The loss of electrons or a rise in the oxidation state of an ion, atom, or specific atoms in a molecule is referred to as oxidation. It’s also known as a reaction in which an element reacts with oxygen. Magnesium oxidation, for example, is the result of a chemical reaction between magnesium metal and oxygen, resulting in magnesium oxide.
The word “reduction” is derived from a Latin term that means “to return.” The gain of electrons or a decrease in the oxidation state of an ion, atom, or specific atoms in a molecule is referred to as reduction. At 20000 degrees Celsius, magnesium oxide and carbon combine to generate magnesium metal and carbon monoxide, resulting in magnesium metal and carbon monoxide.
Oxidation and Reduction in terms of Oxygen Transfer
Oxidation and reduction are chemical terms that refer to the transport of oxygen. Anything that goes back to a free metal state is referred to as a reduction reaction. Magnesium is subjected to both oxidation and reduction reactions.
2Mg (s) + O2 (g) → 2MgO (s) is a Oxidation reaction
MgO (s) + C (s) → Mg (s) + CO (g) is a Reduction reaction.
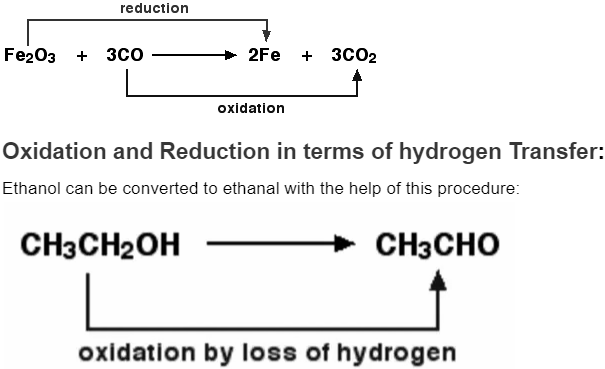
An oxidising agent is required to extract the hydrogen from ethanol. Potassium dichromate (VI) solution acidified with weak sulfuric acid is a common oxidising agent. By adding hydrogen, ethanal can be converted back to ethanol. Sodium tetrahydridoborate, NaBH4, is a suitable reducing agent in this case.
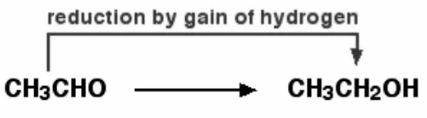
Oxidation And Reduction In Terms of Electron Transfer
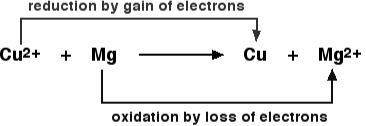
Because electrons are neither generated nor destroyed in a chemical reaction, both oxidation and reduction reactions are always intertwined. As a result, they only appear in pairs, and one cannot exist without the other. Magnesium is an excellent example of this principle. Magnesium is oxidised when it loses two electrons to oxygen, which is then reduced when magnesium accepts two electrons.
2 Mg + O2 → 2 [Mg2+] [O2-]
Because oxidation and reduction cannot occur separately, they are referred to as Redox reactions as a whole. The oxidizing agent is the reactant that oxidises the other reactant, while the reducing agent is the reactant that reduces it. It is critical to perform these processes one by one to determine if oxidizing agents take or give away electrons: Other reactants are oxidized by an oxidizing agent. It signifies that the amount of oxidizing agent is decreasing. As a result, the oxidizing agent will need to obtain electrons.
Comparing Strengths of Oxidants And Reductants
There are different types of oxidizers and reducers. The standard reduction potentials can be thought of as a ranking of compounds based on their ability to oxidize and reduce. Compounds having high oxidation states or high electronegativity that gain electrons in the redox reaction are known as strong oxidizing agents. Hydrogen peroxide, permanganate, and osmium tetroxide are examples of powerful oxidizers. Electropositive elements like hydrogen, lithium, sodium, iron, and aluminum are commonly used as reducing agents because they lose electrons in redox processes. Hydrides, such as sodium hydride, sodium borohydride, and lithium aluminum hydride, are frequently utilized as reducing agents in organic and organometallic processes.
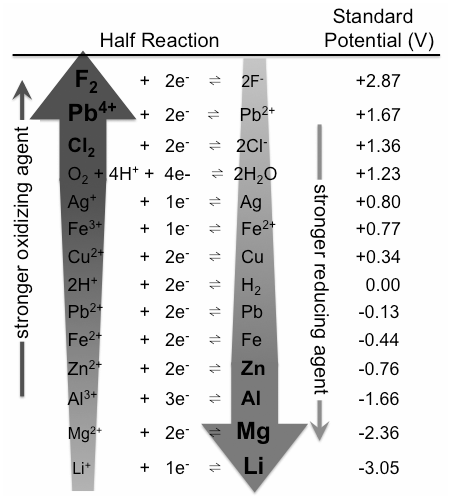
Eo values can be used to forecast the relative intensities of various oxidants and reductants. Standard electrode potentials can be used to assess the oxidative and reductive strengths of a range of compounds. The fact that electrode potentials are measured in aqueous solution, which allows for strong intermolecular electrostatic interactions, rather than in the gas phase, explains apparent irregularities.
Oxidation Vs Reduction
During a reaction, oxidation occurs when there is a loss of electrons.When a reactant acquires electrons during a reaction, on the other hand is reduction. A reduction-oxidation reaction, also known as a redox reaction, is a chemical reaction in which both reduction and oxidation take place at the same time. The reduced species acquire electrons whereas the oxidized species lose them in this process. Furthermore, the oxidation process does not necessitate the presence of oxygen, despite its name.
Conclusion
Many organic chemistry processes are classed as redox reactions. They occur as a result of oxidation state changes that occur without independent electron transport. It’s also known as an oxidation-reduction reaction, and it’s described as a reaction in which one reactant oxidizes while the other is reduced during the process. For example, during the combustion of wood with molecular oxygen, the oxidation state of carbon atoms in the wood increases, while oxygen atoms decrease with the creation of carbon dioxide and water. As a result, oxygen atoms receive electrons while carbon atoms are oxidized and lose electrons. In the above case, oxygen is the oxidizing agent and carbon is the reducing agent.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



