AT A GLANCE
- Chronic, low-grade inflammation is thought to be a primary driver of AMD and geographic atrophy (GA) pathogenesis, and overactivity of the complement system has been specifically implicated.
- The most promising interventions for GA currently involve inhibiting the activation of C3 and C5.
- There is some concern for increased risk of exudative transformation with complement inhibition for GA.
The complement system is strongly implicated in the development and progression of geographic atrophy (GA) and has emerged as an attractive therapeutic target. In February, pegcetacoplan intravitreal injection (Syfovre, Apellis Pharmaceuticals), a complement inhibitor, gained FDA approval for the treatment of GA secondary to AMD, marking a significant milestone in the progress of therapeutics for AMD. This article reviews our understanding of the complement system, its role in GA, and current investigational therapies that target this system.
THE COMPLEMENT SYSTEM AND GA
The complement system serves as the first line of defense against invading pathogens by inducing cell lysis and enhancing the activity of the antibody-driven adaptive immune system (Figure 1). The three complement pathways (classical, alternative, and lectin) converge upon a shared terminal series of reactions that lead to the formation of the membrane attack complex (MAC) on target cell surfaces.1 MAC causes cell lysis, the major endpoint of the complement system. Byproducts of this enzyme cascade recruit leukocytes and tag pathogens for phagocytic destruction, while MAC itself can induce several localized inflammatory reactions.1 The potency of the complement system requires tight regulation to prevent local host tissue damage.
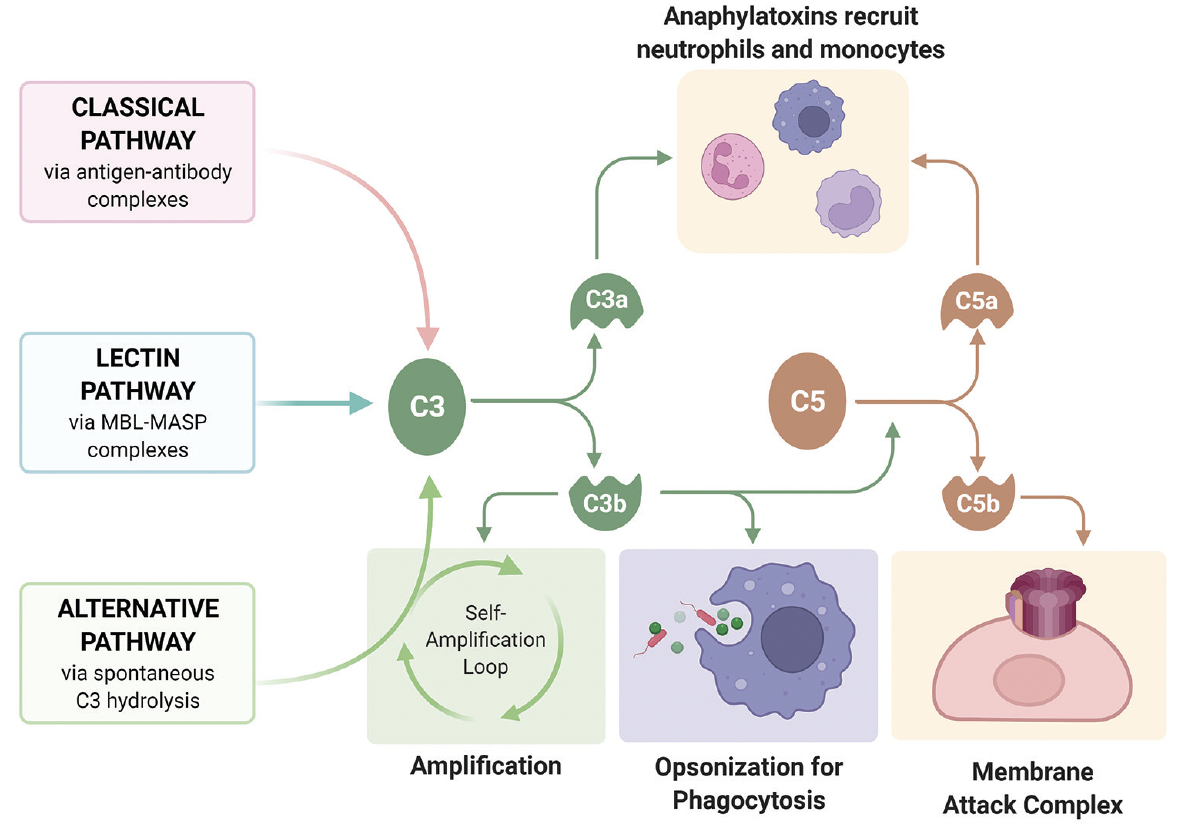
Figure 1. The complement system consists of three distinct pathways. The classical pathway is triggered by IgG or IgM antibody-antigen complexes. The alternative pathway is continuously activated through slow, spontaneous hydrolysis of C3, requiring constant inhibition by endogenous regulatory proteins that pathogens do not possess. The lectin pathway is triggered by recognition of specific carbohydrates on microbial surfaces. Image reproduced from Kislev S. Complement Overview. Wikimedia Commons. 2022. bit.ly/40nW7Hl.
