Alternatived Products of [ 1009-27-4 ]
Product Details of [ 1009-27-4 ]
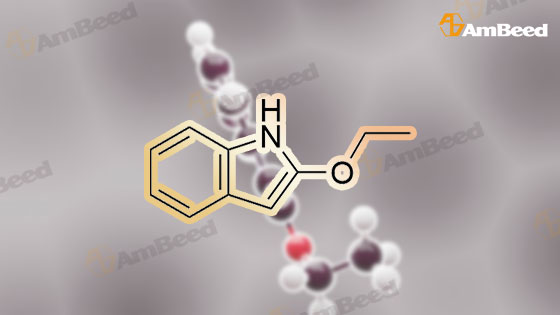
| CAS No. : | 1009-27-4 |
MDL No. : | MFCD13178666 |
| Formula : |
C10H11NO
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | LKEJIEFLJHCIAK-UHFFFAOYSA-N |
| M.W : |
161.20
|
Pubchem ID : | 12287052 |
| Synonyms : |
|
Application In Synthesis of [ 1009-27-4 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 1009-27-4 ]
- 1
-
 [ 108-31-6 ]
[ 108-31-6 ]

-
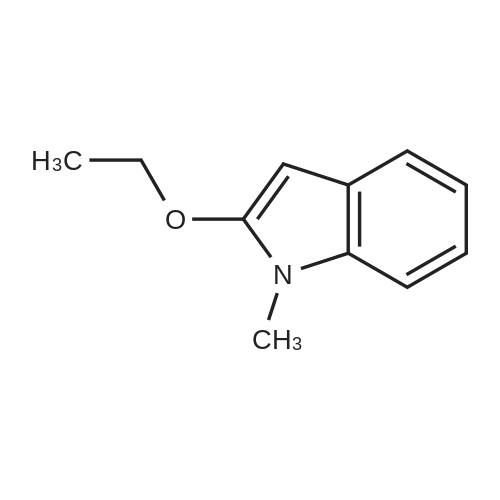
 [ 1009-27-4 ]
[ 1009-27-4 ]

-
 [ 13191-50-9 ]
[ 13191-50-9 ]
| Yield | Reaction Conditions | Operation in experiment |
|
In 1,4-dioxane Heating; |
|
- 2
-
 [ 2085-31-6 ]
[ 2085-31-6 ]

-
 [ 1009-27-4 ]
[ 1009-27-4 ]

-
 [ 61579-91-7 ]
[ 61579-91-7 ]
| Yield | Reaction Conditions | Operation in experiment |
|
In xylene for 0.2h; Heating; |
|
| Yield | Reaction Conditions | Operation in experiment |
|
Rk. in festem od. geloestem Zustand m. Sauerstoff -> Indirubin; |
|
|
Rk. m. CH3-O-C(O)-C-=C-C(O)-O-CH3 in abs. Dioxan <Dampfbad> -> /BRN= 488355/, /BRN= 488354/, /BRN= 492085/; |
|
|
Rk. m. Tetrahydrofuran-boran -> Indol u. Indolin; |
|
|
Rk. m. I2, Methanol-H2SO4 -> Isoindigo; |
|
|
Rk. m. 1) Luft-O2, 2) HCl -> Indirubin; |
|
|
Rk. m. 1) PbO2, 2) HCl -> Indirubin; |
|
|
Rk. mit CH3-O2C-C-=C-CO2-CH3, abs. Dioxan, Dampfbad -> 1 (/BRN= 488355/), 2 (/BRN= 488354/), 3 (/BRN= 492085/); |
|
|
Rk. mit THF-boran -> Indol u. Indolin; |
|
|
fester oder geloester Zustand, Sauerstoffzutritt -> indirubin; |
|
|
Rk. m. Piperidin, 24 h, Kochen, N2 -> 2-Piperidino-3H-indol; |
|

| Yield | Reaction Conditions | Operation in experiment |
|
Oxindol + Triaethyloxonium-borfluorid; |
|
|
(yield)90percent; |
|
|
Oxindol, Triethyloxonium-borfluorid; |
|
- 5
-
 [ 1009-27-4 ]
[ 1009-27-4 ]

-
 [ 14185-81-0 ]
[ 14185-81-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: dioxane / Heating
2: Py |
|
- 6
-
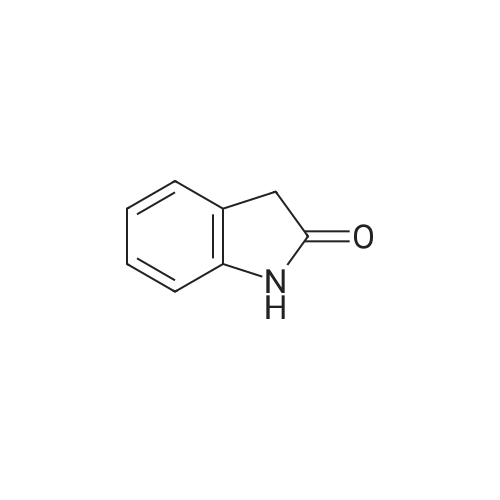 [ 59-48-3 ]
[ 59-48-3 ]

