During a routine eye examination, a 61-year-old White woman was discovered to have two pigmented iris lesions with corectopia and ectropion in her right eye, suspicious for uveal melanoma. She denied any history of ocular trauma and was unaware of the mass but recognized the progressively distorted pupil. She was referred to ocular oncology for evaluation.
On our examination, BCVA was 20/70 OD and 20/80 OS, with an IOP of 18 mm Hg OD and 19 mm Hg OS. The left eye was unremarkable; the anterior segment showed a round pupil with no iris pigmentary abnormality. The anterior segment examination of the right eye showed a nonsuspicious nevus in the inferior iris without seeding that measured 2.0 mm in diameter (Figure 1A). There was ectropion and corectopia with two-point traction pulling the iris inferomedially away from the nevus and toward a second pigmented region that represented iridocorneal touch. There was no tumor or corneal endotheliopathy. The dilated fundus examination was unremarkable.
Imaging with anterior segment OCT (AS-OCT) confirmed broad-based peripheral anterior synechiae (PAS) inferomedially with no evidence of solid melanoma (Figures 1B and 1C). These features were consistent with iridocorneal endothelial (ICE) syndrome and coincident iris nevus, simulating melanoma. Observation and specular microscopy to assess corneal endothelial cell count were recommended.
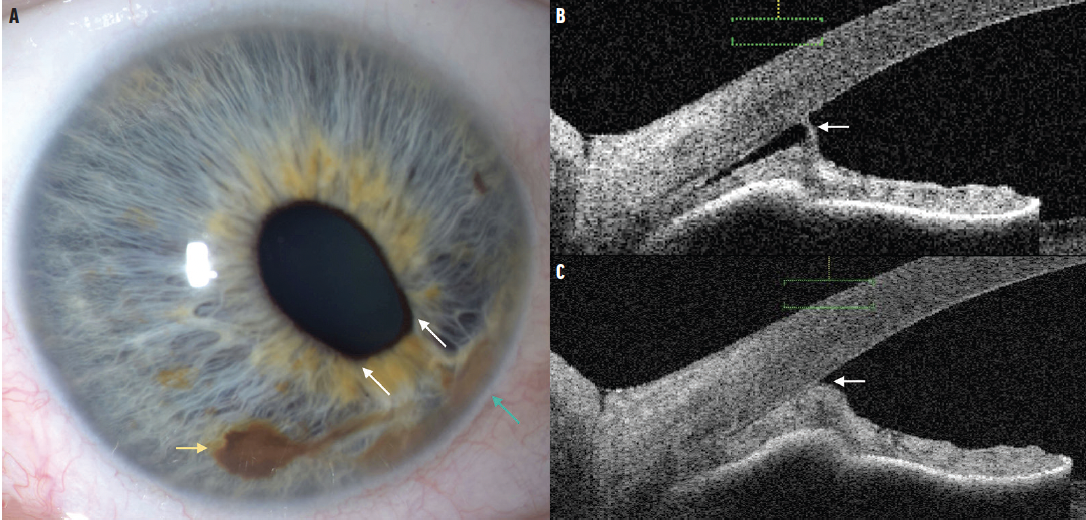
Figure 1. These images depict ICE syndrome masquerading as a suspicious iris nevus in the right eye. Slit lamp photography demonstrates two pigmented iris lesions with classic iris nevus (yellow arrow) and iridocorneal endothelial adhesion (green arrow; A). Note that there is two-point iris traction (white arrows), more consistent with ICE syndrome than iris nevus or melanoma. On AS-OCT there is focal (B) and diffuse (C) iris adhesion to the corneal endothelium with draping of the iris. Note that the iris is otherwise of normal thickness and the iris pigment epithelium is intact without tumor.
DISCUSSION
ICE syndrome is a rare ophthalmic disorder that can mimic iris melanoma due to shared clinical features of corectopia, ectropion uveae, and a gray-brown iris lesion.1-4 In the case presented here, the features were most consistent with ICE syndrome with corectopia showing two-point traction. AS-OCT imaging confirmed iridoendothelial adhesion with iris elevation and no solid mass. Most iris nevi or melanoma with corectopia tend to show only one-point traction directed toward the lesion without iris atrophy or endothelial adhesion.5
Other considerations for iris nevus or melanoma were absent, including feeder or intrinsic vessels; tumor seeding on the iris stroma or anterior chamber angle; and hyphema.5 The coincidental finding of an unrelated iris nevus in this eye with ICE syndrome created a diagnostic challenge.