Chronic, low-grade inflammation is a primary driver of AMD and GA pathogenesis, and overactivity of the complement system has been specifically implicated.2,3 For example, several complement-related gene mutations are strongly associated with increased AMD risk, likely due to complement dysregulation within the retina.4 Accordingly, high concentrations of complement byproducts have been found in the plasma of patients with AMD, as well as within the retinal pigment epithelium (RPE) and photoreceptor outer segments in areas of GA.5 Complement byproducts have also been found in high concentrations within drusen,3 raising the possibility that drusen may represent biomarkers of a localized, complement-mediated inflammatory process driving GA pathogenesis at the RPE-Bruch membrane junction.6
Therefore, the complement system is an attractive target for GA therapy (Figure 2) with the most promising strategy involving the inhibition of C3 and C5 activation.7,8
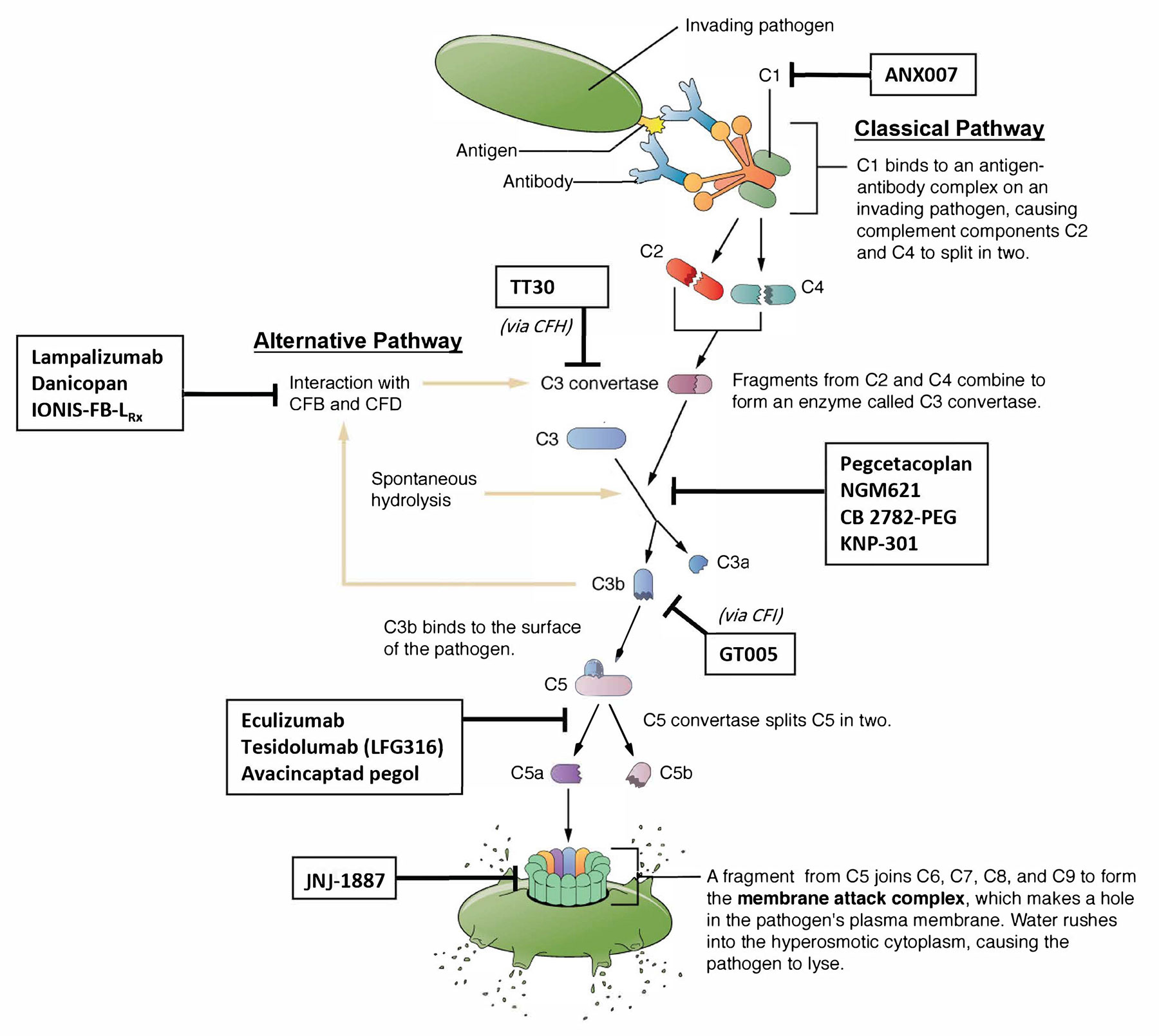
Figure 2. Investigational therapies for GA have largely focused on interfering with C5 and C3 activity. Other therapies have targeted MAC formation and specific components of the alternative and classical pathways. The lectin pathway has not been strongly implicated in AMD and does not currently represent a major therapeutic target. Image adapted from Betts JG, et al. Barrier defenses and the innate immune response. In: Anatomy and Physiology. OpenStax College. 2022. bit.ly/40rvcum.
THERAPEUTIC TARGETS
Common Pathway Target: C3
Pegcetacoplan is a C3 inhibitor that binds and prevents activation of C3 by C3 convertase, blocking activation of downstream effectors and halting the cascade’s progression.
The phase 3 OAKS and DERBY trials enrolled patients with BCVA ≥ 24 ETDRS letters (approximately 20/320 Snellen equivalent) and total GA area between 2.5 mm2 and 17.5 mm2.9 In the OAKS trial, both monthly and every-other-month treatment arms showed statistically significant reductions in GA growth (22% and 18%, respectively) at 24 months when compared with sham.10 DERBY data followed a similar trend with reductions in GA growth of 19% and 16%, respectively.10 A phase 3 extension study evaluating the long-term safety of pegcetacoplan for up to 36 months is underway.11
Another C3 inhibitor, NGM621 (NGM Biopharmaceuticals), is a monoclonal antibody that was recently evaluated in the phase 2 CATALINA trial.12 Although the study failed to reach its primary endpoint,12 a post-hoc analysis suggests a statistically significant reduction in GA growth rate (21.9% and 16.8% with every 4- and 8-week injections, respectively) among a subgroup of eyes exhibiting a narrower range of baseline GA area than the trial inclusion criteria.13
