A century of waiting: description of a new Epictia Gray, 1845 (Serpentes: Leptotyphlopidae) based on specimens housed for more than 100 years in the collection of the Natural History Museum Vienna (NMW)
- Published
- Accepted
- Received
- Academic Editor
- Michael Wink
- Subject Areas
- Biodiversity, Taxonomy, Zoology
- Keywords
- Burrowing snakes, Epictinae, Thread snakes, Fossorial snakes, Slender blind snakes, Systematics, High resolution x-ray tomography, Osteology, Pholidosis, Squamata
- Copyright
- © 2019 Koch et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2019. A century of waiting: description of a new Epictia Gray, 1845 (Serpentes: Leptotyphlopidae) based on specimens housed for more than 100 years in the collection of the Natural History Museum Vienna (NMW) PeerJ 7:e7411 https://doi.org/10.7717/peerj.7411
Abstract
We describe a new species of Epictia based on eight specimens from Nicaragua collected and housed in the collection of the Natural History Museum Vienna for more than a century. The species differs from the congeners by the combination of external morphological characters: midtail scale rows 10; supralabials two, anterior one large and in broad contact with supraocular; infralabials four; subcaudals 14–19; middorsal scale rows 250–267; supraocular scales present; frontal scale distinct; striped dorsal color pattern with more or less triangular dark blotches on each scale; small white blotch in anterior part of dorsal surface of rostral present in five out of six specimens (two further specimens are lacking their heads); terminal spine and adjacent scales white. Eidonomic species separation from other Epictia spp. is also supported by a few qualitative and quantitative differences in vertebrae count and morphology. The new species is putatively assigned to the Epictia phenops species group based on external morphological characters and distribution.
Introduction
The fossorial threadsnakes of the family Leptotyphlopidae represent about 140 currently recognized species (Uetz, Freed & Hošek, 2019) that occur along sub-Saharan Africa and nearby islands, the Arabian Peninsula, in southwest Asia (Leptotyphlopinae and Rhinoleptini) and in the New World (Americas and Antilles) (Adalsteinsson et al., 2009). Despite their wide typical Gondwanan distribution, members of this family still account for one of the least known terrestrial vertebrates (Adalsteinsson et al., 2009). This is mostly due to their secretive habits, which make them rarely encountered in the field, except for a few locally abundant species (McDiarmid, Campbell & Touré, 1999; Curcio, Zaher & Rodrigues, 2002; Passos, Caramaschi & Pinto, 2005). Additionally, the systematics of this group is very controversial mostly due to their relatively conserved external morphology (Passos, Caramaschi & Pinto, 2005; Passos, Caramaschi & Pinto, 2006; Pinto & Curcio, 2011; Martins, Passos & Pinto, 2018). The genus Epictia is the most speciose amongst the subfamily Epictinae, with about 43 species currently recognized (Uetz, Freed & Hošek, 2019). Although subjected to a few systematic studies in recent years (e.g., McCranie & Hedges, 2016; Wallach, 2016) members of this genus still account for several taxonomical issues, which are far from being satisfactorily resolved. Additionally, several species have been described in the past 10 years (e.g., Arredondo & Zaher, 2010; Koch, Venegas & Böhme, 2015; Koch, Santa Cruz & Cárdenas, 2016; Wallach, 2016) reinforcing the need for systematic studies of the group in order to evaluate the presence of new and undescribed taxa in the Neotropical region.
While reviewing specimens of the family Leptotyphlopidae from the herpetological collection of the Natural History Museum Vienna, Austria (NMW), the first author came across a series of eight specimens (NMW 15446:1–8) of threadsnakes, that were assigned by Steindachner, a former curator of the collection, to Epictia albifrons (Wagler, 1824). Steindachner himself donated these specimens to the museum in 1907 and the locality was stated as “Corinto”. Unfortunately the original description of E. albifrons is rather poor, the holotype (ZSM 1348/0) was destroyed during the Second World War and according to Da Cunha & Do Nascimento (1993), Wallach, Williams & Boundy (2014), Pinto, Franco & Hoogmoed (2018) the locality data (“in adjacentibus Urbis Para” = in the proximity of Pará, Brazil) could be erroneous. Furthermore, the species has been confused with E. goudotii (Duméril & Bibron, 1844) in some earlier literature (McCranie & Hedges, 2016). Although a molecular analysis by Adalsteinsson et al. (2009) demonstrated that E. albifrons and Epictia goudotii represent two genetically distinct species, the validity of E. albifrons is still under debate (McCranie & Hedges, 2016). It is recognized by some authors as a valid species (e.g., Peters & Orejas-Miranda, 1970; Wallach, Williams & Boundy, 2014; Natera Mumaw, Esqueda González & Castelaín Fernández, 2015; Wallach, 2016; Murphy, Rutherford & Jowers, 2016), while other authors consider E. albifrons as a nomen dubium and place it in the synonymy of Epictia tenella Klauber, 1939 (e.g., Wilson & Hahn, 1973; Franco & Pinto, 2009). In contrast some authors consider E. tenella as a junior synonym of E. albifrons (e.g., Thomas, 1965; Hoogmoed & Gruber, 1983). Natera Mumaw, Esqueda González & Castelaín Fernández (2015) designated a neotype for E. albifrons (MCZ R-2885) from the vicinity of “Pará, Brasil” which was rejected by Wallach (2016) who designate the topotype BYU 11490 from the vicinity of Belém, Pará State, Brazil, as the neotype of E. albifrons. According to Wallach (2016) E. albifrons is restricted to the vicinity of the topotype series in northeastern Brazil, while E. tenella is a relatively widespread species in cis-Andean South America. According to his (and former) diagnosis the main difference between both species is the presence (E. tenella) or absence (E. albifrons) of a contact between supraoculars and the anterior supralabial. Pinto, Franco & Hoogmoed (2018) state that the neotype proposed by Natera Mumaw, Esqueda González & Castelaín Fernández (2015) is valid, whereas Wallach’s (2016) designation of a neotype was an act against the ICZN (1999) code. They further disagree with Wallach (2016) regarding the absence of a supralabial-supraocular contact in E. albifrons and consider this contact to be present in this species as proposed by Natera Mumaw, Esqueda González & Castelaín Fernández (2015). Whether E. albifrons and E. tenella represent two distinct or the same species is not conclusively clarified. We herein treat both species as being valid. However, after the analysis of both external and internal morphological characters, we verified that the specimens donated by Steindachner do neither represent E. albifrons nor E. tenella, but pertain to a new Epictia species (see data in ‘Results’). Apart from the unequal distribution the new species differs from both species (sensu Wallach, 2016 and Natera Mumaw, Esqueda González & Castelaín Fernández, 2015) by having a higher number of middorsal scale rows (see ‘Comparison’).
