Factors Influencing Corrosion
Intro
Corrosion is quite a complicated phenomenon. Lots of expertise from various fields (engineering, science, physics) have been working together to understand more on it and trying to prevent as well as control the corrosion from happening. It's among the active research area to be conducted by universities and industries. For those who are interested in knowing about the basics of corrosion, I will try to share some fundamentals on corrosion in this post. Hope we all can learn something and appreciate the science behind corrosion.
Potential Difference
When corrosion occurs, there will be chemical reactions (i.e-oxidation,reduction). Often the case, chemical reactions produce heat. However, the heat is quite difficult to detect since the rate of the chemical reactions are quite slow. Instead of measuring the heat, it is more practical to deal with electrical energy that involves with corrosion. In other words, energy that flows during corrosion reactions is viewed in the form of electrical energy.
By using voltmeter, we can measure the electrical potential (voltage) between 2 points, which is the energy differences. By using this method, we can find the potential difference between an anode and a cathode (i.e any 2 different metals), where the anode is in higher energy state than the cathode. In science, energy always travel from high energy state to lower energy state which means, during corrosion; the electrons will flow from high energy state (anode) to low energy state (cathode).
A voltmeter typically has 2 wires of different colours, a red and a black wires. The convention is that, the black wire should be connected to the negative terminal while the red wire connects to the positive terminal. By doing this, a positive value will be displayed by the voltmeter. Now, imagine if we have a zinc plate and a copper plate connected by electrochemical cell. If the black wire is connected to the zinc while the red wire is connected to the copper and the voltmeter is displaying a positive value, this means the zinc is the anode while the copper is the cathode. For visualisation purposes, here is the diagram.

As you can see, the voltmeter is displaying a positive value, meaning that the black wire is connected to the negative terminal (anode) while the red wire is connected to the positive terminal (cathode).
P/S: Note that, the electrolytes usually will be in in 1 mol ions concentration.
EMF Series
When dealing with corrosion topic, EMF series is one of the fundamentals. The EMF series or also called as electromotive force series is quite similar to galvanic series. It lists metals according to their oxidation potentials (active metals having larger negative potentials while passive metals not so negative). The differences are that:
- The list is only for pure metals. For example, it doesn't has stainless steel in the list because stainless steel is not in pure form. It consists of several elements. (i.e - iron (Fe), chromium (Cr), nickel(Ni) and etc..)
- EMF series has only 1 list. The list is generated based on experiment conducted in solutions of standards 1 mol ion concentration. EMF series, only exist for a single environment while for galvanic series, it has many lists, depending on the environment of the metals.
The EMF series is shown here as below:
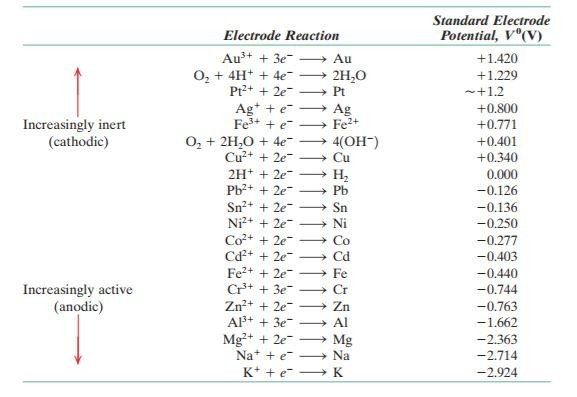
1 important observation is that, we can use EMF series to predict the potentials (voltage) for the image above. The voltage from the image showed a reading of 1.067 V. Let's compared this to values in EMF series. According to EMF series, zinc shows a value of -0.763 V while copper shows a value of +0.34 V. By subtracting 0.34 & (-0.763), this will yield 1.103 V. As you can see, there isn't much difference in value between the experimentation work and the EMF series.
Nernst Equation
As have been mentioned before, EMF series is only applicable to pure metals in 1 mol solutions. If the concentrations is different than 1 mol, Nerst equation has to be used to obtain the right potentials. At 25 deg C (room temperature). Nerst equation is in the form as below:
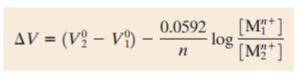
Where V2 and V1 are the electrodes potentials, n is the number of electrons in half cell reactions, M1 and M2 are the concentrations of ions in mol.
pH
Another factor that can affects corrosion is pH. As many people may have already known, pH is a measure of the acidity or alkalinity of a solution. In fact, pH is a short form of potential hydrogen. In actuality, pH refers to the hydrogen ion activity within a solution. Higher hydrogen ion activity corresponds to lower pH value while lower hydrogen ion activity corresponds to higher pH value. For pure water, its hydrogen ion concentration is 10^(-7) which corresponds to pH of 7. So you can see that, pH is taken from the power of 10. For acidic solution, it has lower pH due to it having greater ion concentration, for example in the order of 10^(-4), which correspond to pH of 4. pH can be better visualised in a scale as below:
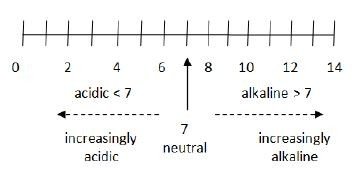
That's all for now. I hope all of you can learn something from here. There are more theories on corrosion but i will post them in multiple different posts.
Thank you.