 records that reported an age class for the crustacean associate (8%). In these nine records, the megalopae belonged to the genera Callinectes, Periclimenes, Metacarcinus, Cancer, and Charybdis, and were all in association with Scyphomedusae (Orders: Rhizostomeae and Semaeostomeae). In addition to megalopae, phyllosoma larvae of the families Scyllaridae and Scyllarinae were reported 12 times. The occurrence of larvae of this type associated with medusae and, more generally, with gelatinous zooplankton is well known, especially along the Japanese coast (Wakabayashi, Tanaka & Phillips, 2019). Within and upon the host, juvenile crustaceans were often coexisting with adult forms. Eighty-one of the associations include juveniles (excluding megalopae, eggs, and copepodites), sometimes embedded in host tissue (Towanda & Thuesen, 2006; Browne, 2015; Yusa et al., 2015; Browne, Pitt & Norman, 2017; Mazda et al., 2019). The presence of eggs and ovigerous females was reported in 39 cases from 23 different species. In at least three papers, females and ovigerous females were present in exceptionally high proportions relative to adult males (Filho et al., 2008; Oliva, Maffet & Laudien, 2010; Mazda et al., 2019). Records of megalopae of the commercial crab, Charybdis feriata were reported in substantial numbers on two separate hosts (Kondo et al., 2014; Boco & Metillo, 2018). In other reports, associations between juvenile Metacarcinus gracilis (Dana, 1852) and medusae are hypothesized to be beneficial to the crab as the medusae supply means of transport and food acquisition, which may be similar across juvenile decapod-scyphozoan associations (Towanda & Thuesen, 2006).
Nature of associations between medusae and crustaceans
There is no agreement between authors on the degree to which medusae and crustaceans’ interactions are parasitic, commensal, or otherwise. In the case of the scyphozoan Phacellophora camtschatica and the decapod Metacarcinus gracilis (Dana, 1852), the interaction may involve a mutualistic cleaning relationship as M. gracilis graduates into adulthood (Towanda & Thuesen, 2006). Other reports of megolopae do not suggest any parasitization of the medusae. Weymouth (1910) also indicates that this is a commensal relationship important to M. gracilis megalopae until they reach ~20mm. In other cases, such as the shrimp Perimincles paivai, the commensals seemed to be feeding on the mucus, not the host tissue (Browne & Kingsford, 2005; Filho et al., 2008). Dittrich (1988) demonstrates an aggressive parasitoidism by Hyperia galba in which a large subset of host medusae was so reduced by predation as to lose almost all morphological features. While the ultimate death of these hosts is not recorded within the text, the loss of all tentacular structure and non-mesoglear tissue would make survival nearly impossible. The numbers in which Hyperia can be found on some of the recorded medusae, occasionally upwards of 100 amphipods engaging in host consumption, may lend credence to the parasitoid rather than classically parasitic nature of this relationship in many hosts (Vader, 1972; Dittrich, 1988; Towanda & Thuesen, 2006). However, additional reports on the same species and other hyperiids reported that this group engages in cradle positioning, facing outwards from the medusa, into the water column with no reported predation, or engage in only limited predation of the gonadal tissue or mesogleal tissue (Bowman, Meyers & Hicks, 1963; Gasca 2005; Browne, 2015). Based on this information it seems likely that the family Hyperidae includes a variety of strategies, and the family Hyperia itself may also encompass non-aggressive parasitism, aggressive parasitism, and parasitoidism. In part, this may be due to temporal behavioral differences within species, with more extreme predation in summer and autumn and limited parasitism in spring as populations raise and fall (Bowman, Meyers & Hicks, 1963; Dittrich, 1988). “Inverted cradle” positioning is a recurring feature of amphipod associates (Bowman, Meyers & Hicks, 1963; Condon & Norman, 1999). While some of the crustaceans fed on the medusae themselves, Towanda & Thuesen (2006) primarily recorded crustaceans engaging in theft of prey collected by medusae. Many crustaceans that were reported feeding on the medusae were feeding entirely or in part on the highly regenerative gonadal tissue (Pagès, 2000; Towanda & Thuesen, 2006; Ohtsuka et al., 2009) or engaging in the excavation of small pits in the host mesoglea (Humes, 1953; Jachowski, 1963; Browne, 2015). Reports of Libinia dubia (H. Milne Edwards, 1834) have the greatest agreement on the parasitic nature of the species’ interactions with their medusa host (Jachowski, 1963; Phillips, Burke & Keener, 1969; Schiariti et al., 2012).