Materials & Methods
We compared the new species with all congeners in the genus Epictia that are currently recognized by at least some authors as valid species (see comments in Koch, Santa Cruz & Cárdenas, 2016). Therefore, we examined 392 specimens (see Appendix) representing 56 species of South American and Mesoamerican Leptotyphlopidae from the following collections: American Museum of Natural History, New York, USA (AMNH), Natural History Museum, London, UK (BMNH), Centro de Ornitología y Biodiversidad, Lima, Peru (CORBIDI), Coleção Herpetológica da Universidade de Brasília, Brasilia, Brazil (CHUNB), Field Museum of Natural History, Chicago, USA (FMNH), Fundación Miguel Lillo, San Miguel de Tucumán, Argentina (FML), Instituto de Bio y Geociencias del Noroeste Argentino, Rosario de Lerma, Argentina (IBIGEO), Instituto Butantan, Sao Paulo, Brazil (IBSP), Museum für Naturkunde, Berlin, Germany (ZMB), Natural History Museum of Los Angeles, Los Angeles, USA (LACM), Laboratório de Zoologia de Vertebrados, Universidade Federal de Ouro Preto, Oure Preto, Brazil (LZV), Museum für Tierkunde in Dresden, Dresden, Germany (MTKD), Museum of Comparative Zoology, Cambridge, USA (MCZ), Muséum National d’Histoire Naturelle, Paris, France (MNHN), Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima, Peru (MUSM), Museu Nacional, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (MNRJ), Museu de Zoologia da Universidade de São Paulo, Sao Paulo, Brazil (MZUSP), Museu Paraense Emílio Goeldi, Belém, Brazil (MPEG), Museo de Historia Natural de la Universidad Nacional de San Agustín, Arequipa, Peru (MUSA), Natural History Museum, University of Kansas, Lawrence, USA (KU), Oklahoma Museum of Natural History, Norman, USA (OMNH), Natural History Museum Vienna, Vienna, Austria (NMW), Museo de Zoología, Pontificia Universidad Católica del Ecuador, Quito, Ecuador (QCAZ), Senckenberg Museum Frankfurt, Frankfurt, Germany (SMF), San Diego Museum of Natural History, San Diego, USA (SDMNH), National Museum of Natural History, Washington, USA (USNM), Coleção Zoológica da Universidade Federal do Mato Grosso, Cuiabá, Brazil (UFMT), Illinois Natural History Survey, Champaign, USA (UIMNH), Zoologische Staatssammlung München, Munich, Germany (ZSM), and Zoologisches Forschungsmuseum Alexander Koenig, Bonn, Germany (ZFMK). In addition, we reviewed data on all known species in the genus Epictia from the following literature sources (original species descriptions and others): Wagler (1824), Schlegel (1839), Duméril & Bibron (1844), Peters (1857), Jan (1861), Cope (1875), Werner (1901), Oliver (1937), Klauber (1939), Taylor (1940), Schmidt & Walker (1943), Smith (1943), Smith & Laufe (1945), Legler (1959), Orejas-Miranda (1961), Orejas-Miranda (1964), Orejas-Miranda (1969), Freiberg & Orejas-Miranda (1968), Peters & Orejas-Miranda (1970), Orejas-Miranda & Zug (1974), Hoogmoed (1977), Zug (1977), Laurent (1984), Lancini & Kornacker (1989), Villa (1990), Vanzolini (1996), Lehr et al. (2002), Duellman (2005), Kretzschmar (2006), Börschig (2007), Boundy & Wallach (2008), Arredondo & Zaher (2010), Pinto et al. (2010), McCranie (2011), Francisco, Pinto & Fernandes (2012), Francisco, Pinto & Fernandes (2018), Esqueda González et al. (2015), Koch, Venegas & Böhme (2015), Koch, Santa Cruz & Cárdenas (2016), Natera Mumaw, Esqueda González & Castelaín Fernández (2015), Martins (2016), McCranie & Hedges (2016), Murphy, Rutherford & Jowers (2016) and Wallach (2016). Data on other South American Leptotyphlopidae were obtained from: Pinto & Curcio (2011), Pinto & Fernandes (2012), Pinto & Fernandes (2017) and Salazar-Valenzuela et al. (2015).
Measurements were taken with a digital caliper to the nearest 0.1 mm or with a ruler to the nearest 1.0 mm. The following abbreviations were used: SVL = snout-vent length, TAL = tail length, TL = total length, HW = head width at largest area (level of the parietal scale), HH = head height at highest point, HL = head length from tip of snout to posterior level of skull, (when feeling it tapering to the neck), ED = eye diameter, RES = relative eye size (eye height/ocular shield height), MB = midbody diameter, MT = midtail diameter, MDS = middorsal scale rows from the rostral scale to the terminal spine, V = number of ventral scales in longitudinal row from mental to cloacal shield, D = number of scales around the body counted at three different points along the body (1. at a head’s length behind the head, 2. at midbody, 3. at a head’s length before the cloaca), SC = number of subcaudal scales counted in longitudinal row from cloaca to tip of tail, TS = number of midtail scale rows counted transversely across the middle of the tail, SL = number of supralabials, IF = number of infralabials, PCV = precloacal vertebrae, CAV = caudal vertebrae, CLV = cloacal vertebrae.
Terminology for cephalic plates, scale features, and measurements follows Wallach (2003), Broadley & Wallach (2007), Arredondo & Zaher (2010) and Francisco, Pinto & Fernandes (2012).
For obtaining information on the number of precloacal and caudal vertebrae and the position of the pelvic girdle, whole specimens were X-rayed in 2D outside of ethanol with a Faxitron X-ray LX60 at ZFMK. For details of the skull and lower jaw, cervical vertebrae and pelvic girdle, the head, midbody and cloacal region of each specimen were X-rayed in 3D by the use of a high-resolution micro-CT scanner (Bruker SkyScan 1173) at ZFMK. Therefore bodies of specimens were placed in a tube filled with ethanol and only the regions of interest were sticking out of the ethanol and were CT-scanned (head and cloacal region were scanned together at the same time). Specimens were CT-scanned in 180° degrees at rotation steps of 0.3° or 0.4° degrees with a tube voltage of 40 kV and a tube current of 200 µA without the use of a filter at an image resolution of 8.4 µm. Scan duration was between 25 min (rotation steps of 0.4°) and 42 min (rotation steps of 0.3°) with an exposure time of 359 ms. The CT-dataset was reconstructed using N-Recon software (Bruker MicroCT) and rendered in three dimensions through the aid of CTVox for Windows 64 bits version 2.6 (Bruker MicroCT). We have also used osteological data from Martins (2016) and (A Martins, 2016, unpublished data), specimens cited in the APPENDIX—Additional specimens examined) to provide additional species comparisons. Anatomical terminology follows Romer (1956), List (1966) and Holman (2000) [atlas and axis], List (1966) [pelvic girdle], Rieppel (1979) [parabasisphenoid complex], Kley (2006) and Martins (2016) [suspensorium], Cundall & Irish (2008), Rieppel, Kley & Maisano (2009), Martins (2016) and (A Martins, 2016, unpublished data) [skull], Curcio (2003) and Rieppel, Kley & Maisano (2009) [skull foramina].
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Commission on Zoological Nomenclature (ICZN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: urn:lsid:zoobank.org:pub:C2392EA5-9957-45BF-AE8C-0998E342F90B. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central and CLOCKSS.
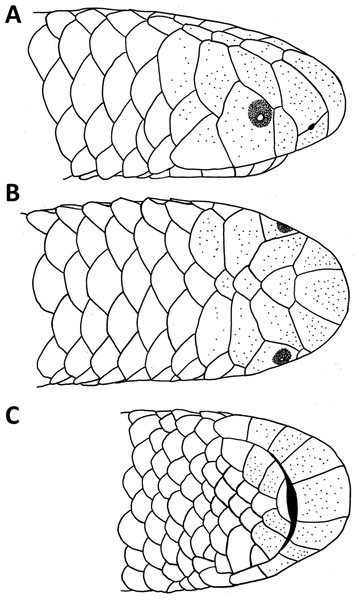
Figure 1: Drawings of the head of the holotype of Epictia rioignis sp. nov. (NMW 15446:6).
(A) Lateral, (B) dorsal and (C) ventral views.Results
Epictia rioignis sp. nov. (Figs. 1–15, Table 1)
urn:lsid:zoobank.org:act:4C7CFC14-FDC7-4B41-B7CF-7CCB8F0E8649
Holotype
NMW 15446:6, from Corinto, presumably Nicaragua (12°29′N, 87°11′W, see Discussion for further details) donated by Steindachner in 1907.
Paratypes (7)
NMW 15446:1–5, NMW 15446:7–8 from the type locality, donated by Steindachner in 1907.
