June 9, 2022 feature
The physicochemical nature of colloidal motion waves among silver colloids
Traveling waves are commonly observed in biological and synthetic systems, and recent discoveries have shown how silver colloids form traveling motion waves in hydrogen peroxide under UV light. In a new report now published in Science Advances, Xi Chen and a team of researchers in smart materials, physics and optics at the Harbin Institute of Technology, and the Shanghai Jiao Tang University, in China, showed the colloidal motion wave as a heterogeneous excitable system.
The silver colloids generated traveling chemical waves via reaction diffusion, and were either self-propelled or advected via diffusion or osmosis. The team observed the fundamental outcomes using hydroxide and pH sensitive dyes, and used a Rogers-McCulloch model to quantitatively and qualitatively produce the characteristic features of colloidal waves. The outcomes pave the way to integrate colloidal waves as a platform to study nonlinear phenomena, and investigate colloidal transport to explore information transmission in biomimetic microrobot ensembles.
Translating biological oscillation in the lab
Oscillatory processes are widely observed in living systems, varying from the circadian rhythm to cytosolic oscillations. The coupling between oscillatory units can lead to synchronization giving rise to traveling waves, as observed with calcium waves spreading across a fertilized egg, action potentials propagating across beating heart cells, mitotic states, and waves of self-organizing amoeba. Biophysicists aim to understand the physicochemical nature of these waves to examine the underlying trends in life. Recent discoveries of the photochemically active, silver-containing oscillating colloids are an exciting addition to the family of nonlinear processes.
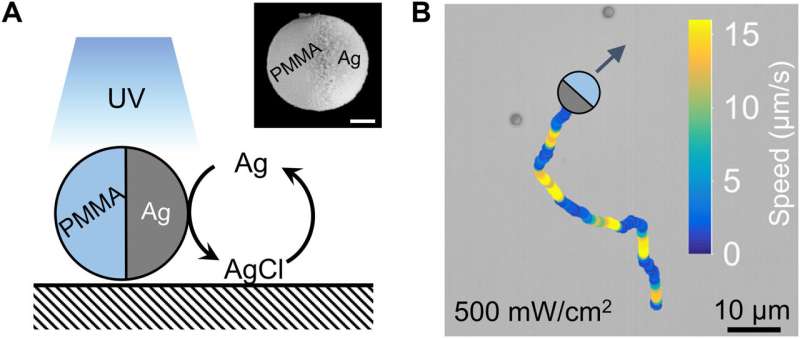
When researchers immersed an inert polymer microsphere half-coated with silver in an aqueous solution of hydrogen peroxide or potassium chloride, and exposed them to light sources, they noted the display of pulses. They proposed that the silver nanoparticles produced during the experiment served as catalytic hotspots to enable further reactions. Regardless of the chemical detail, the team noted how diffusion of chemicals propelled the Janus particles via self-diffusiophoresis, to give rise to similar colloidal motion. In this work, Chen et al offered a first view to generate the chemically active colloids and monitored their response to chemical waves beyond the classical reaction-diffusion systems. The outcomes offer strong possibilities for translational research connecting active matter to nonlinear science in order to regulate swarms of biomimetic microscopic machines.
