Diversity of interstitial nemerteans of the genus Ototyphlonemertes (Nemertea: Monostilifera: Ototyphlonemertidae) in the South China Sea, with a comment on the distribution pattern of the genus
- 1Key Laboratory of Mariculture (Ministry of Education), and Institute of Evolution and Marine Biodiversity, Ocean University of China, Qingdao, China
- 2Faculty of Science, Hokkaido University, Sapporo, Hokkaido, Japan
The genus Ototyphlonemertes Diesing, 1863, consisting of 33 named species and numerous unnamed morphospecies/molecular entities, is a unique group of nemerteans that possess cerebral statocysts and specifically live in coarse-grained sands. Only eight named species of this genus have yet been recorded from the Indo-Polynesian biogeographic province, which harbors the highest marine biodiversity in the world. In recent years, Ototyphlonemertes were collected from eight sites along the South China Sea coasts. Nine species/entities were revealed by four phylogenetic markers (COI, 16S, 18S, 28S) analyzed by three species delimitation methods: Automatic Barcode Gap Discovery (ABGD), Poisson Tree Process (PTP), and Generalized Mixed Yule Coalescent model (GMYC). Six entities are described as new species based on integration of morphological and molecular species delimitations: Ototyphlonemertes conicobasis sp. nov., Ototyphlonemertes coralli sp. nov., Ototyphlonemertes similis sp. nov., Ototyphlonemertes sinica sp. nov., Ototyphlonemertes subrubra sp. nov., and Ototyphlonemertes yingge sp. nov. No morphological differences were detected between two entities and Ototyphlonemertes chernyshevi Kajihara et al., 2018, despite large genetic differences, so are treated as candidate species. Ototyphlonemertes ani Chernyshev, 2007 is first recorded in China. Based mostly on results of phylogenetic analyses, two previously established subgenera are re-defined, and a new subgenus, Procso subgen. nov., is established. Through reviewing the existing studies, we recognize 101 species/entities of Ototyphlonemertes, which are distributed in 18 marine biogeographic provinces. Most (88.1%) of them are endemic to a single biogeographic province, and evolutionary lineages endemic to a geographic area are not uncommon. Maximum diversity has been recorded in the Indo-Polynesian Province (22 species), though sampling to date has covered only a small part of the biogeographic province.
Introduction
Ototyphlonemertes Diesing, 1863 (Nemertea, Hoplonemertea, Monostilifera) is a cosmopolitan genus of interstitial nemerteans, a group with high diversity and special characters, and is very attractive for a variety of potential evolutionary studies (Andrade et al., 2011). Members of the genus mostly inhabit relatively small, isolated, coarse-grained, shallow marine sands (Envall and Norenburg, 2001; Leasi et al., 2016). They are slender, translucent, and usually less than 10 mm in length and not more than 0.5 mm in width (Envall and Norenburg, 2001), but a species up to 158 mm long was reported recently (Liu and Sun, 2018). All possess a pair of cerebral statocysts (diagnostic for the family Ototyphlonemertidae Bürger, 1895) and the adult lacks eyes (Envall and Norenburg, 2001). Currently this genus contains 33 valid species and many unnamed morphospecies and molecular entities (Envall and Norenburg, 2001; Leasi et al., 2016; Norenburg et al., 2022). For the classification of Ototyphlonemertes, several genus/subgenus names have been proposed (Chernyshev, 1993; Envall, 1996; Chernyshev, 1998), but they were not widely accepted in other studies (e.g., Gibson, 1995; Norenburg et al., 2022). Envall and Norenburg (2001) summarized the morphological data from about 100 geographical varieties and classified them into six morpho-groups (called phylomorphs), namely the Cirrula-, Duplex-, Fila-, Lactea-, Macintoshi-, and Pallida-morphs. Leasi et al. (2016) identified their worldwide specimens as seven morphological species/groups (Duplex, Erneba, Fila, Lactea, Macintoshi, Pallida, and Santacruzensis; the morph-group Cirrula seemed not to be included in this study). Their molecular phylogenies supported the monophyletic origin of the morphological species/groups Duplex, Erneba, Fila, and Pallida, while Lactea, Macintoshi, and Santacruzensis were not monophyletic groups. Recently, Kajihara et al. (2018a) proposed a “species group” (infra-sub-generic, supra-specific) rank for the three morpho-groups they studied, Ototyphlonemertes duplex species group (= Duplex morpho-group), Ototyphlonemertes macintoshi species group (= Macintoshi + Lactea morpho-groups), and Ototyphlonemertes parmula species group (= Fila morpho-group). Though Ototyphlonemertes can be classified into some morphospecies/morpho-groups and such classification is to certain extent supported by phylogenetic analyses, studies have revealed that cryptic species were common in Ototyphlonemertes (Andrade et al., 2011; Tulchinsky et al., 2012; Leasi and Norenburg, 2014; Leasi et al., 2016; Mendes et al., 2018). The species within each group are hardly distinguishable from each other solely based on morphological characters (Leasi and Norenburg, 2014; Leasi et al., 2016; Kajihara et al., 2018a).
The integrative approach that united different lines of evidence to solve taxonomic problems has been used in many taxa, such as tardigrades (Stec et al., 2018; Cesari et al., 2019), sea slugs (Jörger et al., 2012; Jörger and Schrödl, 2013), flatworms (Casu et al., 2009; Sluys et al., 2013), and so on. As commented by Sundberg et al. (2016a), molecular evidence proved most efficient for distinguishing nemertean species when crypticism existed. In recent years, DNA-based approaches have been successfully used to delimit species and discover cryptic species in different nemertean groups (e.g., Strand and Sundberg, 2005; Sundberg et al., 2009a; Sundberg et al., 2009b; Chen et al., 2010; Fernández-Álvarez and Machordom, 2013; Leasi and Norenburg, 2014; Kang et al., 2015; Sundberg et al., 2016b; Krämer et al., 2017; Chernyshev et al., 2018; Sagorny et al., 2019). The approach combining molecular and morphological information has been becoming a standard method for describing new species of nemerteans (Strand and Sundberg, 2011; Strand et al., 2014; Hiebert and Maslakova, 2015; Kajihara et al., 2018a).
Indo-Polynesian Province, a biogeographic province as defined by Briggs and Bowen (2012), harbors the highest marine biodiversity in the world (Hoeksema, 2007; Veron et al., 2011; Zhang et al., 2021). Nevertheless, there may be an important gap in knowledge of genus Ototyphlonemertes in this province. Published records of the Ototyphlonemertes are concentrated in the western Atlantic-Caribbean region, European North Atlantic, coasts of Brazil and Chile, the Sea of Japan, and southern Vietnam (Kirsteuer, 1977; Chernyshev, 1993; Chernyshev, 1998; Chernyshev, 2003; Kajihara, 2007a; Andrade et al., 2011; Leasi and Norenburg, 2014; Leasi et al., 2016; Kajihara et al., 2018a; Mendes et al., 2018). A molecular phylogenetics study by Leasi et al. (2016) provided an important world-wide view of the diversity and biogeography of the Ototyphlonemertes, but it included only five individuals from Indo-Polynesian Province (from Okinawa Island, Japan) representing three molecular entities. Only eight nominal species and two(?) unidentified congeners of Ototyphlonemertes are known from the Indo-Polynesian Province (Rao and Ganapati, 1968; Chernyshev, 2007; Kajihara et al., 2007; Kajihara et al., 2018a; Kajihara et al., 2018b; Liu and Sun, 2018).
To address this gap, new specimens were collected from eight localities along the South China Sea coasts. Together with the new sequences of specimens of Kajihara et al. (2018a) and Liu and Sun (2018), we performed molecular species delimitation and reanalyzed the diversity and phylogeny of the genus Ototyphlonemertes based on multiple DNA markers. Six molecular entities with morphological difference are described as new species, and two entities without morphological difference with known species are temporarily treated as candidate species following recent recommendations (Vieites et al., 2009; Padial et al., 2010). This expands to 14 the number of described Ototyphlonemertes species in the Indo-Polynesian Province.