Other C3 inhibitors in development include CB 2782-PEG (Catalyst Biosciences) and KNP-301 (Kanaph Therapeutics), both of which are currently in preclinical testing.14,15
Terminal Pathway Targets: C5 and MAC
C5 is another attractive therapeutic target due to its key role in initiating the formation of MAC.8 The C5 inhibitor avacincaptad pegol (Zimura, Iveric Bio) is a pegylated RNA aptamer that binds and prevents activation of C5. The phase 2/3 GATHER1 trial found a statistically significant decrease in mean rate of GA area growth with monthly injections of 2 mg and 4 mg avacincaptad pegol at 12 months compared with sham (27.4% and 27.8% reduction in rate of square root of GA area growth, respectively).16 The ongoing GATHER2 trial is evaluating monthly injections of 2 mg avacincaptad pegol for 12 months, followed by monthly or every-other-month injections for 23 months. The trial met its primary endpoint, demonstrating a statistically significant 14.3% reduction in mean rate of GA area growth (square root transformed) with monthly treatment compared with sham.17 The FDA has accepted the company’s new drug application, granting priority review status with a Prescription Drug User Fee Act goal date in August.18
MAC inhibition is also a therapeutic strategy for GA. JNJ-1887 (Janssen) is a gene therapy that induces expression of the endogenous MAC-inhibitory protein CD59; the safety of its intravitreal administration in GA is the subject of an ongoing phase 2b trial.19
Alternative Pathway Targets
The alternative pathway accounts for more than 80% of terminal pathway activation and ultimate MAC formation, making it a prime therapeutic target.20 The pathway is constitutively activated by spontaneous hydrolysis of C3 into soluble C3a and C3b fragments, which then participate in a positive feedback amplification loop by creating C3 convertase. The activity of this amplification loop is tightly regulated by several complement factors (CF), including CFH, CFI, CFD, and CFB. Selectively targeting these complement factors in the alternative pathway is theoretically advantageous due to the preservation of host defense via intact classical and lectin pathways.
CFH plays the most prominent role in downregulating alternative pathway activity, degrading C3b and inactivating C3 convertase. CFH deficiency has been strongly implicated in the development of GA, and genetic mutations reducing CFH expression represent the strongest genetic risk factors for AMD, increasing the risk by up to seven-fold.21,22
CFI, an important cofactor of CFH in the degradation of C3b, has been targeted with GT005 (Gyroscope Therapeutics/Novartis), a gene therapy designed to induce local overexpression of CFI following subretinal or suprachoroidal administration.23 Interim data from the phase 1/2 FOCUS trial suggests that GT005 is well tolerated in GA, while two phase 2 trials are ongoing: HORIZON and EXPLORE.24-26 An additional, observational, long-term follow-up cohort trial, ORACLE, is also in progress.27