The largest exception to the above patterns of limited consumption or longer term residence is the scholarship surrounding phyllosoma larvae on gelatinous zooplankton. These larvae have been reported to stab a pair of pereiopods through the exumbrella or exterior of a nectophore and use the medusa as propulsion and food source. This is a common occurrence both in the northern Gulf of Mexico and at various locations along the Japanese coast (Greer et al., 2017; Wakabayashi, Tanaka & Phillips, 2019). In the review on the subject by Wakabayashi, Tanaka & Phillips (2019), it is hypothesized that the flattened body and ventral mouth of these phyllosoma larvae is ideal for consumption of gelatinous zooplankton while attached. The exact length of this parasitoid association is unknown, though it is likely generally ended by the medusa’s eventual death as the larva eats its way through.
The degree to which crustaceans engage in host consumption may be in part obscured by the speed with which medusae regenerate tissues, especially gonadal and oral arm tissues (Towanda & Thuesen, 2006). The number of associates (at least eight crustacean species) found residing within the bell and around the gonads, suggests that gonadal tissue may be common nourishment even when bell and arm tissue is not consumed. Overall, the relationships of crustaceans with their medusa hosts remain largely uncharacterized and require additional study. Few papers have analyzed the gut contents of the epibionts, which would be a helpful tool in determining whether inverted positioning on hosts was actually a signal of lack of consumption, or simply a break from such (Vader, 1972; Pagès, 2000; Towanda & Thuesen, 2006; Oliva, Maffet & Laudien, 2010). Detailed records of the diets of such organisms are difficult to reconstruct. However, specific searches for nematocysts in digestive tract and excretions or stable isotope analysis have proven successful at identifying cnidomedusae as possible food sources (Schiariti et al., 2012; Fleming et al., 2014). Expanding future works to include both these practices, photographs of the host medusae, and notes on swimming strength, tentacular loss and other signs of deterioration would improve our understanding of how detrimental these relationships actually are. This sort of documentation of host condition is impossible when specimens are collected via trawl.
In addition to consumption, the issue of host choice and host specificity has been analyzed only sparsely. There is evidence in multiple studies that while some individual jellyfish host symbionts, others in the same area lack them due to their size or species (Towanda & Thuesen, 2006; Ohtsuka et al., 2011; Boco & Metillo, 2018). While exotic species often have lower amounts of parasitization in their introduced range (Torchin et al., 2003), the degree to which epibionts in medusae are affected by host or epibiont endemicity is unknown. The high number of cryptic species, a history of misidentification, and poor understandings of historical ranges compound issues with sparse research on the topic (Dawson, 2005; Graham & Bayha, 2007; Morandini et al., 2017; De Souza & Dawson, 2018).
Only one study provides an indication of how nuanced the relationship between gelatinous zooplankton hosts and epibionts may be; 6 years of monthly observation showed that single adult females of the amphipod Oxycephallus clausi (Bovallius, 1887) had a broad range of gelatinous hosts, but shifted to primarily Ocyropsis fusca (Rang, 1827), a lobate ctenophore, during brood release (Mazda et al., 2019). While ctenophores are not the focus of this review, it shows that the nature of interactions may change during the crustacean lifecycle. These sorts of long-term analyses are hard to pursue, but provide a fascinating look at the range of information that can be collected with observational methods. Uneven sex ratios, such as those seen in the case of Oxycephallusclausi (97% female), are present across many associations (Condon & Norman, 1999; Filho et al., 2008; Oliva, Maffet & Laudien, 2010; Mazda et al., 2019). The most common explanation for this higher ratio of females and often ovigerous females is use of scyphozoan and hydrozoan hosts primarily as nursery habitat for movement and protection of juveniles (Gonçalves et al., 2016; Gonçalves et al., 2017; Mazda et al., 2019). Potential territoriality in some females, like those of P. paivai, may help ensure more resources for their brood, and is in line with other symbiont crustaceans (Baeza et al., 2017). For deep sea crustaceans, such as Pseudolubbockia dilatata (Sars, 1909), more even sex ratios would be expected, as there is evidence of long-term resident brooding pairs, and mate scarcity is a feature of deep sea life. Evidence for long-term association and pairing has not been found for other deep water crustaceans, although understanding these deep sea interactions is generally hampered but small sample sizes and difficulty of observation (Gasca, Suárez-Morales & Haddock, 2007; Baeza et al., 2017; Gasca & Browne, 2018).