-
 [ 368-39-8 ]
[ 368-39-8 ]

-
 [ 1009-27-4 ]
[ 1009-27-4 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Stage #1: 2-oxoindole; triethyloxonium fluoroborate
Stage #2: With potassium carbonate |
|
|
Stage #1: 2-oxoindole; triethyloxonium fluoroborate In chloroform at 0 - 20℃; for 16h;
Stage #2: With tert-butyl methyl ether; triethylamine In chloroform; water at 20℃; for 0.5h; |
|
Reference:
[1]Location in patent: experimental part
Golovko; Solov'Eva; Anisimova; Smirnova; Evstratova; Kiselev; Granik
[Russian Chemical Bulletin, 2008, vol. 57, # 1, p. 177 - 185]
[2]Varlet, Thomas; Matišić, Mateja; Van Elslande, Elsa; Neuville, Luc; Gandon, Vincent; Masson, Géraldine
[Journal of the American Chemical Society, 2021, vol. 143, # 30, p. 11611 - 11619]
- 7
-
 [ 1009-27-4 ]
[ 1009-27-4 ]

-
 [ 4006-43-3 ]
[ 4006-43-3 ]
| Yield | Reaction Conditions | Operation in experiment |
| 30% |
In nitromethane at 195℃; |
|
- 8
-
 [ 1009-27-4 ]
[ 1009-27-4 ]

-
 [ 109-77-3 ]
[ 109-77-3 ]

-
 [ 156774-76-4 ]
[ 156774-76-4 ]
| Yield | Reaction Conditions | Operation in experiment |
| 77% |
In nitromethane at 100 - 110℃; |
|
- 9
-
 [ 1009-27-4 ]
[ 1009-27-4 ]

-
benzyl 2'-ethoxy-5-methyl-6-phenylspiro[cyclohexane-1,3'-indol]-3-en-2-yl-carbamate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: N,N,N',N'-tetramethylguanidine / water / 24 h / 20 °C / Darkness
2: C44H37O4P / dichloromethane / 48 h / 0 °C / Inert atmosphere |
|
Reference:
[1]Varlet, Thomas; Matišić, Mateja; Van Elslande, Elsa; Neuville, Luc; Gandon, Vincent; Masson, Géraldine
[Journal of the American Chemical Society, 2021, vol. 143, # 30, p. 11611 - 11619]
- 10
-
 [ 1009-27-4 ]
[ 1009-27-4 ]

-
benzyl ((1S,2S,5R,6R)-2'-ethoxy-5-methyl-6-phenyl-spiro[cyclohexane-1,3'-indol]-3-en-2-yl)carbamate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: N,N,N',N'-tetramethylguanidine / water / 24 h / 20 °C / Darkness
2: C48H37O4P / dichloromethane / 48 h / 0 °C / Inert atmosphere |
|
Reference:
[1]Varlet, Thomas; Matišić, Mateja; Van Elslande, Elsa; Neuville, Luc; Gandon, Vincent; Masson, Géraldine
[Journal of the American Chemical Society, 2021, vol. 143, # 30, p. 11611 - 11619]
- 11
-
 [ 100-52-7 ]
[ 100-52-7 ]

-
 [ 1009-27-4 ]
[ 1009-27-4 ]

-
3-(4-bromobenzylidene)-2-ethoxy-3H-indole
[ No CAS ]

-
3-(4-bromobenzylidene)-2-ethoxy-3H-indole
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 63.636 % de |
With N,N,N',N'-tetramethylguanidine In water at 20℃; for 24h; Darkness; Overall yield = 596 mg; |
|
Reference:
[1]Varlet, Thomas; Matišić, Mateja; Van Elslande, Elsa; Neuville, Luc; Gandon, Vincent; Masson, Géraldine
[Journal of the American Chemical Society, 2021, vol. 143, # 30, p. 11611 - 11619]
- 12
-
 [ 100-52-7 ]
[ 100-52-7 ]

-
 [ 1009-27-4 ]
[ 1009-27-4 ]

-
3-benzylidene-2-ethoxy-3H-indole
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 192 mg |
With N,N,N',N'-tetramethylguanidine In water at 20℃; for 24h; Darkness; |
|
Reference:
[1]Varlet, Thomas; Matišić, Mateja; Van Elslande, Elsa; Neuville, Luc; Gandon, Vincent; Masson, Géraldine
[Journal of the American Chemical Society, 2021, vol. 143, # 30, p. 11611 - 11619]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping