In 1841, a ship docking at Alexandria unleashed a panzootic with dire consequences for Africa (a panzootic is the equivalent of pandemic, but for animals). Within two years, 665,000 cattle had died throughout Egypt [Cattle Plague Chapter 22]. Much worse was yet to come, though. During 1888-1896, the “rinderpest” plague blasted from the horn of Africa all the way South to the Cape Colony and all the way West to Senegal. The rinderpest caused over 90% mortality in the previously flourishing cattle populations of Africa. Wild herds of buffalo and wildebeests also suffered tremendous losses, sometimes acting as disease carriers between distant kraals of cattle.
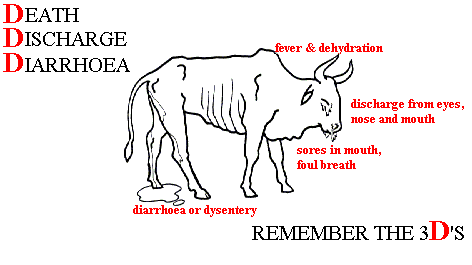
Cattle have long held great socioeconomic significance in the traditional pastoral societies of Africa. Cattle’s role in these societies is not simply one of economics and survival because cattle play a key role in ritual and social definition. A person’s wealth and social importance could be shown in the size of herds; a man’s ability to accumulate and loan cattle defines the role he plays within his community. Even the spatial layout of villages is defined by cattle/male spaces and non-cattle/female spaces. The central kraal is where male elders would conduct their work. Cattle meat is exclusively reserved for the consumption of chiefs and for ritual practice. Dairy products, however, are a key part of the agro-pastoralist diet. Cow milk and blood from living animals may be mixed with millet to create a nutritious porridge. The fermentation of milk to produce amasi is commonplace. Cattle knit society together; for a leader to entrust part of the herd to a follower’s care could represent a statement of alliance. In some cultures, a young man cannot marry until he has acquired cattle. Wars have been initiated by ceremonially stealing a single animal from another group (such as the 1863 ovita vyongombongange described in Wallace, p.63). The great rinderpest plague destroyed far more than a food source; it undermined the fabric of traditional societies across Africa.

In 2011, the rinderpest virus was announced to be eradicated in the wild, only the second virus to reach this status (after smallpox in 1979). Today, laboratories that still house samples of this virus are being urged to “sequence and destroy” them to prevent their ever escaping into the wild. In this post, I’d like to detail the rinderpest virus and describe how we eradicated this threat.
An introduction to Rinderpest morbillivirus

Rinderpest is an example of the morbillivirus genus within family Paramyxoviridae. This genus harbors some genuine killers. The one everybody cares about is rubeola, the virus that causes measles. Rubeola is one of the most contagious viruses in the world for person-to-person transmission, as summarized in the “R naught” metric of 12 or higher (rinderpest scores 4.6 on this scale). Until recently, almost every child was vaccinated against rubeola infection, typically in the “Measles Mumps Rubella (MMR)” vaccine. This vaccine definitely counts as one of the miracles of modern medicine, so I am frankly irritated that people make up nonsense to suggest that ordinary children should not receive this vaccine. Save a life, perhaps your own child’s, by ensuring your kids are vaccinated.

Superficially, paramyxoviridae such as rinderpest seem quite similar to the SARS-CoV-2 virus that causes COVID-19. Both are enveloped viruses of around 150 nm diameter that contain a single-stranded copy of the genome in RNA. Rinderpest, though, has a “negative sense” genome rather than the “positive sense” genome of SARS-CoV-2. The rinderpest genome is only about half the size, at 15.88 kb (thousands of nucleotide bases). The set of genes for paramyxoviridae is pretty different from those of SARS-CoV-2. Here’s how the changes play out:

