This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Same protein, different shape explains protein's effectiveness against bacteria
Shigella bacteria can infect humans but not mice. In the March 29 issue of Nature, a team from UConn Health explains why. Their findings may explain the multifariousness of a key weapon of our immune system.
Shigella infections cause fever, stomach pain, and prolonged, sometimes bloody diarrhea for as long as a week. The bacteria sicken 450,000 people each year in the U.S. alone. Although most people recover on their own, children and those with weakened immune systems are at risk of Shigella infections spreading to their bloodstream and causing kidney damage. Shigella infections are a significant cause of sickness and disability, but it's difficult to study the bacteria because it only sickens primates like humans and apes—not animals easy to study in a lab. The bacteria cannot infect more typical lab animals such as mice.
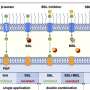
Previous research had looked at how Shigella interacts with gasdermin-B, a critical part of our immune system that helps protect us against infection. Gasdermin-B is member of a protein family called gasdermin, which includes gasdermin-A, -B, -C, -D, -E and -F. It was thought that when gasdermin-B detects an invader, such as bacteria, it begins to poke holes in the cell's wall, causing it to burst open and release chemicals that induce inflammation and call reinforcements from the immune system. But the past research studies on gasdermin-B were contradictory; some confirmed its role in cell death during infection, but others contradicted the idea.
UConn School of Medicine immunologist Jianbin Ruan and a team of colleagues from UConn Health wanted to clarify whether gasdermin-B actually does cause cell death in the case of microbial invasion; they also wanted to figure out why it doesn't do this when Shigella is the invader.
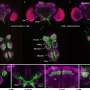
The team needed to take a close look at gasdermin-B. They expressed the protein, purified it, and then cooled the protein down to very low temperatures so it would hold still while they took pictures of it with an electron microscope.
"We collected hundreds of thousands of images to build the 3D models of protein molecules at the atomic level. Through these models we will understand what these proteins look like and how they do their job," said Chengliang Wang, research fellow in the Ruan lab and first author of the study.
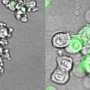
Their research confirms previous research and provides evidence that Shigella bacteria grab onto a specific segment of gasdermin-B in humans. However, the mouse version of the protein has a different shape that prevents Shigella from latching onto it, resulting in the rapid clearance of the bacteria and preventing infection. This finding helps explain why Shigella is unable to infect mice.
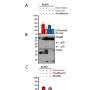
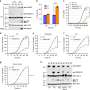
Since human gasdermin-B can be configured in six slightly differing proteins, or isoforms, the team expressed all six then looked at how these isoforms behaved inside cells, and they found something surprising: some of the isoforms of gasdermin-B did indeed poke holes to cause cell death—but other isoforms did not.
"Previously, people didn't understand why studies contradicted each other. We show that only two of the isoforms of gasdermin-B cause pyroptosis, or cell death," says Ruan. Those two isoforms contain a specific protein segment that is absent in the other gasdermin-B isoforms, as shown by their cryogenic electron microscopy structure.
The finding may explain many mysteries of cell death, and life. Cancer cells, for example, are notoriously long lived and unlikely to die via pyroptosis. It may be that these cancer cells express only gasdermin-B isoforms that don't poke holes in cell walls.
However, we don't yet know what these other isoforms are doing. It may be that the different isoforms of gasdermin-B play significant and distinctive roles depending on where they are in the body, and different cell types preferentially express different isoforms.
"The protein structures that our team discovered have significant implications for drug development. Specifically, they can inform the design of small molecule drugs that modulate gasdermin-B activity," explains Ruan. "These drugs could potentially be used to treat a range of conditions, including cancer, inflammatory and autoimmune diseases, and infectious diseases by either suppressing or enhancing the immune response. Our findings thus hold promise for the development of novel therapies to address these pressing medical needs."
More information: Chengliang Wang et al, Structural basis for GSDMB pore formation and its targeting by IpaH7.8, Nature (2023). DOI: 10.1038/s41586-023-05832-z
Journal information: Nature
Provided by University of Connecticut