Years and locations
The oldest records examined were only available from earlier literature reviews (Pagès, 2000; Towanda & Thuesen, 2006; Schiariti et al., 2012). The first record is the Bate (1862) account of the amphipod Iphimedia eblanae on the scyphozoan Rhizostoma pulmo (Macri, 1778) from 1862, also reported in the Vader (1972) review on amphipod associations with medusae. Thiel (1976) refers to older records from as far back as 1791. Overall, the number of records detailing interactions has risen over time but has not exceeded ten papers during any 5 years. While these numbers are increasing modestly, the number of distinct interactions that any given paper reports have increased. Pre-1990s articles, on average put forward information on 1.24 associations per paper. In contrast, the average number of associations reported in papers published from 1990 to 2018 increased more than twofold (an average of 2.83 records per paper). These surveys provide useful records of separate associations found in one area or on one organism and are informative of ecosystem features on a regional level. Still, given the studies’ breadth, they often lack depth, not characterizing relationships between individual host species and their associates.
Records were unevenly distributed globally, with Africa and Europe completely devoid of records from the past 30 years with the exception of a single note on an accidental observation from Gran Canaria, Spain. The eastern coast of North America (one record since 1984 (Tunberg & Reed, 2004) and China (no direct records)), as well as West Africa (one record from 1972 (Bruce, 1972)) and the Mediterranean Sea (last collections 1985 (Dittrich, 1988)) also lack records from the last 30 years. The areas consistently covered by recent papers are Australia (1968–2009), the Philippines (2014, 2018), the eastern coast of South America (1980–2016), and the western United States (1966–2015). Japanese records represent the longest continuity over time, with 33 records between 1902 and 2019. The association that consistently appears throughout time is that of Alepas pacifica (Thoracica, Lepadiformes) with Nomura’s Jellyfish (Nemopilema nomurai) (Pagès, 2000; Yusa et al., 2015). The first record of this association was in 1902 (Pagès, 2000), and the most recent in 2015 (Yusa et al., 2015). Phyllosoma larvae of multiple species, Chlorotocella gracilis (Balss, 1914), and Latreutes spp. also have records spanning multiple decades and papers.
It is worth mentioning that the uneven geographic distribution of associations reported herein may be an artifact of lack of readily available English translations of works from some areas. Reports from Japan and China of crustacean and gelatinous zooplankton associations are mentioned by Hayashi, Sakagami & Toyoda (2004) and Wakabayashi, Tanaka & Phillips (2019), but were not available in English and therefore are not accounted for in this review. Similarly, European records may be underestimated, as non-English records are absent. Other locations’ lack of records may be a more accurate representation of a gap in academic knowledge. Africa’s west and eastern coasts are known to be understudied ecosystems, and so the missing research here is likely not just untranslated (Berkström et al., 2019). As in other ecological inquiries, the expansion of Local Ecological Knowledge into the study of gelatinous zooplankton should be considered, as fishermen and coastal communities often have a deep knowledge of organisms and their associations (Berkström et al., 2019). Fishermen are often well acquainted with specific gelatinous zooplankton species and know their harms, and may have knowledge of symbionts living upon or within them (Al-Rubiay et al., 2009).
Commercial species
Many commercial crustaceans and jellyfish were found to have associations that may be of ecological and commercial importance. Twelve records reported the edible jellyfish Rhopilema spp. as hosts (Berggren, 1994; Pagès, 2000; Hayashi, Sakagami & Toyoda, 2004; Towanda & Thuesen, 2006; Ohtsuka et al., 2010; Ohtsuka, Boxshall & Srinui, 2012; Boco & Metillo, 2018). The commercially harvested shrimp, Penaeus stylirostris (Stimpson, 1871), was found on Stomolophus meleangris (Riascos et al., 2018). Notably, young Callinectes sapidus, the Chesapeake Blue Crab, was reported by Jachowski (1963) as regularly found on Chrysaora quinquecirrha (Desor, 1848) medusae without consuming them. This association was reported again briefly in the Mississippi Sound by Phillips, Burke & Keener (1969). This interaction between a jellyfish and the blue crab has never been corroborated further except for a nonspecific report of a Callinectes sp. associated with jellyfish reported by Towanda & Thuesen (2006) as unpublished data. The commercially valuable crab, Charybdis feriata, has been reported in association with ten jellyfish species (Berggren, 1994; Towanda & Thuesen, 2006; Ohtsuka et al., 2010; Schiariti et al., 2012; Boco, Metillo & Papa, 2014; Boco & Metillo, 2018). These reports involve juveniles (Trott, 1972; Towanda & Thuesen, 2006; Schiariti et al., 2012; Kondo et al., 2014; Boco & Metillo, 2018) and megalopae (Kondo et al., 2014; Boco & Metillo, 2018) of C. feriata, and this association has been recorded in Hong Kong, Japan, the Philippines, Mozambique, and Indonesia, suggesting a consistent pattern over time (first record in 1965 (Schiariti et al., 2012) and last record in 2014 (Boco & Metillo, 2018)) and across their range.