The experiments
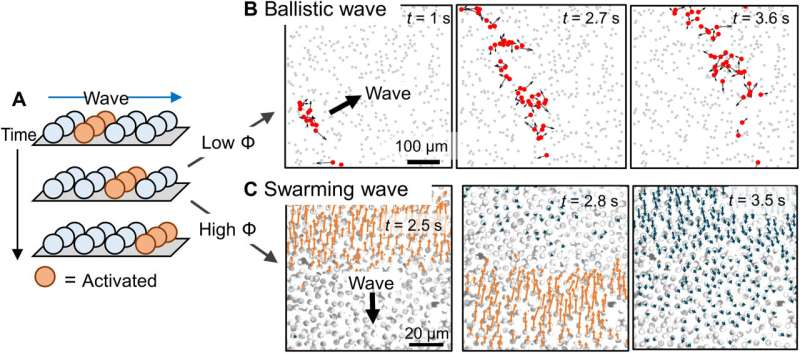
The team noted the development of periodic colloidal motion waves in synchronized propagation. They had previously recorded ballistic waves at an intermediate population density, where activated colloids at the wavefront moved in all directions on account of phoretic self-propulsion. The researchers noted the emergence of qualitatively different types of waves, known as swarming waves at even higher population densities. In this instance, the team developed polymethylmethacrylate microspheres half-coated with silver (PMMA-Ag), suspended in hydrogen peroxide and potassium chloride and illuminated with 365 nm light. The colloidal particle containing silver could in principle emit swarming waves. The experimental results indicated an outcome similar to the "Mexican wave" seen in football stadiums. The team then quantified the swarming wave via single-particle tracking and micro-particle image velocimetry by considering the colloidal particles as flow tracers. In this instance, the wave traveled at a speed of 16 µm/s, with tunable parameters. Changes in light intensity only mildly changed the period and speeds of a swarming wave. The team distinguished the swarming waves from ballistic waves via their characteristic mobility and physicochemistry
Chemical waves: The physicochemical nature of a colloidal wave
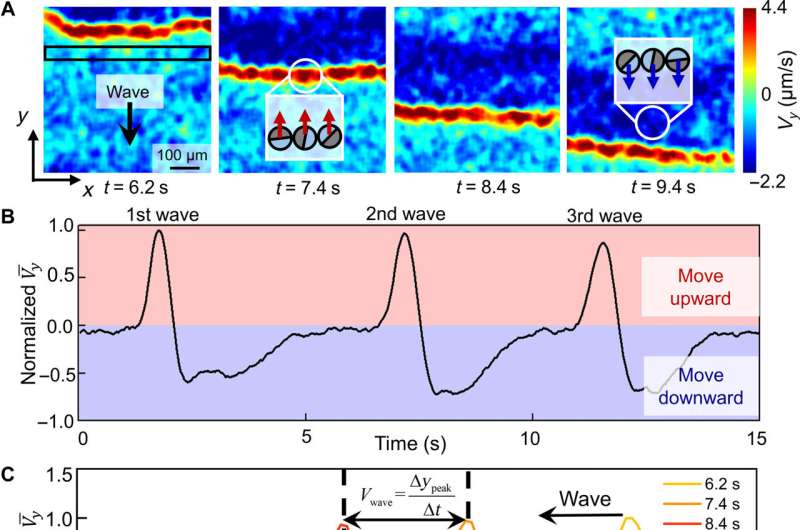
Chen et al described the physicochemical nature of the activation and recovery of colloidal waves. Since the wave phenomenon is inspired by traveling waves in reaction-diffusion systems, they hypothesized colloidal waves to be underpinned by a traveling chemical wave, due to reaction-diffusion mechanisms. For instance, hydrogen peroxide can decompose faster in higher pH to form a burst of highly oxidative intermediates that oxidized silver into silver chloride. The resulting chemical reactivity activated the silver-colloid to release a burst of chemicals to maintain chemical wave propagation. They confirmed the production of hydroxide anions during silver oxidation, and the formation of hydrogen cations during silver chloride photodecomposition, at and behind the chemical wavefront by using fluorescence mapping and pH measurements.
Colloids respond to a chemical wave: Modeling a reaction-diffusion colloidal wave
The scientists next studied the dynamics of colloidal particles in a chemical wave to dictate the type of colloidal wave formed. They noted ionic self-diffusiophoresis, and at higher ionic densities they noted weaker electro-kinetic effects for reduced self-propulsion. They identified the dynamics of neutral diffusio-osmosis dynamics, which moved colloid particles via advection, in addition to self-propagation. As self-propagation weakened and diffusio-osmosis intensified in a crowded solution with rising ionic strength, the colloidal wave switched to swarming wave. The team observed a range of effects, including electrokinetic effects, advection support via osmosis, and self-propulsion during the experiments. Chen et al next reproduced and corroborated the proposed reaction-diffusion colloidal wave via numerical simulations. At the first step, they used the Rogers-McCulloch model to simulate a chemical wave, the resulting numerical models qualitatively reproduced key features, to explore the dynamics of colloidal waves.