Diagnosis
Epictia rioignis sp. nov. can be distinguished from all congeners by the following combination of characters: (1) midbody scale rows 14; (2) midtail scale rows 10; (3) supralabials two, anterior one large and in broad contact with supraocular; (4) infralabials four; (5) subcaudals 14–19; (6) middorsal scale rows 250–267; (7) total number of precloacal vertebrae 231–248; (8) supraocular scales present; (9) frontal scale distinct, not fused with rostral; (10) striped dorsal color pattern with more or less triangular dark blotches on each scale; (11) upper half of eyes visible in dorsal view; (12) some caudals in posterior part of tail are fused in 50% of the specimens; (13) small white blotch in anterior part of dorsal surface of rostral present in about 83% of the specimens; (14) terminal spine and adjacent scales white.
Figure 2: Holotype of Epictia rioignis. sp. nov. (NMW 15446:6).
(A) Dorsal and (B) ventral or lateral views.Figure 3: Comparison of dorsal views of the heads of holotype (NMW 15446:6, A) and paratypes of Epictia rioignis sp. nov. (B–F).
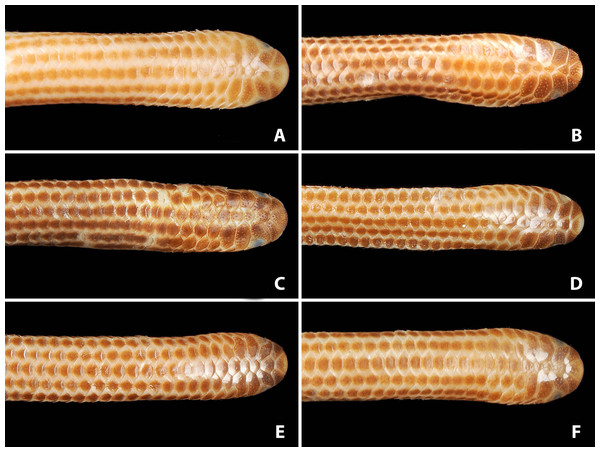
(A) Holotype (NMW 15446:6). (B) Paratype (NMW 15446:2). (C) Paratype (NMW 15446:4). (D) Paratype (NMW 15446:5). (E) Paratype (NMW 15446:7). (F) Paratype (NMW 15446:8). Photo credits: Alice Schumacher & Josef Muhsil.Figure 4: Comparison of lateral views of the heads of holotype (NMW 15446:6, A) and paratypes of Epictia rioignis sp. nov. (B–F).
(A) Holotype (NMW 15446:6). (B) Paratype (NMW 15446:2). (C) Paratype (NMW 15446:4). (D) Paratype (NMW 15446:5). (E) Paratype (NMW 15446:7). (F) Paratype (NMW 15446:8). Photo credits: Alice Schumacher & Josef Muhsil.Comparisons
The new species differs from Epictia albipuncta, Epictia striatula, Epictia unicolor and Epictia weyrauchi by having 10 midtail scale rows [vs. 12]. The number of 14 midbody scale rows distinguishes the species from E. undecimstriata [16]. By the presence of an unfused frontal and rostral scale it is differentiated from Epictia ater (including Epictia nasalis), Epictia bakewelli and Epictia schneideri. By having the anterior supralabial in broad contact with the supraocular the new species can be distinguished from E. albipuncta, Epictia amazonica, E. ater, Epictia australis, E. bakewelli, Epictia borapeliotes, Epictia clinorostris, Epictia collaris, Epictia columbi, Epictia diaplocia, Epictia fallax, Epictia goudotii, Epictia magnamaculata, Epictia martinezi, Epictia melanura, Epictia munoai, Epictia pauldwyeri, Epictia peruviana, Epictia phenops, Epictia resetari, E. schneideri, Epictia signata, Epictia subcrotilla, Epictia vellardi, Epictia vindumi, and Epictia wynni [vs. anterior supralabial and supraocular separated by supranasal-ocular contact]. The number of 250–267 middorsal scale rows distinguishes Epictia rioignis sp. nov. from E. albifrons [206–218 sensu Wallach, 2016; 242 sensu Natera Mumaw, Esqueda González & Castelaín Fernández, 2015], Epictia alfredschmidti [267–279], E. amazonica [208–245], Epictia antoniogarciai [195–208], E. collaris [155–166], E. diaplocia [205–233], Epictia hobartsmithi [191–208], E. melanura [395–396], E. munoai [184–226], E. pauldwyeri [202–226], E. peruviana [185–199], E. subcrotilla [318–333], Epictia tenella [215–233 sensu Wallach, 2016 ], Epictia tricolor [276–310], E. unicolor [246], Epictia vanwallachi [188], Epictia venegasi [211–221], and Epictia vonmayi [196–205]. The number of 14–19 subcaudal scales differentiates this species from E. columbi [22–25], E. munoai [10–14], E. nasalis [21], and E. pauldwyeri [10–14]. By the presence of four infralabials [vs. three] Epictia rioignis sp. nov. differs from E. australis, E. borapeliotes, E. collaris, E. munoai, and E. wynni. The presence of a light blotch on the dorsal part of the rostral further differentiates the new species from E. columbi, Epictia rufidorsa, E. vanwallachi, and E. weyrauchi, and the presence of a whitish terminal spine distinguishes it from E. columbi, E. melanura, Epictia melanoterma, E. rufidorsa, and Epictia septemlineata. By lacking a tricolor pattern (reddish-brown, black, yellow) it differs from E. alfredschmidti, Epictia rubrolineata, Epictia teaguei, and E. tricolor. By lacking a preoral groove in the ventral rostral it differs from E. columbi. The presence of a distinct striped dorsal color pattern with more or less triangular dark blotches on each scale distinguishes the new species from E. amazonica [uniformly black coloration, without any trace of stripes], E. ater, and E. columbi [both species appear uniformly dark, pale outline of the scales is only visible upon closer examination]. From Epictia tesselata which is only known from Lima (Peru) and surroundings, the new species differs by having a very small light blotch on the rostral [light spot on the rostral and lower portion of the nasals] and a darker ventral coloration. It differs from E. ater and E. phenops by presenting unfused neural arches of the atlas [vs. fused]. The number of 231–248 trunk vertebrae distinguishes it from E. magnamaculata [199], E. munoai [207], E. phenops [213–246], E. tenella [190–204], and E. tricolor [282].
Description of holotype (Figs. 1 and 2, and upper left picture of Figs. 3–9)
A large specimen with TL of 211 mm; TAL of 10.3 mm; MB of 4.4 mm; MT of 3.1 mm; TL/TAL of 20.5; TL/MB of 48; TAL/MT of 3.3; HW of 2.8 mm; HL of 3.6 mm; HH of 2.3 mm; ED of 0.4 mm; RES of 0.3. Head subcylindrical, slightly dorsoventrally compressed, hardly distinguishable from neck; body cylindrical; not tapered cranially or caudally. Snout rounded in dorsal, lateral and ventral views. Rostral subtrapezoidal in dorsal view with straight apex, straight in ventral view, reaching the imaginary transverse line between the anterior borders of the eyes, contacting upper and lower nasals laterally and frontal dorsally.
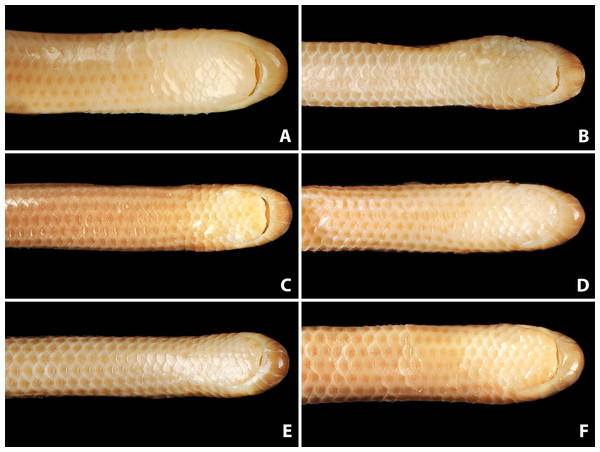
Figure 5: Comparison of ventral views of the heads of holotype (NMW 15446:6, A) and paratypes of Epictia rioignis sp. nov. (B–F).