The alternative pathway amplification loop may also be targeted directly by inhibiting CFB and CFD. A CFD inhibitor, ACH-4471 (Danicopan, Alexion Pharmaceuticals), is being studied as an oral therapy for GA in a phase 2 trial.28 CFB is being targeted by subcutaneous administration of IONIS-FB-LRx (Ionis Pharmaceuticals), an antisense oligonucleotide that reduces CFB protein expression by degrading CFB messenger RNA29; the phase 2 GOLDEN trial is active.30
Classical Pathway Target: C1
The classical pathway has also been a therapeutic target of some interest. This pathway is initiated by antibody-antigen complexes binding and activating the C1 complex. ANX007 (Annexon) is an intravitreally-injected antigen-binding fragment designed to inhibit C1q, the functional component of C1.31 The phase 2 ARCHER trial is assessing the safety of ANX007 in GA, with topline data expected this year.32
SAFETY CONSIDERATIONS
Complement inhibition for GA has generally been well tolerated in clinical trials. However, there is some concern for increased risk of exudative transformation with anti-complement therapy. In the combined phase 3 pegcetacoplan trial data, new-onset exudation was noted in 11.9% of the monthly groups, 6.7% in the every-other-month groups, and 3.1% in the sham groups at 24 months.10 Patients at greatest risk were those with exudative AMD in the contralateral eye and those exhibiting the OCT double-layer sign—a potential marker of subclinical type 1 macular neovascularization.33,34 The GATHER1 and GATHER2 trials also demonstrated a slightly increased risk of exudative transformation with avacincaptad pegol therapy.16,17 The mechanism by which complement inhibition may increase the risk of exudation and neovascular disease remains uncertain, and further research is ongoing. Additional theoretical concerns have been raised regarding endophthalmitis risk with intravitreal injection of immunosuppressive complement inhibitors. However, endophthalmitis rates in the pegcetacoplan and avacincaptad pegol trials have reassuringly been similar to or lower than those reported with other intravitreal therapies.10
FUTURE PERSPECTIVES
Our approach to AMD is rapidly evolving with a growing array of promising treatment options. The development of sustained release technologies and novel drug delivery methods will hopefully increase the durability and lessen the burden on our patients. With ongoing research and innovation, we are confident that new treatments for AMD will continue to advance, helping preserve vision and improve the quality of life of countless individuals around the world.