-
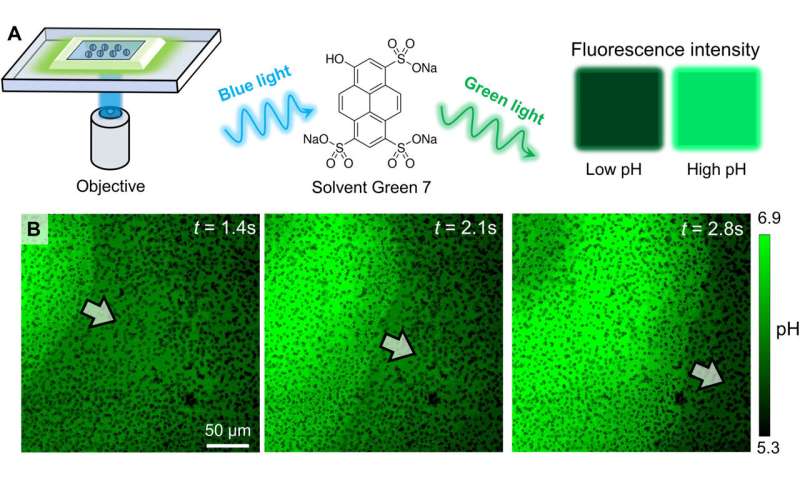
Experimental confirmation of an OH− wave. (A) Schematic diagram of the experimental setup for relating the fluorescence emission of Solvent Green 7 with local pH. (B) Optical micrographs of the pH profile during the propagation of a colloidal wave. PMMA-Ag particles of a population density ϕ of 25% were suspended in an aqueous solution containing 0.5 wt % H2O2, 200 μM KCl, and 100 μM Solvent Green 7. A blue light source (475 nm, 75 mW/cm2) served both to activate the oscillatory reaction and to excite the dye molecules. Credit: Science Advances (2022). DOI: 10.1126/sciadv.abn9130 -
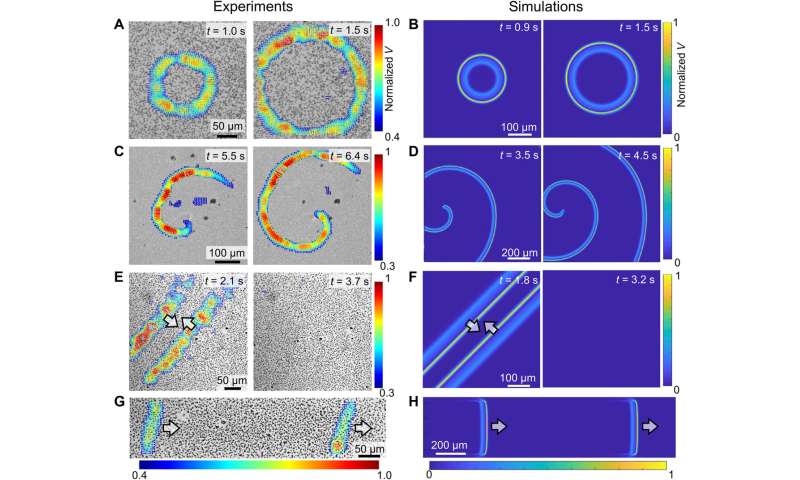
Qualitative comparison of colloidal waves between experiments (left) and simulations (right). (A to D) Evolution of target waves (A and B) and spiral waves (C and D). (E and F) The annihilation of two colloidal waves traveling in opposite directions. (G and H) Two consecutive waves. In all experiments, PMMA-Ag particles [population density ϕ of 20% for (B), 15% for (D), 20% for (F), and 23% for (H)] were suspended in an aqueous solution containing 0.5 wt % H2O2 and 200 μM KCl under a 405-nm illumination of 1.6 W/cm2. Credit: Science Advances (2022). DOI: 10.1126/sciadv.abn9130
Outlook
In this way, Xi Chen and colleagues developed a numerical model to simulate colloidal waves to study the heterogeneity of chemical waves. The outcomes showed good agreement with simulations and experiments to provide key insights to understand microscopic details of chemical waves in experimental systems. Colloidal waves can be integrated with optical tweezers, acoustofluidics or microfluidics to regulate micro- and nanoscopic objects in space and time. The method is useful to swarm physicochemical dynamics of a colloidal wave and can lead to develop wave-mediated information transmission systems to examine autonomous micro-robots. The colloidal waves present a good model system of reaction-diffusion processes at mesoscopic and microscopic scales.
More information: Xi Chen et al, Unraveling the physiochemical nature of colloidal motion waves among silver colloids, Science Advances (2022). DOI: 10.1126/sciadv.abn9130
Ido Nitsan et al, Mechanical communication in cardiac cell synchronized beating, Nature Physics (2016). DOI: 10.1038/nphys3619
Journal information: Nature Physics , Science Advances
© 2022 Science X Network