Materials and methods
Sample collection and morphological studies
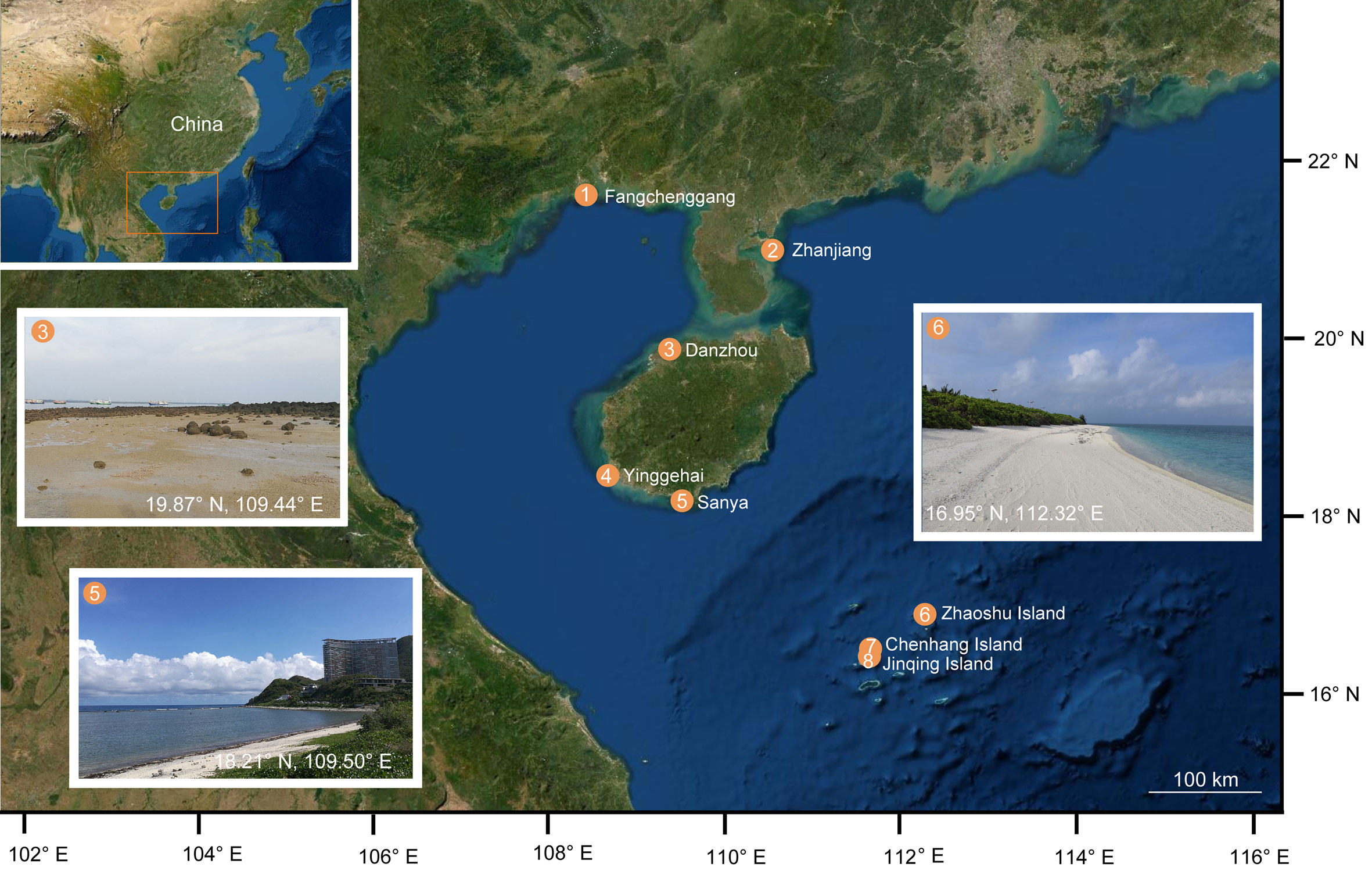
A total of 48 Ototyphlonemertes specimens were collected from eight localities along coasts of South China Sea between 2013 and 2019 (Figure 1). Specimens were extracted from sandy sediments following Norenburg (1988): each sediment sample was placed in a 20-L plastic bucket, covered with seawater, and then stirred; as the sediment settled, the water—while still spinning—was quickly decanted through a 0.25-mm mesh; for a single sediment sample, this procedure was repeated for 3–4 times, and the residue on the mesh was collected and placed in plastic bottles. In the laboratory, the extracted sample was placed in a tray (40×30×10 cm) filled with seawater. Nemertean worms crawling out were transferred to petri dishes with seawater. After being anaesthetized with a MgCl2 solution isotonic with seawater, worms were observed and photographed under microscopes. Some anaesthetized individuals were fixed in Bouin’s fluid for histological study. Serial sections of 7 µm in thickness were stained with Mallory’s trichrome method. Specimens for molecular study were preserved in 95% ethanol.
Molecular analyses
DNA was extracted using the MicroElute Genomic DNA Kit (OMEGA) following the manufacturer’s protocols. Partial sequences of the mitochondrial cytochrome c oxidase subunit I (COI) and 16S ribosomal RNA (16S), and the nuclear small subunit ribosomal RNA (18S) and large subunit ribosomal RNA (28S) genes were PCR-amplified and determined. The PCR was carried out using the universal primers: LCO1490 and HCO2198 (Folmer et al., 1994) for COI; 16sar-L and 16sbr-H (Palumbi et al., 1991) for 16S; EukF and SR7 (Vilgalys and Sun, 1994; Sands et al., 2008) for 18S; LSU5 and LSU3 (Littlewood, 1994) for 28S. Thermal cycling was initiated with 3 min at 94°C, followed by 30 cycles of denaturation at 94°C for 45 s, annealing at 50°C (COI) or 48°C (16S) or 52°C (18S, 28S) for 45 s, and extension at 72°C for 1 min. The cycling ended with a 7-min sequence extension at 72°C. The PCR products were purified using QIA-quick gel purification kit (Qiagen). Double-stranded Sanger sequencing was conducted by BGI (Qingdao, China) using both forward and reverse primers. All sequences obtained in the present study were deposited in the GenBank database under accession numbers shown in Table S1.