Slipper lobster larvae of the genera Scyllarus and Ibacus have been reported many times across various hosts (Wakabayashi, Tanaka & Phillips, 2019). Some slipper lobsters are commercially fished for consumption, and a large number of these larvae (40% in the Gulf of Mexico) have been shown to live attached to gelatinous zooplankton (Greer et al., 2017).
The consumption of some Scyphozoan hosts, such as Catostylus mosaicus and Rhopilema spp., makes their records valuable as well. The fishing pressures on the jellyfish populations may significantly impact the crustaceans that rely on their oral arms and bells for transport and nourishment of their juvenile stages. Further understanding of these relationships may be especially important in cases where both the medusae (e.g., Rhopilema spp., Lobonemoides robustus (Stiasny, 1920) and Catostylus spp.) and crustacean (Charybdis feriata) are subject to fishing (Boco, Metillo & Papa, 2014; Boco & Metillo, 2018, Kondo et al., 2014). Finally, current information on Callinectes sapidus and its relationship to and frequency of interaction with host jellyfish is needed, as the blue crab represents a commercially valuable fishery in the Gulf of Mexico and along the Atlantic Coast of the USA.
Understanding the nature of the relationships between economically valuable species of Crustacea and common scyphozoans and hydrozoans can improve fisheries practices and regulation, as already acknowledged for economically important fish and their jellyfish hosts (Tilves et al., 2018). The importance of maintaining juvenile communities for commercially sized adult populations to recruit from is well established and a frequent impetus for marine protection areas. The fishing of medusae is different from most modern vertebrate fishing. It is temporally highly variable, and blooms, when found, are fished as intensely as possible by local fishermen. It is also comparatively new as an export industry, especially in Southeast Asia (Omori & Nakano, 2001). Additional regulation and management should be considered for jellyfish species known to harbor juveniles of commercially viable crustaceans. It is clear that many crustaceans, fish, and other organisms live in, upon and around medusae, thus indiscriminate efforts to remove or destroy blooms of endemic species are likely unwise (Tilves et al., 2018; Riascos et al., 2018).
Conclusion
Many of the interactions we reviewed are fragmented and not comprehensive. Studies covering timing and breadth of infection of commercially valuable crustaceans on marine scyphozoans are scarce, but may be valuable information to fully understand the complexity of their life cycle, and thus the species’ vulnerability at each life cycle stage. The general picture of the commensal relationships that arise from this review is complex and emphasizes the diversity of jellyfish and crustaceans’ relationships. Any attempt to paint them as uniformly parasitic fails to acknowledge the diversity of crustacean host-use strategies. While some seem to be parasitic or parasitoid, others are life-stage dependent commensals reliant on medusae for transportation. Some deep water crustaceans may be lifelong commensals (Gasca, Suárez-Morales & Haddock, 2007). In each of these cases, the work thus far is far from exhaustive. Additional research on seasonality, maternal care, territoriality, impact on host and other such matters should be further pursued.
The scyphozoans and hydrozoans studied here represent only a small proportion of the globally recognized species. Even shallow water coastal species are poorly covered. This research has been restricted to a small selection of near-shore sites over the past 50 years, leaving inadequate coverage even in regions with a significant scyphozoan research presence (i.e., the Mediterranean, western Europe, China, northeastern North America). Because much of the published research focused on single occurrences, this paper’s overall results do not necessarily capture the broader ecology of the species involved (Bowman, Meyers & Hicks, 1963; Jachowski, 1963; Suzuki, 1965; Ohtsuka et al., 2011). Similarly, species descriptions that mention an association without details on the conditions in which it was found offer little insight on the frequency and ecological role of such interactions (Humes, 1953; Reddiah, 1968; Bruce, 1972; Criales, 1984; Bruce, 1988; Bruce, 1995; Bruce, 2008).
Best practices moving forward should include some of the following elements: in situ imaging pre-collection, observations on medusa health, analysis of epibiont gut contents when possible, preferential use of non-destructive collection methods, observations on symbiont placement within or upon the medusa, and frequency, geographical and temporal variation of the association.
With this review, we hope to highlight a significant knowledge gap and a lack of formal study on the ecology of the crustaceans residing on and around jellyfish, as well as a glimpse of the ecological complexity of these interactions. We provide easy access to a century of ecological research and a framework for analyzing and contextualizing future research on this topic.
Supplemental Information
Expanded medusa crustacean association table.
Every association in all reviewed papers with details on species and higher order classification of host, species of associate, sex and life stage of associate, notes on association, location on host, location association was recorded, date of record, depth of association and literature source. Expanded to include higher taxon labels for both crustaceans and medusae.