Story highlights
FDA says a problem with the machines may prevent needed shock to patient
Agency issues recommendation on how to use device
Keep the AED until you get a replacement, FDA says
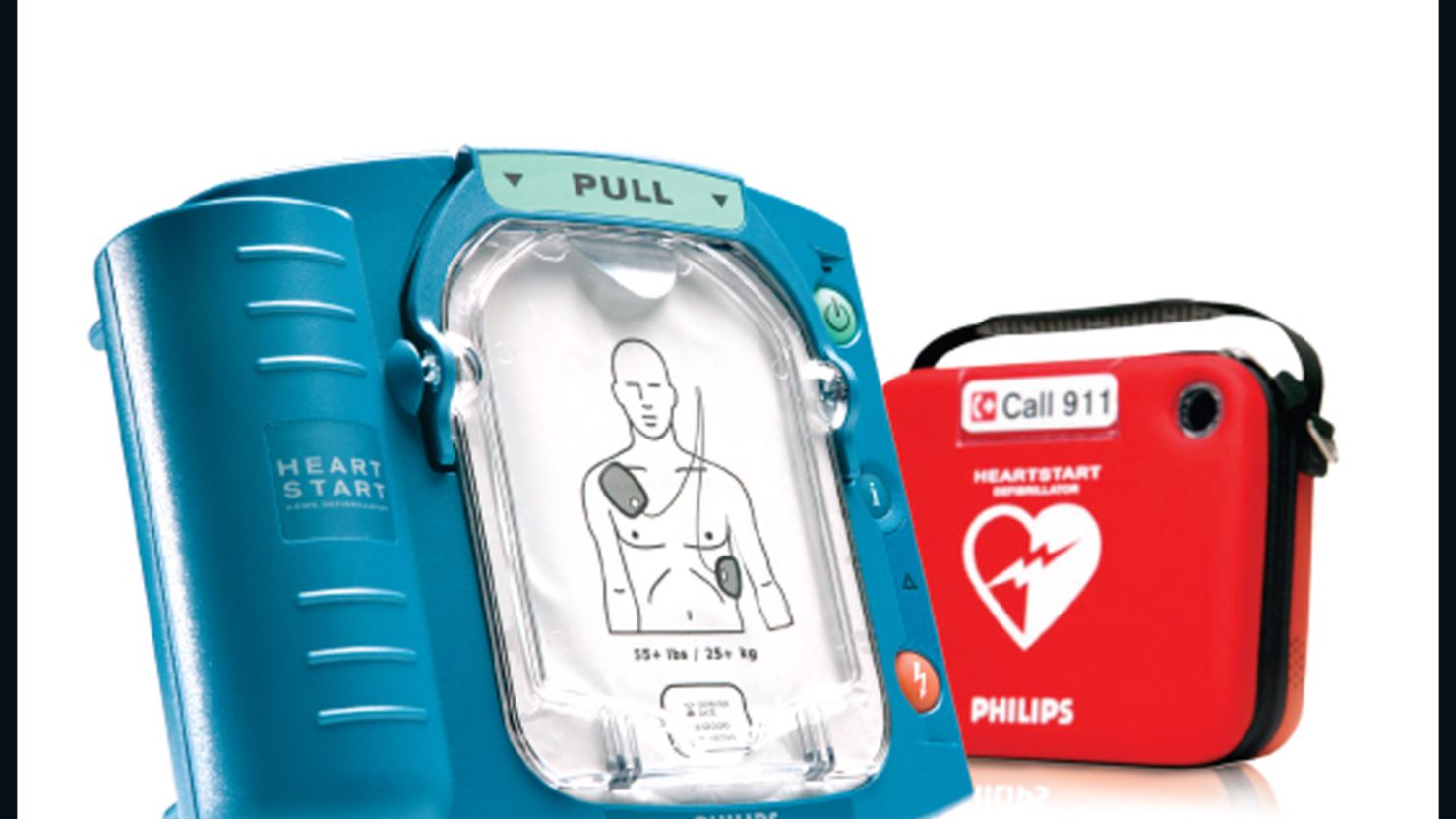
The Food and Drug Administration warned this week that thousands of Philips automated external defibrillators (AED) might not be able to deliver a needed shock in a cardiac emergency situation.
The agency said Tuesday customers should keep the defibrillators – designed for home use and use by emergency responders – until a replacement arrives.
In 2012, Philips recalled about 700,000 of the devices, saying a problem with an internal electrical component problem could falsely indicate the AED was ready to use, according to the FDA.
Last month, Philips updated its customers to say that the part failure could also cause the AEDs to be unable to deliver a shock.
“Despite current manufacturing and performance problems, the FDA considers the benefits of attempting to use an AED in a cardiac arrest emergency greater than the risk of not attempting to use the defibrillator,” said Steve Silverman, director of the office of compliance in the FDA’s Center for Devices and Radiological Health.
The FDA issued recommendations in a safety communication for using the devices during emergency situations and monitoring the readiness of the AED.
AEDs analyze heart rhythm and deliver a shock to return the heart beat to normal.
The devices were sold between 2005 and 2012 by Philips Healthcare with model names HeartStart FRx, HeartStart HS1 Home, and HeartStart HS1 OnSite.
Doctors develop a heart with no pulse
If you think you might have one of these products in your home or office, call Philips HealthCare and they will replace it. The phone number is 1-800-263-3342 or e-mail, HeartStartCapacitorAction@philips.com