As vitreoretinal surgeons, one of the most basic techniques we learn is reattaching the retina following rhegmatogenous retinal detachment (RRD). Many vitreoretinal surgeons were taught by mentors who were trained to favor the use of a scleral buckle (SB) for RRD repair. However, these mentors have also witnessed a tremendous evolution in vitreoretinal surgery over the past 10 to 15 years, with small-gauge pars plana vitrectomy (PPV) becoming an essential procedure for many conditions such as diabetic vitreous hemorrhage, tractional retinal detachment, macular hole repair, and epiretinal membrane removal.
Because of the recent advances in technology, most training programs now focus on PPV, and they vary with respect to their emphasis on segmental, radial, and encircling SB, either alone or in combination with vitrectomy, and pneumatic retinopexy (PnR).
However, there is a paucity of adequately powered randomized clinical trials comparing the functional outcomes associated with the various surgical techniques of RRD repair. Furthermore, the most commonly used measure of success has been the relatively basic and rudimentary outcome of single-operation reattachment rate. We now know that retinal reattachment is necessary, but not sufficient, to achieve the best possible outcomes for our patients.
Until recently, we have been limited in our ability to assess the “integrity” of the retinal reattachment and have had limited evidence regarding which techniques provide patients with the best functional results.
RRD AND EVIDENCE-BASED MEDICINE
We have entered a new era in RRD repair guided by a greater emphasis on evidence-based medicine and significant advances in accessible multimodal imaging. Together, these two factors have enabled us to move closer to determining the best possible treatment approach for a given patient when considering both functional and anatomic outcomes.
Functional outcomes include visual acuity, metamorphopsia, aniseikonia, and vision-related quality of life.
Anatomic outcomes include not only single-operation reattachment rate but also the final reattachment rate in addition to the presence or absence of retinal displacement (Figure 1), outer retinal folds (Figure 2a), persistent subretinal fluid blebs (Figure 2b), discontinuity of the external limiting membrane, and ellipsoid zone integrity (Figure 3), among other imaging biomarkers. These anatomic outcomes of integrity, some of which are not visible on clinical examination with indirect ophthalmoscopy, can be assessed with multimodal imaging.
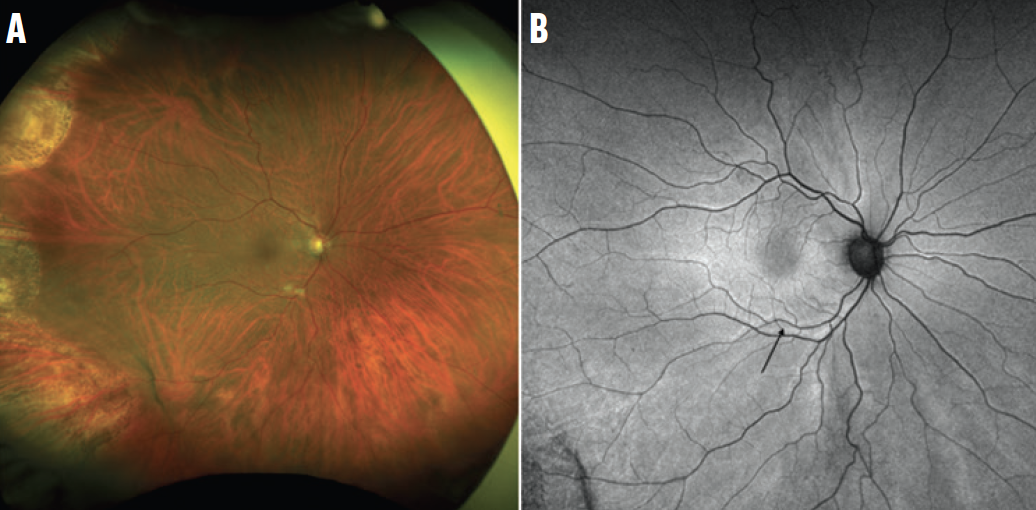
Figure 1. This ultra-widefield color photograph demonstrates a reattached retina following PPV for RRD repair (A). The ultra-widefield fundus autofluorescence image of the same patient shows multiple retinal vessel printings (black arrow, B), indicating that the retina has been displaced from its original position. This patient has had a low-integrity retinal attachment.
