In the first post about carbohydrates, we mentioned that depending on the position of the carbonyl (C1 or C2) the sugar molecule can be an aldehyde or a ketone which are classified as an Aldose or a Ketose.

Today, we will go over the structure and stereochemistry of aldoses and ketoses with 4-7 carbon atoms. Let’s start with aldotereoses.
Four Carbon Aldehydes – Aldotetroses
There are two chiral centers and therefore, 22=4 stereoisomers are possible. These are the two pairs of enantiomers of threose and erythrose.

Notice that, D-erythrose and D-threose, for example, are diastereomers since only one of the stereogenic centers has a different (or identical in this case) configuration. You can read about the D and L notation for carbohydrates and amino acids here.
In general, two diastereomers that differ in the configuration of one chiral center only are called epimers. And when this pertains to cyclic hemiacetals like furanose and pyranose, we classify them as anomers.
The threo and erythro notation is a general approach for naming compounds with two stereogenic centers and is not restricted to carbohydrates only. In short, erythro is when the two common substitutes on the stereogenic centers are on the same side, and when they are on opposite sides, it is the threo.
Five Carbon Aldehydes – Aldopentoses
Aldopentoses have three stereogenic centers, and therefore there are eight (23) possible stereoisomers (four pairs of enantiomers). Four of the possible aldopentoses are D sugars, while the other four have an L configuration:

Among these, D-Ribose is the most common and perhaps important as it is the sugar building block of the DNA backbone in the deoxy form (Deoxyribonucleic acid).
Six Carbon Aldehydes – Aldohexoses
Aldohexoses have four stereogenic centers which means there are eight (24 = 16) possible stereoisomers This corresponds to eight pairs of enantiomers – eight D aldohexoses, and eight L aldohexoses:

Among aldopentoses, glucose is the most common and important since it is the building unit of starch, cellulose, and sucrose which is the table sugar.
As mentioned in the picture, D and L isomers are enantiomers and any other pair represents diastereomers.
For example, D-Glucose and D-mannose are diastereomers since the configuration of only one stereogenic center is changed.
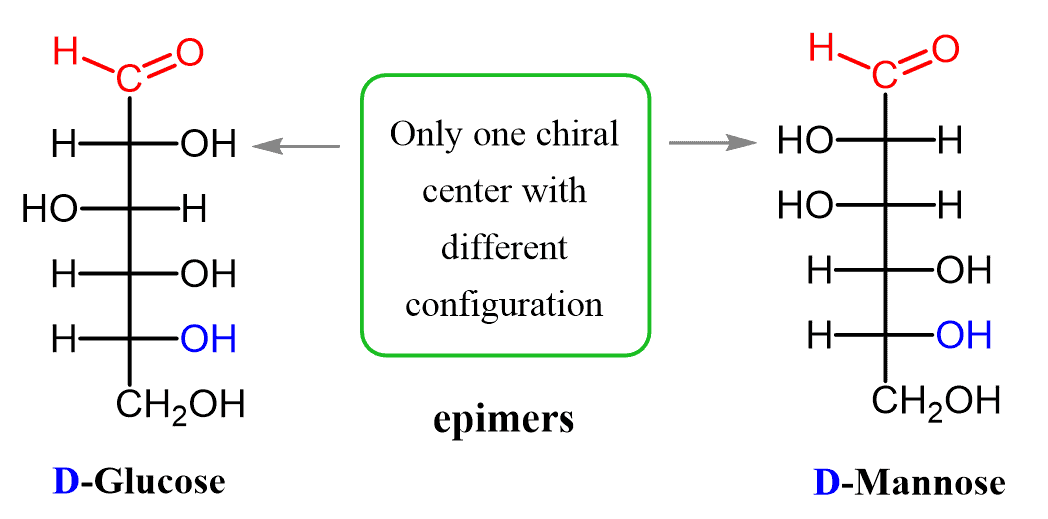
Diastereomers that differ in the configuration of only one chiral center are called epimers and we will talk about them in separate post to address more details.
All the aldoses can also be further classified into a family tree. This done for the D isomers since these are the naturally occurring sugars. We start with d-glyceraldehyde and keep adding a new chiral center just below the carbonyl group. For example, adding a new chiral center to glyceraldehyde generates two additional stereoisomers – D-Erythrose and D-Threose (check the relation of D-Erythrose and D-Threose with the threo and erythro notation) which are aldotetroses. These, in turn, generate four possible aldopentoses and then aldohexoses:

Ketoses
Two main differences between aldoses and ketoses:
1) ketoses contain a ketone rather than an aldehyde C=O,
2) because the C=O is on carbon number two, ketoses have one less chiral center than the corresponding aldehydes.
The family tree starts from the simplest ketose, dihydroxyacetone, and is built by adding a new stereogenic carbon between C2 and C3. D-fructose is the most common naturally occurring ketose found in many plants, where it is often bonded to glucose thus forming sucrose.

Need some practice on carbohydrates?
Check this Multiple-Choice, summary quiz on the structure and reactions of carbohydrates with a 40-min video solution!
Check also in Carbohydrates
- Carbohydrates – Structure and Classification
- Erythro and Threo
- D and L Sugars
- Aldoses and Ketoses: Classification and Stereochemistry
- Epimers and Anomers
- Converting Fischer, Haworth, and Chair forms of Carbohydrates
- Mutarotation
- Glycosides
- Isomerization of Carbohydrates
- Ether and Ester Derivatives of Carbohydrates
- Oxidation of Monosaccharides
- Reduction of Monosaccharides
- Kiliani–Fischer Synthesis
- Wohl Degradation


Please tell me the mechanism of this reaction.