In the Literature
In 2011, Shields et al reviewed 71 consecutive cases of individuals with ICE syndrome misconstrued as possible iris nevus or melanoma referred for ocular oncology consultation.2 The study provided a comparative analysis of eyes with ICE syndrome versus those with iris melanoma (n = 169) and found that the following findings were suggestive of ICE syndrome: corneal guttatae (46% vs 0%), corneal edema (10% vs 0%), iris atrophy (58% vs 0%), PAS (80% vs 0%), and polycoria (1% vs 0%). Features more suggestive of iris melanoma versus ICE syndrome included episcleral sentinel vessels (25% vs 8%), extrascleral extension of the tumor (6% vs 0%), iris mass or nodule (72% vs 7%), iris tumor seeds (56% vs 0%), solid mass in angle (46% vs 0%), and angle seeding (57% vs 0%). Some overlapping features for ICE syndrome and iris melanoma included mean age at presentation (51 vs 48 years), female sex (76% vs 50%), corectopia (75% vs 62%), ectropion iridis (34% vs 44%), and IOP greater than 22 mm Hg (8% vs 30%) or greater than 30 mm Hg (4% vs 17%).
The mechanism of glaucoma in ICE patients is uniformly angle-closure.2,6 In patients with iris melanoma and glaucoma, the glaucoma is typically secondary to tumor infiltration of the anterior chamber angle.2 Shields et al also highlighted corneal guttatae, corneal edema, multidirectional corectopia, iris atrophy, PAS, and elevated IOP from angle-closure as the major differentiating features of ICE syndrome from circumscribed or diffuse iris melanoma.2
Lakosha et al similarly found that essential iris atrophy, a clinical subtype of ICE syndrome, can mimic iris neoplasms and that longitudinal ultrasound biomicroscopy was useful in establishing a diagnosis by demonstrating progressive iris thinning and contraction of PAS.4
Pathogenesis and Treatment
The hypothesized pathogenesis of ICE syndrome is corneal endothelial cell proliferation triggered by herpes simplex virus or Epstein-Barr virus infection.7,8 Proliferating endothelial cells migrate toward the iridocorneal angle and onto the iris, precipitating corneal, iris, angle, and pupillary abnormalities. Most believe that ICE syndrome is sporadic, unilateral, and more prevalent among middle-aged women.2,9 The location and degree of pathology differentiates the three ICE syndrome subtypes—Chandler syndrome, Cogan-Reese syndrome, and progressive iris atrophy. ICE syndrome is well documented to mimic malignancy, especially iris melanoma, and assessment by an ocular oncologist is often necessary.
Several cohort studies and case reports support the use of in vivo confocal microscopy as a diagnostic tool for ICE syndrome, particularly in borderline presentations or in cases where corneal edema precludes visualization by specular microscopy.10-12 In a study of 12 patients with unilateral ICE syndrome, Le et al showed that affected eyes demonstrated a decrease in the percentage of hexagonal endothelial cells (20.3% vs 63.3%, P < .05) and greater variation in endothelial cell size (0.512 vs 0.357, P < .05) compared with contralateral healthy eyes, which falls in line with previous studies.10,13 These findings reflect the transformation of uniform corneal endothelial cells to pleomorphic epithelioid “ICE” cells that underlies the pathogenesis of this syndrome.
Treatment of ICE syndrome primarily involves the management of glaucoma with topical IOP-lowering eye drops and glaucoma surgery, including trabeculectomy with antifibrotic agents, implantation of shunt devices, goniotomy, and ciliary body ablation.6,14 Management of corneal decompensation includes corneal graft with penetrating keratoplasty, Descemet membrane endothelial keratoplasty, or Descemet stripping automated endothelial keratoplasty.15,16
CONCLUSION
ICE syndrome can closely simulate iris melanoma; however, there are special features that suggest ICE syndrome, including the corneal endothelial “beaten metal” appearance of guttatae, iris transillumination defects, multipoint ectropion iris and corectopia, and broad-based PAS (iridocorneal adhesion). In contrast, iris melanoma tends to demonstrate a solid iris mass, occasionally with iris ectropion or corectopia, but usually single-point and with additional iris stromal seeding, angle seeding, secondary glaucoma, and evidence of growth.2,4
Support provided in part by the Eye Tumor Research Foundation, Philadelphia, PA (CLS). The funders had no role in the design and conduct of the study, in the collection, analysis and interpretation of the data, and in the preparation, review or approval of the manuscript. Carol L. Shields, MD, has had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. No conflicting relationship exists for any author.
