Thermogravimetric analysis (TGA) is a technique utilized to measure alterations in mass or the overall mass of a substance in relation to variations in temperature. During the process of thermogravimetric analysis, the continuous monitoring of the mass of a given material is conducted as it undergoes heating or cooling within a predetermined atmospheric environment. Consequently, thermogravimetric analysis (TGA) is predominantly employed for the purpose of understanding distinct thermal phenomena, including desorption, adsorption, sublimation, vaporization, reduction, and oxidation.
Thermogravimetric Analysis (TGA) is a highly notable methodology employed for the characterization of materials utilized in diverse domains such as pharmaceuticals, food science, environmental studies, and petrochemical industries. This technique can be employed to investigate various phenomena, including decomposition, reaction equilibrium, pyrolysis, oxidation, filler mass, ash percentage, metallic residue, and the loss of solvents, water, or plasticizer while heating or aging.
Interesting Science Videos
Types of Thermogravimetric Analysis
Thermogravimetric methods can be classified into three distinct categories:
Static (isothermal) Thermogravimetry
This particular approach entails conducting an analysis wherein the weight of the sample is meticulously recorded over a period of time under controlled temperature conditions.
Quasistatic Thermogravimetry
The sample undergoes controlled thermal treatment within various temperature intervals, wherein it is carefully maintained for a specific duration, typically until its mass reaches a state of equilibrium. This presents an exceptional opportunity to delve into substances renowned for their diverse decomposition patterns at varying temperatures, thereby enhancing our understanding of their intricate decomposition mechanisms.
Dynamic Thermogravimetry
The specimen undergoes thermal treatment within a controlled environment, wherein the temperature progressively ascends or descends at a predetermined pace, typically following a linear trajectory. The majority of scientific examinations applying the thermal technique of analysis commonly employ the dynamic thermogravimetric analysis method.
Thermogravimetric Analyzer
Thermogravimetric analysis (TGA) is performed using a sophisticated device known as a thermogravimetric analyzer. A thermogravimetric analyzer, with its remarkable capability, diligently records the weight shifts of the specimen as the temperature undergoes gradual changes. Mass, temperature, and time serve as fundamental parameters in thermogravimetric analysis, forming the bedrock upon which a multitude of obtained metrics can be built.
Thermogravimetric Analysis Principle
- The technique of Thermogravimetric Analysis (TGA) provides a valuable means of quantitatively assessing alterations in weight resulting from thermally induced transitions. The procedure entails subjecting a sample to controlled heating, wherein the temperature is incrementally increased over time.
- The thermo-gravimetric analysis involves subjecting the sample to controlled heating within a specified environment, such as air, nitrogen, carbon dioxide, helium, argon, and others. The alteration in the mass of the substance is documented as a variable dependent on either temperature or time.
- The temperature is systematically raised at a consistent pace with a predetermined initial weight of the substance, and the alterations in weight are documented as a function of temperature at various time intervals.
The graphical representation depicting the correlation between weight variation and temperature is commonly referred to as the thermo-gravimetric curve or thermo-gram. This fundamental principle serves as the foundation for TGA, an analytical technique of great significance.
The results may be presented in the following two ways: TG curve and TG Curve Derivative (DTG)
TG curve
The thermogravimetric curve, commonly referred to as the TG curve, is a graphical representation that captures the weight variation of a substance in relation to either temperature or time.
TG Curve Derivative (DTG)
The derivative of the thermogravimetric (TG) curve, known as the derivative thermogravimetry (DTG), involves plotting the first derivative of the TG curve with respect to either temperature or time. The plotted graph is commonly referred to as the pyrolysis curve.
The unique nature of the TG curve for a particular substance or material arises from the specific sequence of physical transitions and chemical reactions that occur within defined temperature intervals. Weight changes occur as a consequence of physical transformations and the formation and disruption of chemical bonds under elevated temperatures. Thermogravimetry (TG) is commonly conducted within the temperature range of 1200°C, regardless of whether the atmosphere is inert or reactive. The thermally induced reaction rates are often influenced by the molecular structure.