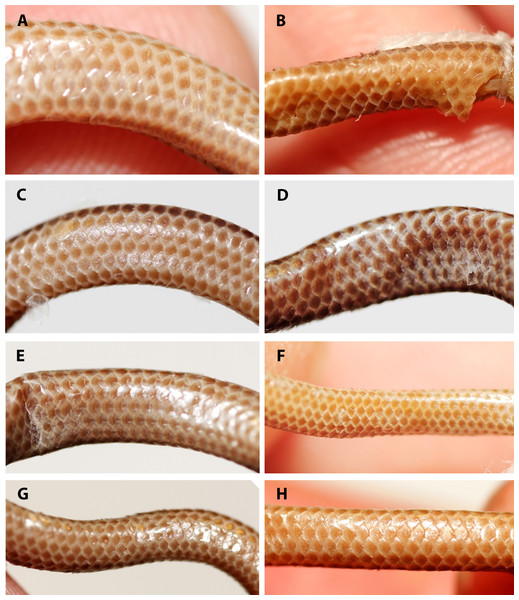
(A) Holotype (NMW 15446:6). (B) Paratype (NMW 15446:2). (C) Paratype (NMW 15446:4). (D) Paratype (NMW 15446:5). (E) Paratype (NMW 15446:7). (F) Paratype (NMW 15446:8). Photo credits: Alice Schumacher & Josef Muhsil.Figure 6: Comparison of dorsal body scales of holotype (NMW 15446:6, A) and paratypes of Epictia rioignis sp. nov. (B–H).
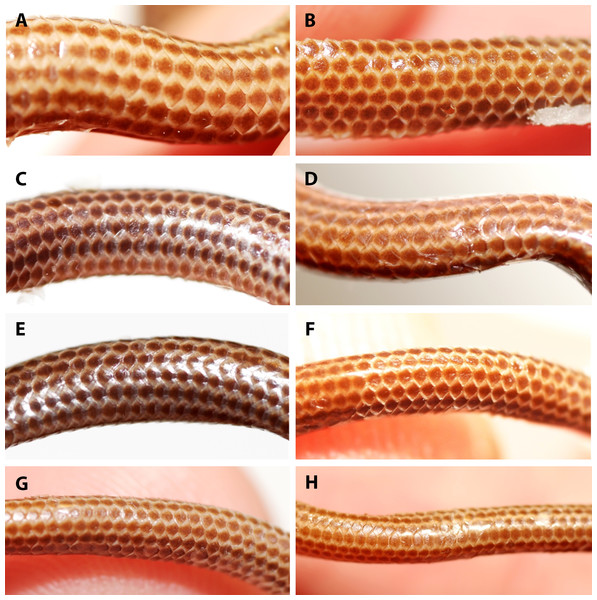
(A) Holotype (NMW 15446:6). (B) Paratype (NMW 15446:1). (C) Paratype (NMW 15446:2). (D) Paratype (NMW 15446:3). (E) Paratype (NMW 15446:4). (F) Paratype (NMW 15446:5). (G) Paratype (NMW 15446:7). (H) Paratype (NMW 15446:8).Figure 7: Comparison of ventral body scales of holotype (NMW 15446:6, A) and paratypes of Epictia rioignis sp. nov. (B–H).
(A) Holotype (NMW 15446:6). (B) Paratype (NMW 15446:1). (C) Paratype (NMW 15446:2). (D) Paratype (NMW 15446:3). (E) Paratype (NMW 15446:4). (F) Paratype (NMW 15446:5). (G) Paratype (NMW 15446:7). (H) Paratype (NMW 15446:8). Photo credits: Alice Schumacher & Josef Muhsil.Figure 8: Comparison of dorsal view of tails of holotype (NMW 15446:6, A) and paratypes of Epictia rioignis sp. nov. (B–H).
(A) Holotype (NMW 15446:6). (B) Paratype (NMW 15446:1). (C) Paratype (NMW 15446:2). (D) Paratype (NMW 15446:3). (E) Paratype (NMW 15446:4). (F) Paratype (NMW 15446:5). (G) Paratype (NMW 15446:7). (H) Paratype (NMW 15446:8). Photo credits: Alice Schumacher & Josef Muhsil.Nasal completely divided horizontally by an oblique suture, reaching rostral and first supralabial; ellipsoid nostril located in the center of the suture between upper and lower nasal, having the major axis obliquely oriented along the suture; supranasal higher than wide, contacting rostral anteriorly, infranasal inferiorly, first supralabial and supraocular posteriorly, and frontal dorsally; infranasal not visible in dorsal view, contacting rostral anteriorly and first supralabial posteriorly; two supralabial scales positioned anterior and posterior to ocular scale (1 + 1), respectively, resulting in an upper lip border formed by rostral, infranasal, anterior supralabial, ocular, and posterior supralabial; first supralabial 2.2 times higher than wide, exceeding nostril, reaching central level of eye, dorsally acuminate and in contact with supraocular scale; second supralabial subtrapezoidal 1.3 times higher than wide, slightly exceeding central level of eye, about as high as and at widest point 1.7 times wider than first supralabial; posterior margin of second supralabial in broad contact with temporal and first scale of lateral body row, dorsal margin in contact with parietal; temporal scale of same size as dorsal scales of lateral rows, but distinct from lateral body scales by its oblique orientation; ocular scale pentagonal with dorsal apex acuminate and anterior border slightly rounded at eye-level, 1.8 times higher than wide, contacting anteriorly first supralabial, anterodorsally supraocular, posterodorsally parietal and dorsally second supralabial; eye distinct, located at level of maximum width of ocular, with lower eye margin at nostril level, positioned anteriorly and almost contacting scale sutures; upper half of eyes visible in dorsal view; supraocular scale oriented obliquely, about twice as long as wide, contacting supranasal anteriorly, parietal and postfrontal posteriorly, frontal dorsally, and first supralabial inferiorly; supraocular, parietal and occipital scales visible in lateral view; middorsal head plates (frontal, postfrontal, interparietal, and interoccipital) imbricate, subhexagonal, except for subtriangular frontal, with frontal and interoccipital being slightly larger than the other two scales; middorsal head plates narrower than posterior middorsal scales; frontal contacting rostral, supranasals, supraoculars, and postfrontal; postfrontal contacting frontal, supraoculars, parietals, and interparietal; interparietal contacting postfrontal, parietals, occipitals, and interoccipital; interoccipital contacting interparietal, occipitals, nuchal and first pair of paravertebral dorsal scales; parietal slightly larger than occipital, both irregularly hexagonal and about twice as high as wide; lower margin of parietal contacting upper border of posterior supralabial and temporal, posterior margin in broad contact with occipital, dorsal margin contacting postfrontal and interparietal, anterior margin in broad contact with ocular and supraocular; lower margin of occipital contacting temporal and first scale of lateral body row, posterior margin in broad contact with first paravertebral and first scale of dorsolateral body row, dorsal margin in contact with interparietal and interoccipital, anterior margin in broad contact with parietal; four infralabials per side, subequal in size, first three higher than wide, fourth wider than high, first two pairs of infralabials almost rectangular, larger than third infralabials; mental scale single, small, lunulate; labials, chin and gular scales, and dorsal and lateral head scales with numerous scattered pores.
Figure 9: Comparison of ventral view of tails of holotype (NMW 15446:6, A) and paratypes of Epictia rioignis sp. nov. (B–H).