1. Eagle RC Jr, Font RL, Yanoff M, Fine BS. Proliferative endotheliopathy with iris abnormalities. The iridocorneal endothelial syndrome. Arch Ophthalmol. 1979;97(11):2104-2111.
2. Shields CL, Shields MV, Viloria V, Pearlstein H, Say EA, Shields JA. Iridocorneal endothelial syndrome masquerading as iris melanoma in 71 cases. Arch Ophthalmol. 2011;129(8):1023-1029.
3. Silva L, Najafi A, Suwan Y, Teekhasaenee C, Ritch R. The iridocorneal endothelial syndrome. Surv Ophthalmol. 2018;63(5):665-676.
4. Lakosha HM, Pavlin CJ, Simpson ER. Essential iris atrophy mimicking iris neoplasm: an ultrasound biomicroscopic study. Can J Ophthalmol. 2000;35(7):390-393.
5. Shields CL, Kaliki S, Hutchinson A, et al. Iris nevus growth into melanoma: analysis of 1611 consecutive eyes: the ABCDEF guide. Ophthalmology. 2013;120(4):766-772.
6. Laganowski HC, Kerr Muir MG, Hitchings RA. Glaucoma and the iridocorneal endothelial syndrome. Arch Ophthalmol. 1992;110(3):346-350.
7. Alvarado JA, Underwood JL, Green WR, et al. Detection of herpes simplex viral DNA in the iridocorneal endothelial syndrome. Arch Ophthalmol. 1994;112(12):1601-1609.
8. Tsai CS, Ritch R, Straus SE, Perry HD, Hsieh FY. Antibodies to Epstein-Barr virus in iridocorneal endothelial syndrome. Arch Ophthalmol. 1990;108(11):1572-1576.
9. Shields MB. Progressive essential iris atrophy, Chandler’s syndrome, and the iris nevus (Cogan-Reese) syndrome: a spectrum of disease. Surv Ophthalmol. 1979;24(1):3-20.
10. Le QH, Sun XH, Xu JJ. In-vivo confocal microscopy of iridocorneal endothelial syndrome. Int Ophthalmol. 2009;29(1):11-18.
11. Chiou AG, Kaufman SC, Beuerman RW, Ohta T, Yaylali V, Kaufman HE. Confocal microscopy in the iridocorneal endothelial syndrome. Br J Ophthalmol. 1999;83(6):697-702.
12. Malhotra C, Seth NG, Pandav SS, et al. Iridocorneal endothelial syndrome: Evaluation of patient demographics and endothelial morphology by in vivo confocal microscopy in an Indian cohort. Indian J Ophthalmol. 2019;67(5):604-610.
13. Lucas-Glass TC, Baratz KH, Nelson LR, Hodge DO, Bourne WM. The contralateral corneal endothelium in the iridocorneal endothelial syndrome. Arch Ophthalmol. 1997;115(1):40-44.
14. Sacchetti M, Mantelli F, Marenco M, Macchi I, Ambrosio O, Rama P. Diagnosis and management of iridocorneal endothelial syndrome. Biomed Res Int. 2015;2015:763093.
15. Rotenberg M, Downward L, Curnow E, et al. Graft survival after penetrating and endothelial keratoplasty in iridocorneal endothelial syndrome. Cornea. 2020;39(1):18-22.
16. Ao M, Feng Y, Xiao G, Xu Y, Hong J. Clinical outcome of Descemet stripping automated endothelial keratoplasty in 18 cases with iridocorneal endothelial syndrome. Eye (Lond). 2018;32(4):679-686.




-1_1643727930.jpg?auto=compress,format&w=70)

-1_1643042672.jpg?auto=compress,format&w=70)



