1. Janeway CA. How the immune system protects the host from infection. Microbes Infect. 2001;3(13):1167-1171.
2. Fleckenstein M, Keenan TDL, Guymer RH, et al. Age-related macular degeneration. Nat Rev Dis Primers. 2021;7(1):31.
3. Anderson DH, Radeke MJ, Gallo NB, et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res. 2010;29(2):95-112.
4. Shughoury A, Sevgi DD, Ciulla TA. Molecular genetic mechanisms in age-related macular degeneration. Genes (Basel). 2022;13(7):1233.
5. Katschke KJ, Xi H, Cox C, et al. Classical and alternative complement activation on photoreceptor outer segments drives monocyte-dependent retinal atrophy. Sci Rep. 2018;8(1):7348.
6. Hageman GS, Luthert PJ, Chong NHV, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20(6):705-732.
7. Kassa E, Ciulla TA, Hussain RM, Dugel PU. Complement inhibition as a therapeutic strategy in retinal disorders. Expert Opin Biol Ther. 2019;19(4):335-342.
8. Kim BJ, Mastellos DC, Li Y, Dunaief JL, Lambris JD. Targeting complement components C3 and C5 for the retina: Key concepts and lingering questions. Prog Retin Eye Res. 2021;83:100936.
9. Goldberg R, Heier JS; Wykoff CC, et al. Efficacy of intravitreal pegcetacoplan in patients with geographic atrophy (GA): 12-month results from the phase 3 OAKS and DERBY studies. Invest Ophthalmol Vis Sci. 2022;63:1500.
10. Apellis announces 24-month results showing increased effects over time with pegcetacoplan in phase 3 DERBY and OAKS studies in geographic atrophy (GA) [press release]. Apellis Pharmaceuticals. August 24, 2022. Accessed April 5, 2023. bit.ly/3MZJFub
11. An extension study to evaluate the long-term safety and efficacy of pegcetacoplan (APL-2) in subjects with geographic atrophy secondary to AMD (GALE). Accessed April 3, 2023. clinicaltrials.gov/ct2/show/NCT04770545
12. NGM Bio announces topline results from the CATALINA phase 2 trial of NGM621 in patients with geographic atrophy (GA) secondary to age-related macular degeneration [press release]. NGM Bio. October 17, 2022. Accessed April 5, 2023. bit.ly/40qS4Kg
13. NGM Bio announces presentation of post-hoc analyses from CATALINA phase 2 trial of NGM621 in patients with geographic atrophy (GA) secondary to age-related macular degeneration (AMD) at the Retina Society annual scientific meeting [press release]. NGM Bio. November 3, 2022. Accessed April 5, 2023. bit.ly/3AisQmV
14. Blouse GE. CB 2782-PEG: a complement factor C3- inactivating protease and potential long-acting treatment for dry AMD. Presented at the 3rd annual Complement-Based Drug Delivery Summit. November 13-15, 2019; Boston.
15. Chung EDP, Kim D, Ryu S, Chang J, Lee BC. KNP-301, a dual inhibitor of the complement pathway and angiogenesis, effectively suppresses angiogenesis and atrophy. Invest Ophthalmol Vis Sci. 2021;62:183.
16. Jaffe GJ, Westby K, Csaky KG, et al. C5 inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration: a randomized pivotal phase 2/3 trial. Ophthalmol. 2021;128(4):576-586.