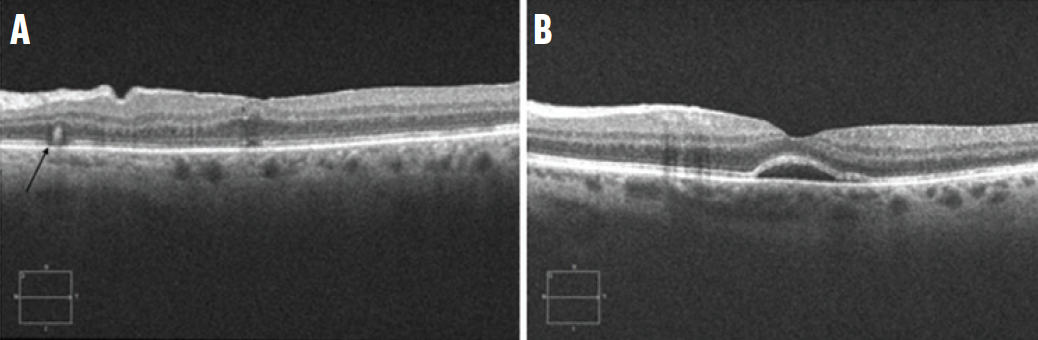
Figure 2. The cross-sectional OCT in a patient following PPV for RRD repair demonstrates a prominent outer retinal fold (black arrow, A). In another patient, OCT reveals a persistent subfoveal fluid bleb (B).

Figure 3. The cross-sectional OCT in a patient following PPV with an intact foveal external limiting membrane (white arrow) demonstrates discontinuity of the ellipsoid zone and interdigitation zone (red arrow). Image courtesy of Barbara Parolini, MD
Although we are early in our understanding of how abnormalities in the anatomic outcomes of integrity impact patients, stretching, folding, or lack of continuity in retinal layers may have some impact on functional outcomes. Knowing why these anatomic abnormalities occur can help us begin to understand how they may be prevented or minimized, leading to refinements in our surgical techniques.
A DIFFERENCE IN INTEGRITY
The PIVOT trial, a single-center randomized trial of 176 patients, compared PPV to PnR for patients with RRD who met specific inclusion/exclusion criteria.1 The study found that patients treated with PnR had superior ETDRS visual acuity at every postoperative study visit, including the 1-year primary endpoint, compared with patients who were treated with PPV. Furthermore, patients treated with PnR had superior vision-related quality of life in the first 6 months.2 Surprisingly, patients treated with PnR had less vertical metamorphopsia compared with those treated with PPV.
This finding is intriguing, considering postoperative vertical metamorphopsia is most likely the result of a structural and/or functional abnormality of the photoreceptors, and raises the question, “is there a difference in the integrity of retinal reattachment with different surgical procedures?”
THE ANSWER IS IN THE DATA
The first step in answering this question is reviewing a series of studies that assessed whether the retina was reopposed as closely as possible to its original position following retinal reattachment. A multicenter retrospective study found that those treated with PPV had a substantially greater risk of retinal displacement compared with patients who underwent PnR.3
On fundus autofluorescence imaging, hyperautofluorescent lines indicating the location of retinal vessels before the RRD were compared with the new location of the corresponding retinal vessels after RRD repair. These lines are hyperautofluorescent because they occur where the retinal pigment epithelium (RPE)—previously shielded by retinal vessels—became exposed to light following reattachment of the retina in the presence of retinal displacement. The prior lack of exposure to light likely results in a different composition of fluorophores and metabolic activity in the RPE, which leads to a difference in the autofluorescence. We refer to a case with retinal displacement as a low-integrity retinal attachment (LIRA) and a case without retinal displacement as a high-integrity retinal attachment (HIRA).
Further multicenter studies have confirmed the substantially greater risk of retinal displacement associated with PPV compared with PnR.4 They have also demonstrated that patients with LIRA have a greater risk of aniseikonia compared with patients with HIRA.4 Following retinal detachment repair, many patients complain of micropsia; retinal displacement may stretch the retina and causes changes in the spacing between photoreceptors, leading to subsequent changes in the perceived size of an object.
Following the studies on retinal displacement, other anatomic outcomes of retinal reattachment integrity were investigated.5 Eyes in the PIVOT trial were imaged with spectral-domain OCT at 1 year, and a higher risk of ellipsoid zone and external limiting membrane discontinuity was found in eyes treated with PPV compared with those treated with PnR.5 In addition, other post-hoc analyses of the PIVOT trial showed that the rate of outer retinal folds was higher in the PPV group compared with the PnR group at 1 month (34.1% vs 14.3%, P = .034). Eyes that underwent PPV and presented with outer retinal folds at 1 month had reduced visual acuity at 1 year compared with PPV eyes without outer retinal folds (62.8 ± 24.7 ETDRS vs 75.4 ± 9.2 ETDRS, P = .04).6
Another area of interest has been how exactly the retina reattaches. Until recently, the understanding of the physiology of retinal reattachment in vivo was limited. What information we had came from landmark studies in owl monkeys in the 1960s by Machemer.7 Recently, the in vivo physiology of retinal reattachment in humans was characterized using swept-source OCT imaging of eyes that underwent PnR.8 Five stages of retinal reattachment, from the initial approach of the retina toward the RPE (stage 1) to the restoration of the foveal bulge (stage 5c), were described. By studying these stages in detail, we are able to understand how certain anatomic abnormalities, such as outer retinal folds and persistent subfoveal fluid blebs, can form and, in some cases, be avoided.