The grappling hook: Hemagglutinin, made famous by the influenza virus, is one of those proteins that makes me think “sinister!” Our cells frequently use sugar molecules on their exteriors as doorbells of a sort. Hemagglutinin attaches to sialic acid, triggering the cell to engulf the virus. While it’s ringing the doorbell, the Fusion protein causes the viral envelope and cell membrane to merge so that the viral contents can enter the cell. Nasty.
The production line: Negative-stranded RNA viruses need to send messenger-RNA to the cell’s ribosomes to produce viral proteins. It cannot use the cell’s transcription machinery to do that, though, so it must contribute its own RNA-dependent transcription equipment. We think of the Central Dogma of molecular biology allowing mRNA to be built from a dsDNA template, but viruses don’t really care about our “rulebook.”
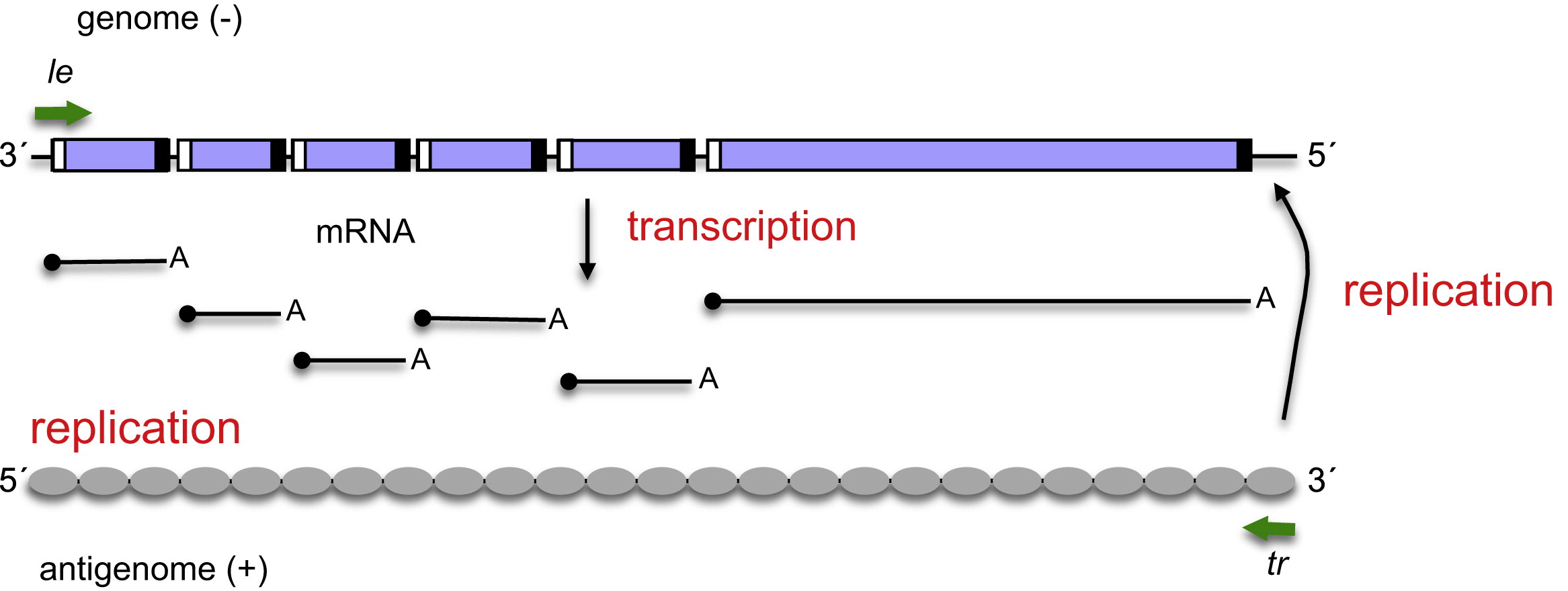
The genome copier: Every virus wants to manufacture more viruses once it has sufficient control of cellular machinery. The same enzyme that built the transcripts for individual genes of the virus can switch to another mode that creates a complementary copy of the virus genome. “Not so fast,” you say, “the complement of a negative sense viral genome is a positive sense strand. You can’t ship that out and get a functional virus!” Here’s the tricky part: once the RNA-dependent RNA polymerase has made a full-length positive-sense complement to the genome (“antigenome”), it can build a complementary strand to the positive-sense genomic RNA that will then be a negative-sense genomic RNA, ready for packaging. One gene is capable of making 1) mRNAs from the viral genome, 2) full-length complementary RNA genome copies, and 3) a full-length negative-sense RNA genome ready for viral packaging. The virus has placed our Central Dogma in the trash and closed the lid!
How we beat the rinderpest