A representative thermal gravimetric (TG) curve illustrating the behavior of CuSO4.5H2O can be presented as follows:
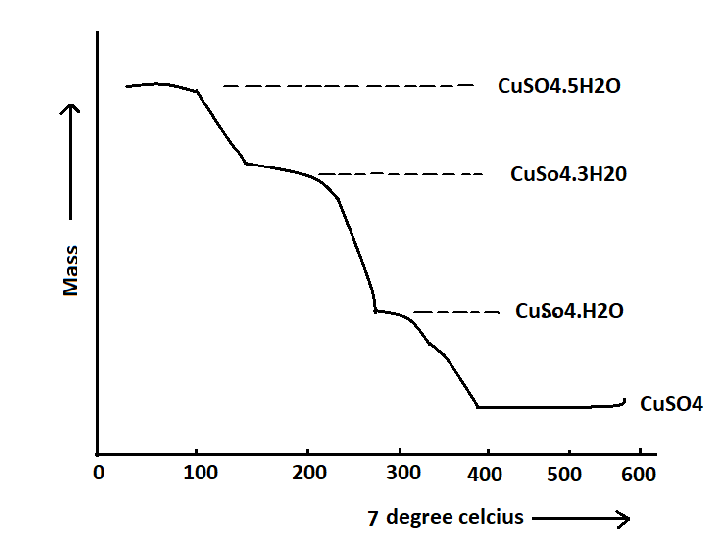
The graph depicted in this illustration can be classified into both horizontal and vertical segments. The horizontal segments represent areas of weight stability, whereas the curved segment in the vertical portion indicates a decrease in weight. Compound stoichiometry calculations can be performed at any given temperature due to the quantitative nature of the TG curve equation.
The compound CuSO4.5H2O can be observed to undergo decomposition in four distinct regions, as depicted in the following illustration:
Derivative Thermogravimetric (DTG)
Plotting the weight change over time (dW/dT) vs temperature results in the derivative thermogravimetric (DTG) curve. In the DTG curve, dW/DT equals zero when there is no weight loss. The peak of the derivative curve corresponds to the maximum slope on the TG curve. An inflection, or change in slope, occurs on the TG curve when dW/dT is minimal but not zero.
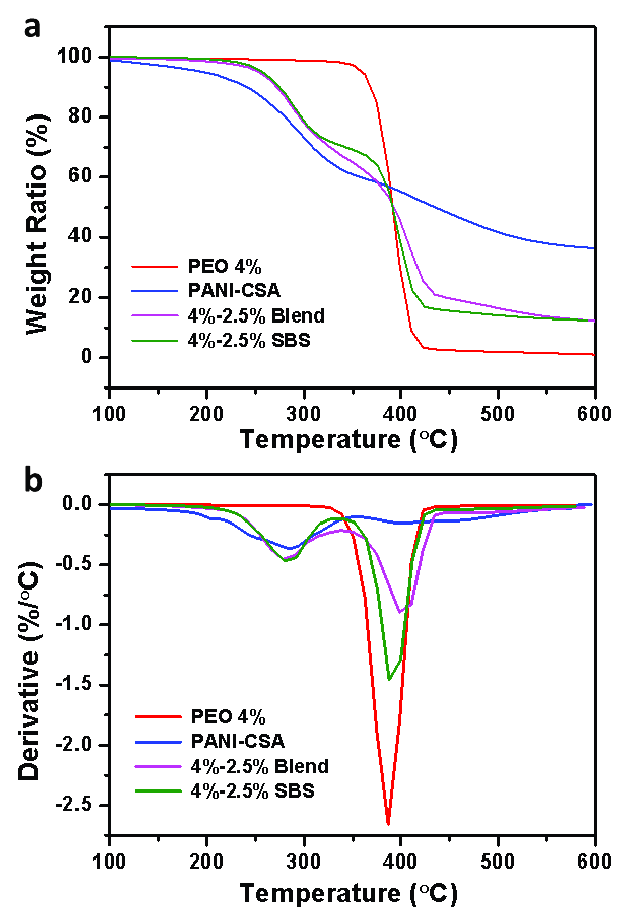
[Image source: http://dx.doi.org/10.3390/polym11060954]
Applications of Thermogravimetric Analysis
Thermogravimetric analysis (TGA) is a widely employed technique in materials characterization, which involves the measurement of the mass variation of a substance as a function of temperature. Through the utilization of this methodology and the precise calculations that can be derived from it, Thermogravimetric Analysis (TGA) can be effectively employed to ascertain the characteristics of a given substance, including:
- Alterations in mass result from processes such as decomposition, oxidation, evaporation, or combustion.
- The moisture content that has been absorbed
- The rate of volatilization of materials
- The thermal cracking point of the base fluids or formulations present within the substance is of interest.
Thermogravimetric analysis (TGA) is particularly advantageous in the examination of polymer-based materials that necessitate tailored responses to elevated temperatures or swift fluctuations in temperature.