WHAT WE LEARNED
These data have taught us important lessons about the comparison of outcomes associated with PPV versus PnR and how PPV may be modified to potentially minimize the risk of adverse anatomic outcomes of integrity such as retinal displacement and outer retinal folds.
In PnR, the technique involves a slow and natural resolution of subretinal fluid after closure of the retinal break(s) and a small-volume gas tamponade. These two features may serve to reduce the risk of unfavorable anatomic outcomes of integrity following PnR. The corollary is that these factors also may be modified in PPV in an attempt to improve outcomes.
Minimal gas vitrectomy and minimal gas vitrectomy buckle have been developed to reduce the risk of retinal displacement following RRD repair in certain appropriate cases.9,10 In these procedures, although a complete PPV is performed, the retina is left detached and no fluid-air exchange is performed. A small gas bubble is injected at the end of PPV after the wounds are closed. The patient is then positioned in a manner similar to PnR. The retinal break is treated with cryopexy during surgery or with laser retinopexy once the retina is reattached; this is facilitated by endodiathermy marking of the break(s) at the time of PPV. In cases with inferior breaks, a segmental buckle is added. These procedures may serve to reduce the risk of retinal displacement, outer retinal folds, and ellipsoid zone/external limiting membrane discontinuity associated with PPV.
We are entering an exciting time in vitreoretinal surgery, where we may be guided by multimodal imaging and randomized trial data to optimize case selection and surgical techniques, with subsequent improvements in the integrity of retinal attachment and functional outcomes for patients. Looking beyond the single-procedure reattachment rate will serve us and our patients well.
1. Hillier RJ, Felfeli T, Berger AR, et al. The pneumatic retinopexy versus vitrectomy for the management of primary rhegmatogenous retinal detachment outcomes randomized trial (PIVOT). Ophthalmology. 2019;126(4):531-539.
2. Muni RH, Francisconi CLM, Felfeli T, et al. Vision-related functioning in patients undergoing pneumatic retinopexy vs vitrectomy for primary rhegmatogenous retinal detachment: a post hoc exploratory analysis of the PIVOT randomized clinical trial. JAMA Ophthalmol. 2020;138(8):826-833.
3. Brosh K, Francisconi CLM, Qian J, et al. Retinal displacement following pneumatic retinopexy vs pars plana vitrectomy for rhegmatogenous retinal detachment. JAMA Ophthalmol. 2020;138(6):652-659.
4. Muni RH, Francisconi CLM, Marafon SB, et al. Retina displacement following pneumatic retinopexy vs pars plana vitrectomy for retinal detachment repair. Paper presentation at Virtual Retina Society 2020.
5. Muni RH, Felfeli T, Sadda SR, et al. Postoperative photoreceptor integrity following pneumatic retinopexy vs pars plana vitrectomy for retinal detachment repair: a post hoc optical coherence tomography analysis from the pneumatic retinopexy versus vitrectomy for the management of primary rhegmatogenous retinal detachment outcomes randomized trial JAMA Ophthalmol. 2021;139(6):620-627. Published correction in: JAMA Ophthalmol. 2021;139(6):679.
6. Lee WW. Postoperative outer retinal folds in pneumatic retinopexy vs pars plana vitrectomy for rhegmatogenous retinal detachment: PIVOT post hoc analysis. Presented at ASRS 2021; San Antonio, Texas; October 12, 2021.
7. Machemer R. Experimental retinal detachment in the owl monkey. IV. The reattached retina. Am J Ophthalmol. 1968;66(6):1075-1091.
8. Bansal A, Lee WW, Felfeli T, Muni RH. Real-time in vivo assessment of retinal reattachment in humans using swept-source optical coherence tomography. Am J Ophthalmol. 2021;227:265-274.
9. Muni RH, Felfeli T, Figueiredo N, Marafon SB, Escaf LS, Juncal VR. Minimal gas vitrectomy technique for reducing risk of retinal displacement following rhegmatogenous retinal detachment repair. Preprint. Published online November 6, 2020. Retin Cases Brief Rep.
10. Muni RH, Bansal A, Lee WW, Escaf LC. Minimal gas vitrectomy with scleral buckle to minimize retinal displacement in rhegmatogenous retinal detachment with inferior breaks. Preprint. Published online June 14, 2021. Retin Cases Brief Rep.