Initial efforts to prevent the spread of rinderpest into South Africa were reminiscent of zombie movies. A pair of massive fences along the border with Botswana was intended to prevent infected cattle from wandering from Matabele lands. The area near Mafeking was proclaimed a “cattle-free zone,” and many herds were shot and buried. Despite these efforts, rinderpest repeatedly appeared within South Africa. Any herd with a single infection reported was immediately slaughtered. Thousands of animals were destroyed to forestall the spread of the disease, but eventually a transport rider brought the disease to the Eastern Cape. An estimated 2.5 million cattle died in Southern Africa between 1896 and 1899.
The agro-pastoral communities understandably did not have a lot of trust for the white settlers’ claims about the best way to manage the spreading plague. The people of these communities didn’t generally appreciate the disinfection of their persons, particularly when it was used as retribution rather than a clinical necessity. The destruction of a cattle herd was eviscerating to people living in traditional communities in a way that it was not for white farmers. At a high level, the relationships among leaders were destroyed when the cattle that represented those links were dead. At the ground level, people were forced to eat “roots, grubs, locusts, and even decaying meat” to survive. Rinderpest played an out-of-scale role in the destruction of traditional society throughout sub-Saharan Africa, just as the Scramble for Africa was reaching full steam.
Local researchers Dr. Arnold Theiler and Dr. Herbert Watkins-Pitchford banded together to fight rinderpest, but the government wasted their time by compelling them to test the spurious “remedies” put forward by farmers such as water deprivation and snake venom. European expertise was recruited at substantial expense. German Scientist Dr. Robert Koch resided in Kimberley from 1886 to 1897 working this problem, and a team of French scientists from the Pasteur Institute came to South Africa near the end of Koch’s period in country.
Dr. Koch, already famous for his work in anthrax and in enunciating “Koch’s Posulates,” arrived with considerable star-power. He brought Dr. Paul M.J. Kohlstock to assist him. Koch was initially skeptical of Russian Professor Eugene Semmer‘s findings that fluids removed from infected animals could evoke a protective response if injected into an animal that had not yet developed illness. When Koch announced in February, 1897 a treatment based on this strategy, using 5 mL of bile from sick animals, he was celebrated as a hero. Kohlstock accepted an invitation to German Southwest Africa (the colony that later became Namibia) to introduce a refined technique there.