(A) Holotype (NMW 15446:6). (B) Paratype (NMW 15446:1). (C) Paratype (NMW 15446:2). (D) Paratype (NMW 15446:3). (E) Paratype (NMW 15446:4). (F) Paratype (NMW 15446:5). (G) Paratype (NMW 15446:7). (H) Paratype (NMW 15446:8).Figure 10: Three-dimensional reconstruction of the skull of Epictia rioignis sp. nov. based on Micro-CT data of the holotype (NMW 15446:6).
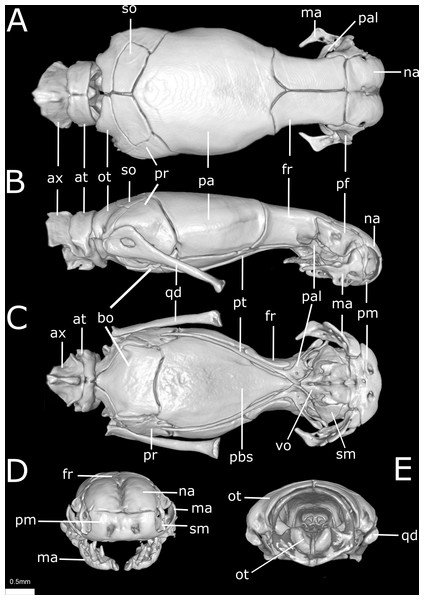
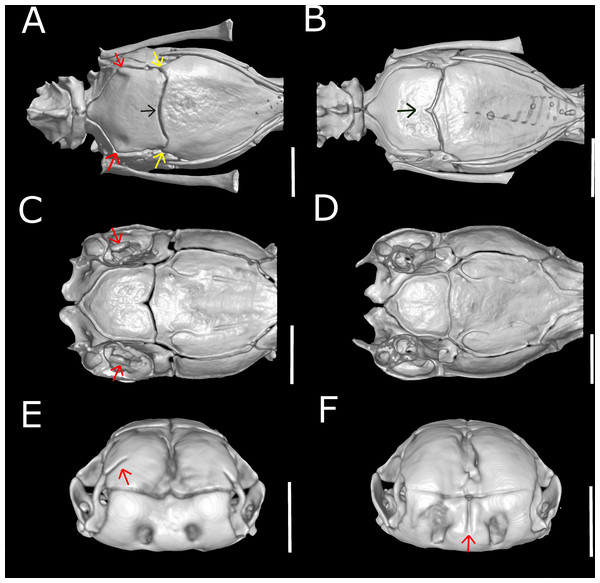
(A) Dorsal, (B) lateral, (C) ventral, (D) anterior, and (E) posterior views. at, atlas; ax, axis; bo, basioccipital; fr, frontal; ma, maxilla; na, nasal; ot, otooccipital; pa, parietal; pal, palatine; pbs, parabasisphenoid; pf, prefrontal; pm, premaxilla; pr, prootic; pt, pterygoid; qd, quadrate; sm, septomaxilla; so, supraoccipital; vo, vomer.Figure 11: Three-dimensional cutaway views of the skull of Epictia rioignis sp. nov. based on Micro-CT data of the holotype (NMW 15446:6).
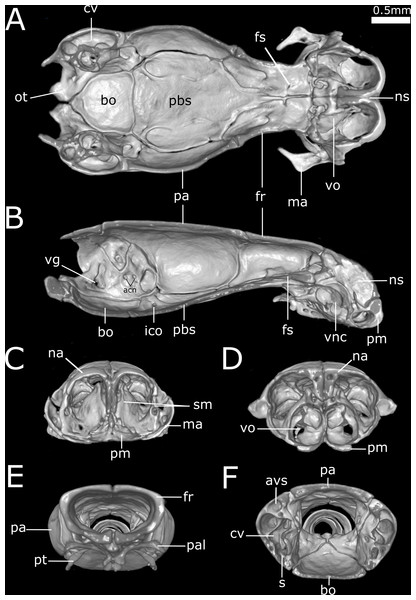
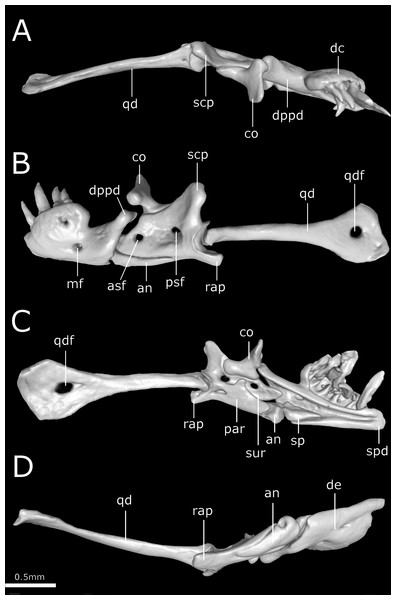
Cuts along the (A) frontal, (B) sagittal and (C–F) transverse axis. acn, acoustic nerve; avs, anterior vertical semicircular canal; bo, basioccipital; cv, cavum vestibuli; fs, frontal subolfactory process; fr, frontal; ico, internal carotid opening; ma, maxilla; na, nasal; ns, nasal septum; ot, otooccipital; pa, parietal; pal, palatine; pbs, parabasisphenoid; pm, premaxilla; pt, pterygoid; s, stapes; sm, septomaxilla; vg, vagus nerve foramen; vnc, vomeronasal cupola; vo, vomer.Figure 12: Three-dimensional reconstruction of the suspensorium (quadrate + lower jaw) of Epictia rioignis sp. nov. based on Micro-CT data of the holotype (NMW 15446:6).
(A) Dorsal, (B) lateral, (C) medial, and (D) ventral views. an, angular; asf, anterior surangular foramen; co, coronoid; dc, dental concha; de, dentary; dppd, dorsoposterior process of dentary; mf, mental foramen; par, prearticular lamina of compound bone; psf, posterior surangular foramen; qd, quadrate; qdf, quadrate foramen; rap, retroarticular process; scp, supracotylar process of surangular; sp, splenial; spd, symphyseal process of dentary; sur, surangular lamina of compound bone.Dorsal scales imbricate, smooth, homogeneous, rhomboid or elliptical in shape, about 1.5 times wider than long; 267 MDS; 14-14-14 D; 258 V; 10 TS. Cloacal shield large, subtriangular in shape, about 2.2 times wider than long, bordered anteriorly and posteriorly each by five scales; 15 SC, becoming successively narrower distally, no fused scales dorsally or ventrally on tail; terminal spine conical and shorter than wide.
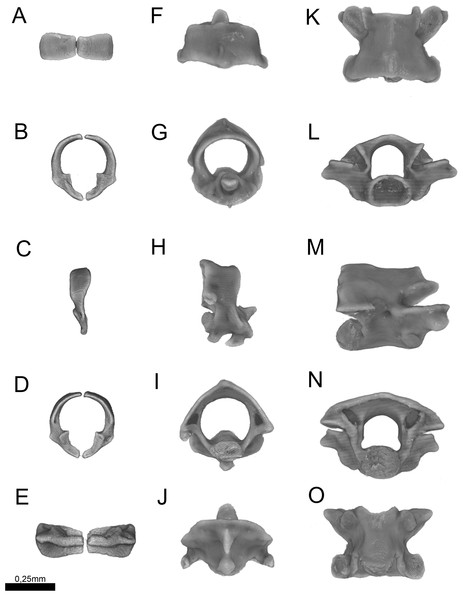
Figure 13: Three-dimensional reconstruction of the (A–E) atlas, (F–J) axis and (K–O) midtrunk vertebrae of Epictia rioignis sp. nov. based on Micro-CT data of the holotype (NMW 15446:6).