17. Iveric Bio announces positive topline data from Zimura GATHER2 phase 3 clinical trial in geographic atrophy [press release]. Iveric Bio. September 6, 2022. Accessed April 5, 2023. bit.ly/41LktMa
18. Iveric Bio announces FDA accepts new drug application and grants priority review for avacincaptad pegol for the treatment of geographic atrophy [press release]. Iveric Bio. February 16, 2023. Accessed April 5, 2023. bit.ly/41HnJZ9
19. A study to evaluate intravitreal JNJ-81201887 (AAVCAGsCD59) compared to sham procedure for the treatment of geographic atrophy (GA) secondary to age-related macular degeneration (AMD). Accessed June 7, 2023. clinicaltrials.gov/ct2/show/NCT05811351
20. Harboe M, Ulvund G, Vien L, Fung M, Mollnes TE. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin Exp Immunol. 2004;138(3):439-446.
21. Tzoumas N, Hallam D, Harris CL, Lako M, Kavanagh D, Steel DHW. Revisiting the role of factor H in age-related macular degeneration: insights from complement-mediated renal disease and rare genetic variants. Surv Ophthalmol. 2021;66(2):378-401.
22. Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385-389.
23. Ellis S, Buchberger A, Holder J, Orhan E, Hughes J. GT005, a gene therapy for the treatment of dry age-related macular degeneration (AMD). Invest Ophthalmol Vis Sci. 2020;61:2295.
24. FOCUS: first in human study to evaluate the safety and efficacy of GT005 administered in subjects with dry AMD. Accessed April 3, 2023. clinicaltrials.gov/ct2/show/NCT03846193
25. HORIZON: A phase II study to evaluate the safety and efficacy of two doses of GT005. Accessed April 3, 2023. bit.ly/3UV17lu
26. EXPLORE: A phase II study to evaluate the safety and efficacy of two doses of GT005. Accessed April 3, 2023. bit.ly/43PNAzL
27. ORACLE: A long-term follow-up study to evaluate the safety and durability of GT005 in a Gyroscope-sponsored antecedent study. Accessed April 3, 2023. clinicaltrials.gov/ct2/show/NCT05481827
28. Boyer D, Ko Y-P, Podos SD, et al. Danicopan, an oral complement factor D inhibitor, exhibits high and sustained exposure in ocular tissues in preclinical studies. Transl Vis Sci Technol. 2022;11(10):37.
29. Jaffe GJ, Sahni J, Fauser S, Geary RS, Schneider E, McCaleb M. Development of IONIS-FB-LRx to treat geographic atrophy associated with AMD. Invest Ophthalmol Vis Sci. 2020;61:4305.
30. GOLDEN STUDY: A study to assess safety and efficacy of multiple doses of IONIS-FB-LRx in participants with geographic atrophy secondary to age-related macular degeneration (AMD). Accessed April 3, 2023. clinicaltrials.gov/ct2/show/NCT03815825
31. Grover A, Sankaranarayanan S, Mathur V, et al. Pharmacokinetic and target engagement measures of ANX007, an anti-C1q antibody fragment, following intravitreal administration in nonhuman primates. Invest Ophthalmol Vis Sci. 2023;64:3.
32. A study investigating the efficacy and safety of intravitreal injections of ANX007 in patients with geographic atrophy (ARCHER). Accessed April 3, 2023. clinicaltrials.gov/ct2/show/NCT04656561
33. Wykoff CC, Rosenfeld PJ, Waheed NK, et al. Characterizing new-onset exudation in the randomized phase 2 FILLY trial of complement inhibitor pegcetacoplan for geographic atrophy. Ophthalmol. 2021;128(9):1325-1336.
34. Shi Y, Motulsky EH, Goldhardt R, et al. Predictive value of the OCT double-layer sign for identifying subclinical neovascularization in age-related macular degeneration. Ophalmol Retina. 2019;3(3):211-219.