One prevalent utilization of Thermogravimetric Analysis (TGA) in the realm of business and industry involves assessing the thermal resistance of various products, primarily for the purposes of ensuring quality and safety. Industries such as Polymers Manufacturing, Plastic Manufacturing, Pharmaceutical Manufacturing, and General Manufacturing may employ Thermogravimetric Analysis (TGA) for the following purposes:
- quality control and quality assurance.
- Determination of Thermal Stability
- Research on Oxidation and Combustion for Determining Product Storage and Utilization
Some of the most important applications of TGA are listed below:
In Analytical Chemistry
Thermogravimetric analysis has been extensively utilized in various analytical investigations. These include the exploration of appropriate weighing methods for numerous elements, the examination of materials that serve as current or potential analytical standards, and the direct implementation of the technique in analytical determinations.
Previous studies have demonstrated the utilization of thermogravimetric analysis as a method for assessing hygroscopic moisture, organic matter, and inorganic carbonates in soils. Dupuis and Dupuis (2015) employed thermogravimetry as a method for quantifying the presence of calcium and magnesium in dolomite rock.
In Inorganic Chemistry
The utilization of thermogravimetry in conjunction with infrared investigations has been proposed as a means to differentiate between constitutional water and adsorbed water. The 8-hydroxyquinolines derived from various bivalent metals, including uranium and plutonium, have been subject to extensive investigation. Thermogravimetric studies have been conducted on bivalent metal anthranilates, as well as 298 substituted anthranilates of lanthanum and other related chelate complexes
In organic Chemistry
Thermogravimetric and differential thermal analytical investigations were conducted to examine the pyrolysis process of wood and wood samples treated with inorganic salts. TGA was used in comparative analysis between differential thermogravimetry and differential thermometry as techniques for investigating the pyrolysis process of polymers.
Complementary Procedures
The applications of thermogravimetric analysis have been thoroughly examined. However, it is worth noting that many of the referenced papers have employed additional techniques to supplement the insights obtained from thermogravimetry. In specific investigations, the utilization of a supplementary method becomes crucial when attempting to interpret the progression of thermal decomposition. Without such an approach, the interpretation would rely solely on speculation.
It is recommended to conduct either a chemical analysis of the solid material extracted from the thermobalance at a suitable position on the thermogravimetric curve or alternatively, an analysis of the gas released during the process. The data obtained from thermogravimetry can be further enhanced and expanded through the utilization of solid-state chemistry techniques, thereby increasing its significance and applicability.
Differential thermal analysis (DTA)
It is a technique used in materials science and chemistry to study the thermal behavior of substances. The reason for selecting this method as the initial approach is due to its strong correlation with thermogravimetry. Both methods are characterized by their dynamic nature and reliance on procedural detail. The assertion that the optimal technique for thermogravimetric analysis is universally applicable to differential thermal analysis is unfounded. Undoubtedly, the utilization of equipment capable of simultaneously conducting thermogravimetry and differential thermal analysis on a single sample yields valuable and significant information.
References
- https://aurigaresearch.com/pharmaceutical-testing/thermogravimetric-analysis-tga/
- https://wcnt.wisc.edu/thermogravimetric-analysis/
- H. H. Willard, L. L. Merritt, J. R. Dean, and F. A. Settle, Instrumental Methods of Analysis (7th Edition), CBS Publishers and Distributions, India, 1986.
- https://www.innovatechlabs.com/newsroom/2333/applications-thermogravimetric-analysis/
- https://analyzing-testing.netzsch.com/en/contract-testing/methods/thermogravimetric-analysis
- https://www.hitachi-hightech.com/global/en/knowledge/analytical-systems/thermal-analysis/basics/tg.html
- https://chem.libretexts.org/Courses/Franklin_and_Marshall_College/Introduction_to_Materials_Characterization__CHM_412_Collaborative_Text/Thermal_Analysis/Thermogravimetric_analysis_(TGA)
- Vogel’s Textbook of Quantitative Chemical Analysis, 6th Edition, 2008.
- D.A. Skoog, F.J. Holler and T.A. Nieman, Principle of Instrumental Analysis, 5th Edition.
- https://chemistnotes.com/analytical_chemistry/thermogravimetric-analysis-principle-instrumentation-and-reliable-application/
- Coats, A. W.; Redfern, J. P. (1963). “Thermogravimetric Analysis: A Review”. Analyst. 88 (1053): 906924. Bibcode:1963Ana….88..906C. doi:10.1039/AN9638800906.
- https://www.sciencedirect.com/topics/materials-science/thermogravimetric-analysis