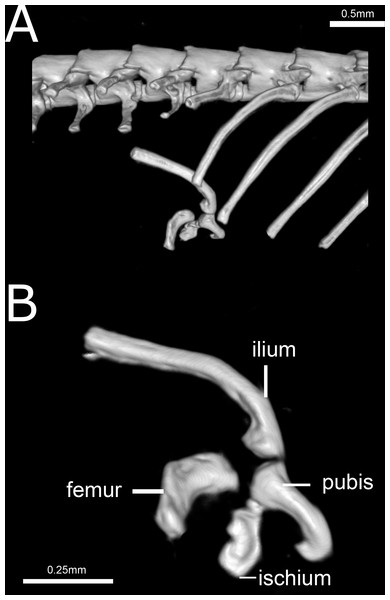
(A, F, K) Dorsal, (B, G, L) anterior, (C, H, M) lateral, (D, I, N) posterior, and (E, J, O) ventral views.Figure 14: Three-dimensional reconstruction of the pelvic girdle of Epictia rioignis sp. nov. based on Micro-CT data of the holotype (NMW 15446:6).
(A) Overview with position and orientation. (B) Pelvic girdle digitally isolated in lateral view.Figure 15: Three-dimensional reconstruction of skulls of different specimens of Epictia rioignis sp. nov. based on Micro-CT data, showing variations in skull parameters.
(A) Ventral views showing shape variation of basioccipital of holotype (NMW 15446:6) lacking medial recess and (B) paratype (NMW 15446:7) with medial recess. (C) Cutaway views along the frontal axis of paratype (NMW 15446:7) with presence of statolythic mass and (D) absence in the holotype (NMW 15446:6). (E) Anterior views of paratype (NMW 15446:4) with lateral recess of nasal and (F) paratype (NMW 15446:8) with medial sulcus of premaxilla.Color of holotype after more than 100 years of preservation in ethanol (Fig. 2, and upper left picture of Figs. 3–9)
Dorsal head scales (supranasal, frontal, supraoculars, postfrontal, parietals, interparietal, occipitals, interoccipital) except for rostral, reddish-brown with cream-colored sutures; rostral reddish-brown in posterior part and with a small cream-colored blotch on the anterodorsal part, lower (ventral) part of rostral light greyish-brown; infranasal and anterior supralabial light reddish-brown; ocular scale mostly cream-colored; posterior supralabial light reddish-brown in upper half and cream-colored in lower half; ventral head scales (mental, infralabials, scales of chin and gular region) cream-colored; each dorsal body scale with a more or less triangular, median to dark reddish-brown blotch in the center of the scale and with cream-colored lateral margins, forming a pattern of dark longitudinal stripes with cream-colored interspaces, dark stripes slightly broader than interspaces, reaching to the penultimate scale of the tail; each ventral body scale, anal plate and scales on ventral part of tail light or median brown in central part and with cream-colored margins; terminal spine cream-colored.
Osteology of holotype
Skull (Figs. 10 and 11)
Premaxilla roughly rectangular in anterior view and hexagonal in ventral view, edentulous, pierced by six foramina (two in anterior view and four in ventral view); transverse process of premaxilla absent and vomerian process single; premaxilla with internal septum composed by two laminae that support the septum nasii dorsally, expanding posteriorly to fit medially in the septomaxilla (internally); nasals paired, approximately rectangular in dorsal view, being pierced by a pair of foramina in lateral border of contact with prefrontals (foramen for the apicalis nasi); an additional pair of foramina pierce the medial contact within both nasals; a single additional foramen pierces the anterior-medial region of the nasal dorsal lamina; nasal septum descending as double medial vertical flanges that contact the premaxilla, septomaxilla and vomer ventrally (internally); prefrontals paired, subtriangular in dorsal view, in contact with septomaxilla and maxilla ventrally; septomaxillae paired, complex in shape, expanding dorsally into the naris; conchal invagination absent; ascending process of premaxilla pierced by single large foramen; internally, dorsal surface of each septomaxilla pierced by a foramen, and with a medial deep sulcus that extends from its posterior to anterior regions; vomers paired, located midventral to vomeronasal cupola, bearing transversal arms, and with short posterior arms in contact with each other posteriorly; a pair of foramina pierce the ventral lamina of the vomer; frontals paired, nearly rectangular in dorsal view, the left element bearing short anterolateral projections to attach to prefrontals; frontal pillars absent; optic nerve restricted to lateral descending surface of frontals; maxilla edentulous, irregular in shape, pierced by four large foramina in lateral view, two in the dentigerous process of maxilla; posterior process of maxilla reaching the level of the optic nerve foramen; posterior orbital element absent; parietal single, wide, representing the largest bone of braincase; parietal internal pillars (sensu Martins, 2016) absent; parabasisphenoid arrow-like, with tapered anterior tip lying dorsally to palatine, and fitting in medial line of vomeronasal cupola; in ventral view, parabasisphenoid bearing posterior-lateral projections to provide insertion for the neck muscles (Martins, Passos & Pinto, 2019); parabasisphenoid with shallow pituitary fossa and lateral sulcus; anterior opening for the palatine artery indistinct or absent in parabasisphenoid dorsal (internal) surface, internal carotid artery foramen and abduscens nerve foramen present; opening for the palatine ramus of the facial nerve formed by the lateral edge of the parabasisphenoid and the ventral edge of the parietal; basioccipital single and approximately pentagonal in ventral view, bearing lateral process to attach tendons for the neck muscles (A Martins, 2016, unpublished data); basioccipital does not participate in the formation of the foramen magnum; supraoccipitals paired, approximately rectangular in dorsal view, pierced medially (internally) by a large endolymphatic foramen; prootics paired and triangular in lateral view; prootics forming the trigeminal nerve foramen together with the parietal; prootics pierced medially (internally) by two acoustic nerve foramina, and an additional foramen ventral to the former; statolythic mass in cavum vestibuli absent; stapedial footplate apparently not co-ossified with prootic; otooccipitals paired and irregular in dorsal view, descending to contact each other ventrally to exclude the basioccipital in the formation of the foramen magnum and forming a short but distinct atlantal process (sensu Cundall & Irish, 2008); medial surface (internal) of otooccipitals pierced by an internal opening for the recessus scalae timpani and a wide foramen that forms the internal opening for the vagus nerve foramen; a reduced foramen pierces the posterior (external) surface of the otooccipital, posterior to the external opening for the vagus nerve foramen; palatines paired and triradiate; anterior margin of maxillary process flexing ventrally; palatines pierced by a foramen in its ventral surface; pterygoids slender and rod-like, not contacting quadrate posteriorly, and not extending beyond the anterior margin of basioccipital; ectopterygoid indistinct.