In practice, the Koch “Bile Method” was nearly as problematic as the absence of a cure. The death rate of treated cattle was approximately half; the protective benefits only began days after treatment and lasted less than half a year. In German Southwest Africa, some indigenous farmers objected when they saw that cows were repeatedly taken from their farms for bile production to save cattle at other farms (this process was, of course, painfully fatal for the cow). The French team of Mr. Jean Danysz and Dr. Jules Bordet built upon experiments by Theiler and Watkins-Pitchford to develop the “Serum Method,” which soon bore the moniker the “French Method.” It was announced just six months after the Bile Method and soon came to supplant it. When war was declared at the start of the Second Anglo-Boer War in 1898, the panzootic was nearly under control within South Africa.
The modern world was not freed of rinderpest by these early strategies. A tissue culture-based vaccine was created by English Research Veterinarian Walter Plowright at Kenya in 1960. I feel a sense of kinship with him because he also left his homeland to join in the amazing story of Africa! His vaccine was applied aggressively in cattle throughout Africa by Joint Project 15 project to reduce the rinderpest pockets to just the Niger Inland Delta and an area of southern Sudan. A renewed effort by the Pan-African Rinderpest Campaign in the late 1980s followed another wave of the disease out of these pockets. A 1992 effort created a thermostable vaccine that could be used even if left unrefrigerated for a week!
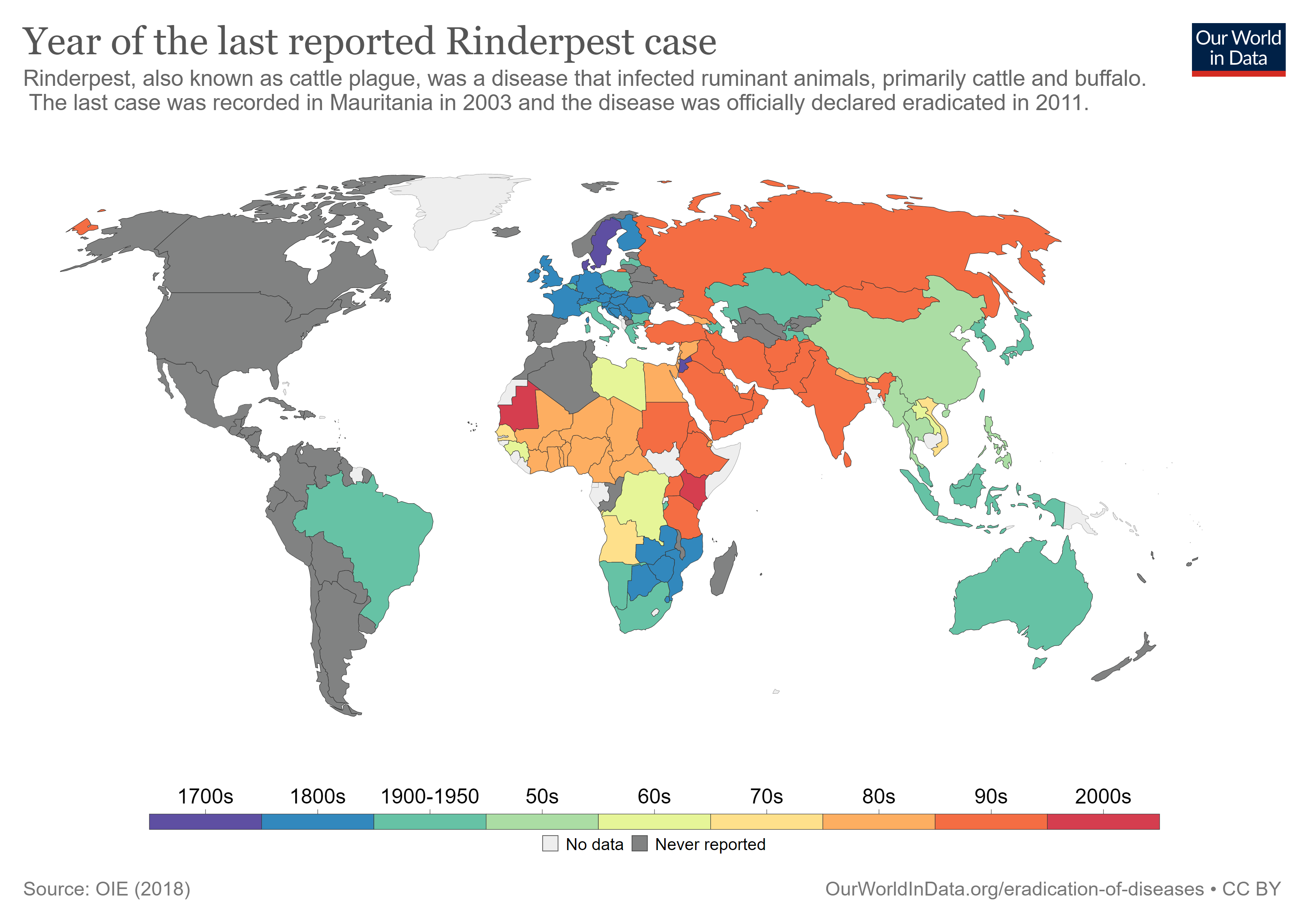
As detailed in Mariner et al, rinderpest eradication required much more than just a viable vaccine. Teams of “local intermediaries called community-based animal health care workers” were recruited and trained, though some locals perceived this effort as competition for their jobs. Eradication was not a strictly top-down effort. Instead, community knowledge of herds and migration schedules and made a significant difference in handling local outbreaks quickly. The network created by the PARC effort may be useful in controlling other diseases such as “peste des petits ruminants,” bird flu, Rift Valley fever, and foot and mouth disease.
Rinderpest posed a tremendous challenge to African communities in the late nineteenth century, but the rapidly evolving field of veterinary medicine was able to control it long enough that it could be eradicated by modern techniques. When the chips are down, it’s a good idea to have some scientists on call!
[I want to offer a special thank you to Natasha for helping me understand the importance of cattle in indigenous societies.]

Very informative! Thanks!
Sent from my iPad
>
LikeLiked by 1 person
Pingback: Who are the heroes among Namibia's Herero? | Picking Up The Tabb