| No. | MDS | V | PCV | SC | CAV | CLV | TL (mm) | TAL(mm) | MB(mm) | MT(mm) | HL(mm) | HW(mm) | HH(mm) | ED(mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMW 15446:1 | / | / | / | 14 | / | / | / | 9.3 | 3.4 | 2.6 | / | / | / | / |
| NMW 15446:2 | 250 | 237 | 231 | 19 | 20 | 4 | 113 | 7.7 | 2.6 | 1.8 | 2.6 | 2 | 1.3 | 0.4 |
| NMW 15446:3 | / | / | / | 19 | / | / | / | 9.2 | 3.4 | 2.8 | / | / | / | / |
| NMW 15446:4 | 261 | 243 | 235 | 17 | 18 | 4 | 149 | 8.2 | 3 | 2.4 | 2.9 | 2.2 | 1.9 | 0.5 |
| NMW 15446:5 | 262 | 245 | 246 | 18 | / | / | 110 | 5.9 | 2 | 1,7 | 2.4 | 2 | 1.4 | 0.4 |
| NMW 15446:6 | 267 | 258 | 248 | 15 | 17 | 4 | 211 | 10.3 | 4.4 | 3.1 | 3.6 | 2.8 | 2.3 | 0.4 |
| NMW 15446:7 | 265 | 248 | 240 | 18 | 14 | 5 | 157 | 7.9 | 2.7 | 2.2 | 2.7 | 2.2 | 1.6 | 0.4 |
| NMW 15446:8 | 260 | 252 | 238 | 18 | 21 | 3 | 157 | 9.3 | 2.8 | 2.1 | 2.9 | 2.4 | 1.8 | 0.4 |
Notes:
- CAV
-
caudal vertebrae
- CLV
-
cloacal vertebrae
- ED
-
eye diameter
- HH
-
head height
- HL
-
head length
- HW
-
head width
- MB
-
midbody diameter
- MDS
-
middorsal scale rows
- MT
-
midtail diameter
- PCV
-
precloacal vertebrae
- SC
-
subcaudals
- TAL
-
tail length
- TL
-
total length
- V
-
ventral scales
Suspensorium (Fig. 12)
Dentary supports a series of six teeth ankylosed to the inner surface of the anterolateral margin of dental concha; mental foramen nearly under the 6th tooth; splenial conical, visible in lateral view, representing smallest bone in lower jaw, extending from the level of the 5th tooth to contact the angular posterior; anterior mylohyoid foramen absent on splenial; posterior mylohyoid foramen on the ventral and dorsal surface of angular; angular conical, extending posteriorly to level of the posterior surangular foramen; compound bone pierced by two foramina in the surangular lamina which are approximately similar in size, anterior surangular foramen located anterior to the coronoid; foramen for the chorda tympani of the hyomandibular ramus of the facial nerve (VII) present; a small foramen pierces the retroarticular process in medial view; prearticular lamina of compound bone presenting dorsal process to support the coronoid; quadrate long and slender, about 50% of skull length, presenting a posterior process (sensu Martins, 2016); a dorsal foramen in the anterior half of quadrate absent.
Cervical vertebrae (Fig. 13)
Atlas composed by neural arches, not fused dorsally but fused ventrally to a reduced ventral element (intercentrum I sensu Holman, 2000); short lateral projections of the atlas are present; axis with short spinal process; lateral foramina of axis indistinct or absent; short lateral processes present. Odontoid process of axis osseous and sutured to axis, approximately losangular in anterior view, with an anterior tapered process; intercentra II and III ventral, compressed laterally, bearing hypapophysis pointed in lateral view (sensu Holman, 2000).
Trunk vertebrae (Fig. 13)
Trunk vertebrae at midbody dorsoventrally flattened and bearing ribs; neural arches flattened; neural spines absent; spinal processes short and distinct; neural canal in anterior view about as high as wide; epyzygapophyseal spines absent; zygosphenes present, emerging from the neural canal; zygosphenal crests laterally expanded and tapered distally; zygosphenal articular facets absent; prezygosphenal articular facets ellipsoidal; prezygapophyses expand into prezygapophyseal accessory processes, distally rounded and visible in dorsal view; zygantrum wide laterally, limited by the relatively developed zygantral articular facets; postzygapophyses laterally expanded and flattened; cotyle ellipsoidal in anterior view; supracotilar and paracotylar foramina indistinct or absent; condyle ellipsoidal; vertebrae centrum bearing lateral foramen at each side; synapophyses with no distinction between diapophyseal and parapophyseal areas; hemal keel absent; subcentral ridges absent.
Pelvic girdle (Fig. 14)
Composed by ilium, ischium, femur, and pubis. Ilium and pubis rod-like; ischium approximately rectangular, apparently fused to pubis; ilium represents the longest bone of pelvic girdle; femur approximately rectangular and curved, slightly larger than ischium, located at between the last trunk vertebrae and second cloacal vertebrae.
Variation
The paratype series consists of seven specimens, two of which are lacking the head and anterior part of body (NMW 15446:1 and NMW 15446:3). Thus information of measurements and scalation of the tail and the coloration of body and tail is based on all specimens, whereas information on other characters (e.g head measurements, skull conditions) is based only on the complete specimens.
Variation of scale counts and measurements (see Table 1 for individual data of each specimen)
250–267 MDS (, n = 6); 237–258 V (, n = 6); 14–19 SC (, n = 8); TL of 110–211 mm (, n = 6); TAL of 5.9–10.3 mm (, n = 6); MB of 2–4.4 mm (, n = 8); MT of 1.7–3.1 mm (, n = 6); TL/TAL of 14.7–20.5 (, n = 6); TL/MB of 43.5–58.1 (, n = 6); TAL/MT of 3.3–4.3 (, n = 8); HW of 2–2.8 mm (, n = 6); HH of 0.4–0.5 mm (, n = 6); HL of 2.4–3.6 mm (, n = 6); ED of 1.3–2.3 mm (, n = 6); RES of 0.3–0.4 (, n = 6).
Variation of color pattern
Color pattern of paratypes mostly resembles that of the holotype except for the following (Figs. 3–9): no cream-colored blotch on anterodorsal part of rostral recognizable in one paratype (NMW 15446:4), cream-colored blotch on rostral indistinct in two paratypes (NMW 15446:7, NMW 15446:8), cream-colored blotch more distinct and larger than in holotype, covering more than half of rostral in dorsal view in one paratype (NMW 15446:5); ocular scale almost entirely light to median reddish-brown in all paratypes; posterior supralabial almost entirely reddish brown in one paratype (NMW 15446:4); some infralabials and some adjacent chin scales exhibit a light brown pigmentation in some paratypes; in some paratypes the cream-colored ultimate part of the tail covers also the scales adjacent to the terminal spine.
Qualitative and quantitative variation of skull and lower jaw (n = 6; holotype condition indicated with asterisk)
Dentary teeth 5 (n = 3; 50%) or 6* (n = 3; 50%); premaxilla pierced by two foramina in anterior view and two large foramina in ventral view (n = 1),three in ventral view and two in anterior view (n = 1) or two in anterior view and four in ventral view (n = 5*); medial sulcus of premaxilla present (n = 1) or absent* (n = 5); maxilla perforated by one (n = 1), three (n = 1), four* (n = 2), five (n = 1) or six (n = 1) foramina; projections for the attachment of neck muscles in parabasisphenoid absent (n = 5; Fig. 15B) or present* (n = 1; Fig. 15A, yellow arrows), projections for the attachment of neck muscles in basioccipital absent (n = 5; Fig. 15B) or present* (n = 1; Fig. 15A, red arrows); statolythic mass present (n = 3; Fig. 15C) or absent* (n = 3; Fig. 15D); basioccipital with medial recess (n = 2; Fig. 15B) or not* (n = 4; Fig. 15A, black arrow); lateral recess of nasal present (n = 1; Fig. 15E) or absent* (n = 5; Fig. 15F).
Postcranial quantitative variation
Precloacal vertebrae 231–248 (, n = 6); caudal vertebrae 14–21 (, n = 5). Correlation (n = 5) between middorsal scales and precloacal + cloacal + caudal vertebrae (0.99:1), between midventral scales and precloacal vertebrae (0.97:1), and between subcaudal scales and cloacal + caudal vertebrae (1:1.3). Pelvic girdle located at the level of the 229th (=penultimate) trunk vertebrae and first cloacal vertebrae (NMW 15446:2), 233–234th (=penultimate and last) trunk vertebrae (NMW 15446:4), 246th (=last) precloacal and second cloacal vertebrae (NMW 15446:5), 248th (= last) trunk and second cloacal vertebrae (NMW 15446:6), 240th (=last) trunk and first cloacal vertebrae (NMW 15446:7) and 238th (=last) trunk and second cloacal vertebrae (NMW 15446:8).
Postcranial qualitative variation (n = 6; holotype condition indicated with asterisk)
Ventral element of atlas absent or not ossified to neural arches (n = 3) or absent with neural arches fused ventrally* (n = 3); anterior hypapophysis of axis pointed* (n = 5) or rounded (n = 1); posterior hypapophysis of axis rounded (n = 1), truncated (n = 3) or pointed* (n = 2); hypapophysis of axis fused (n = 1) or not* (n = 5); femur fused (n = 1) or not* (n = 5) to ischium.
Etymology
The specific epithet is an agglutination of the Latin nomen “ignis” which means fire and the proper noun “Rio” as an acronym for the Brazilian city of Rio de Janeiro. This name was chosen in honour to the Museu Nacional do Rio de Janeiro/UFRJ, Brazil’s oldest scientific institution with the largest South American collections of zoology, anthropology, geology and paleontology. Many of the precious collections pertaining to the zoology department (mostly invertebrates), anthropology, geology and paleontology were completely destroyed in the disastrous fire in its main building on September 2nd 2018. Due to historical neglection of this institution from the Brazilian government, added with substantial funding decrease in the past 5 years the museum did not receive sufficient money to fullfil basic safety standards—such as fire protection. The description of this new species, with specimens housed in a scientific collection for more than 100 years highlights one of the several importances of zoological collections in housing relevant material to understand the diversity of life, and also reinforce that such collections are timeless treasures for science. Such collections should receive strong attention in government investments as they contribute to the global development of science.
Distribution and natural history
Epictia rioignis is currently known exclusively from its type series, from Corinto, Nicaragua (See comments under Discussion).
Discussion
Steindachner (1834–1919) was curator of Ichthyology and Herpetology in the NMW from 1860–1919. On the original label of NMW 15446 (Fig. 16A) he only noted “Corinto, 1907, Steind. don”. but failed to mention a country name. Unfortunately and in addition, Steindachner was not always consistent with respect to the event, to which the year mentioned on his labels refered to. In some occasions the year refers to the collecting event, whereas in other occasions the year refers to the acquisition of the specimen(s) or even to the year the vouchers were inventoried by Steindachner (Neumann, 2011). At least, the color of the labels of the Herpetological collection always referred to a certain continent. The green labels were used for “America” and thus one can conclude that this is the continent of origin of the voucher specimens NMW 15446. Later Eiselt (curator of the herpetological collection from 1952–1977) added “S-Amerika” on a newer label (Fig. 16B) and likewise mentioned “Corinto, S-Amerika, 1907” in the inventory book. Considering the trips of Steindachner and the specimens in the ichthyological and herpetological collection of the NMW, the name Corinto may refer to at least three localities in South- or Central America: Corinto, Minas Gerais (Brazil), Corinto, Cauca (Colombia), and Corinto (Nicaragua) (http://geonames.nga.mil/namesgaz). Corinto, Minas Gerais (Brazil) was named “Curralinho” at the time when the specimens were collected. In 1923, and thus many years after the collecting event, it became a municipality and was renamed as Corinto (Barbosa, 1995). Thus, if Steindachner had acquired the specimens from this locality he would have assigned them to “Curralinho” rather than Corinto. Corinto, Cauca (Colombia) is a town and municipality in the Cauca department (29,308 km2). In the collections of the NMW are indeed specimens from the “Cauca region” inventoried by Steindachner, but the city of Corinto was never mentioned. An extended query for the name “Corinto” in the databases of the NMW yielded a single result: eight marine fish species from “Corinto, Nicaragua, collected in 1901, Schiff Donau” were found in the inventory of the fish collection. When Steindachner acquired several specimens from the same collecting event he often provided just one main label with further information (e.g., the country of origin) and used labels with reduced information for the other specimens, independent of the fact that the specimens belonged to different taxonomic groups (e.g., fishes and reptiles). As no other label for “Corinto” with more specific information could be found, we assume that the only detailed label found in the fish collection represents the “main” label of Steindachner for all specimens from the locality Corinto. Thus we further assume that the specimens of Epictia rioignis sp. nov. found in the Herpetological Collection of the NMW derive from the same collecting event and thus originate from Corinto in Nicaragua. The Nicaraguan Corinto is an important port town in the Northwest Pacific Coast which was founded in 1858, and has been historically important as a point of entry to Nicaragua since early 19th century. When labelling Corinto, Steindachner might have referred to both, the locality where the specimens were collected or the locality from where they were acquired. Therefore, considering the data provided herein, the locality of Corinto might still be considered with certain care, as the specimens might have been brought from other localities in Nicaragua.
Figure 16: Handwritten labels of NMW 15446.
(A) By Steindachner and (B) by Eiselt. Photo credits: Alice Schumacher.Nicaragua is an important megadiverse country, however, its snake fauna is less diverse in comparison to other neighboring countries in Central America (Sunyer, 2014). Even if snake diversity is relatively low, studies on the Nicaraguan herpetofauna are still incipient and additional efforts on herpetological research might reveal novel data on the snake fauna of this country (Sunyer, 2014). So far, amongst Epictia spp., only E. ater has been previously reported to occur in Nicaragua, inhabiting lowland arid forests, premontane wet forests and dry tropical forests at elevations between 40–100 m above sea level in the western portion of the country (Wallach, 2016). Therefore, our study increases the number of Epictia species currently found in Nicaragua to n = 2. As previously mentioned (Comparisons section), E. rioignis sp nov. differs from E. ater mostly by quantitative characters, such as the number of middorsal scales and snout-vent length as well as qualitative characters such as the presence/absence of a frontal scale, supralabial-supraocular contact, pattern of dorsal stripes on body and tail (present study, (Wallach, 2016)), and also based on osteological evidences. Even if there is an apparent overlap of the amplitude of middorsal scales (250–267 in E. rioignis sp nov. versus 212–266 in E. ater), general means for such counts ( for E. rioginis sp nov. versus x = 237.3 for E. ater; present study; (Wallach, 2016)) provide evidence for their distinction. Additionally, albeit both supraocular-supralabial contact, as well as the fusion of the frontal and rostral scale can be subject to intraspecific variation (Wallach, 2016; Francisco, Pinto & Fernandes, 2018), such variation usually occurs in a very limited number of the samples. Thus, these characters are still useful for the differentiation of threadsnake species, especially with respect to the relatively conservative and simplified external morphology of these snakes. The combination of scale size, arrangement, and proportion, color pattern and internal anatomy has proven to be sufficient to distinguish the different taxa, which have also been previously identified in molecular studies (Wallach, 2016). Additional studies on species anatomy (such as hemipenial morphology) might reveal further differences between both species. In fact, with respect to the relatively conservative external morphology of threadsnakes (Wallach, 2016; Martins, Passos & Pinto, 2018), data on hemipenial morphology (and other internal systems) would be very useful to clarify the systematics of threadsnakes.
Wallach (2016) recognizes three groups among the Epictia species: one from Mesoamerica (Epictia phenops species group) and two from South America (Epictia tesselata species group and Epictia albifrons species group). According to the author, the Mesoamerican assemblage consists of 11 species (E. ater, E. bakewelli, E. columbi, E. magnamaculata, E. martinezi, E. pauldwyeri, E. phenops, E. resetari, E. schneideri, E. vindumi, and E. wynni). Given the phenotypic similarities and also similar distribution of E. rioignis sp nov., we assign this species to the E. phenops species group. Nevertheless, the composition of the three groups still needs to be clarified with further molecular analyses (present study; Wallach, 2016).
Conclusions
The biodiversity of scolecophidians worldwide is greatly underestimated and often unexpected (Wallach, 2016). Although most of the systematic changes in Epictia spp. have arisen from molecular studies (or at least provided a start point for additional taxonomical arrangements, see Wallach, 2016), morphological studies including both external morphology and internal anatomy are very important for systematic clarification of this group (present study). Even if molecular studies have aided on the identification of cryptic species, this study shows that the morphological analysis of collection specimens still reveals novel data for threadsnakes. This further emphasizes the importance of zoological collections in housing specimens that still allow description of new taxa based on specimens collected more than a century ago.