A total of 187 individuals of Ototyphlonemertes were used for molecular analyses (Table S1). Four molecular markers were aligned separately using MAFFT ver. 7 (Katoh and Standley, 2013), employing the G-INS-I strategy for COI and 16S, and the Q-INS-I algorithm for 18S and 28S genes (Katoh et al., 2009). The 16S sequences of 20190728I1 and 20190728I2 were manually adjusted based on results from BLASTN queries (NCBI) to achieve better matches. Ambiguous regions were removed with the web version of Gblocks ver 0.91b using default parameters (Castresana, 2000). The final fragment lengths were 564 bp, 439 bp, 590 bp, and 911 bp for COI, 16S, 18S, and 28S, respectively.
Phylogenetic trees were reconstructed using haplotypes of concatenated COI, 16S, 18S, and 28S sequences (using Amphiporus lactifloreus (Johnston, 1828) and Emplectonema buergeri Coe, 1901 as outgroups). Haplotypes were generated by DnaSP ver 5 (Librado and Rozas, 2009). The models for each separated locus were general time reversible plus gamma-distribution plus a proportion of invariant sites (GTR + G + I) for COI, 16S, and 28S, and Hasegawa–Kishino–Yano plus gamma-distribution plus a proportion of invariant sites gamma (HKY + G + I) for 18S, selected by MrModeltest ver 2.3 (Nylander, 2004) according to the Akaike information criterion. The Maximum likelihood (ML) analysis was performed at the CIPRES Science Gateway web with RAxML (Stamatakis, 2014) on XSEDE, in which 1000 rapid bootstrap replicates and the GTRGAMMA model were used to evaluate and optimize the likelihood of the final tree. The Bayesian inference (BI) was performed using MrBayes ver 3.2.2 (Ronquist et al., 2012), and the concatenated dataset was divided into four partitions (genes), each having its own best-fit substitution models and parameters determined by MrModeltest as aforementioned. MrBayes was run for 10 million generations with a tree being sampled every 1000 generations, with two parallel runs and four independent Markov chains per run. The standard deviation of the split frequencies (<0.01) is used as the criterion to validate the convergence of the analysis. After discarding the first 25% of the generations as burn-in, the 50% majority-rule consensus trees from the remaining trees are constructed to estimate posterior probabilities for each clade. Uncorrected p-distances were calculated in MEGA ver 10 (Kumar et al., 2018).
For species delimitation analyses, the General Mixed Yule-Coalescent Model (GMYC) (Pons et al., 2006; Monaghan et al., 2009), Bayesian Poisson Tree Processes (bPTP) (Zhang et al., 2013), and Automatic Barcode Gap Discovery (ABGD) (Puillandre et al., 2012) methods were used. Because taxonomic entities of Ototyphlonemertes estimated from analyses of 18S and 28S were not reliable proxies for diversity and very likely underestimated true species richness (Leasi et al., 2016), only the COI, 16S, and concatenated datasets were applied in these analyses. An ultrametric gene tree obtained with BEAST ver 1.10.4 (Suchard et al., 2018) was used as input for the GMYC analysis in R ver 3.4.4 (R Core Team, 2018) with the SPLITS package. For BEAST analysis, the best substitution models were GTR + G + I for COI, 16S and concatenated dataset, which were evaluated by MrModeltest. A relaxed lognormal clock and the Yule prior models were used. MCMC chains were run for 100 million generations and sampled every 10000 generations. The first 10% samples were discarded as burn-in with the TreeAnnotator program. We checked convergence by Tracer ver 1.7.1 (Rambaut et al., 2018). For bPTP analysis, a ML tree was firstly generated at the CIPRES Science Gateway web with RAxML (Stamatakis, 2014) on XSEDE with 1000 rapid bootstrap replicates and the GTRGAMMA model. Then, the tree was used as the input for the bPTP analysis, which was run at the web server (http://species.h-its.org/ptp/) with parameters: MCMC, 500000 generations; Thinning, 100; Burn-in, 0.1; Seed, 123, and always checked convergence. The ABGD analysis was performed in a web-based interface (http://wwwabi.snv.jussieu.fr/public/abdg/abdgweb.html) with the following settings: Pmin, 0.001; Pmax, 0.1; Steps, 10; X (relative gap width), 1.0; Nb bins (for distance distribution), 20; distance, Kimura (K80) TS/TV 2.0.
Results
Phylogenetic analyses
We obtain 156 new sequences, 35 for COI, 40 for 16S, 42 for 18S and 39 for 28S (Table S1). Based on the concatenated dataset, the two phylogenetic methods (BI and ML) yield almost identical topologies (Figure 2; Figure S1). The members of Ototyphlonemertes are divided into two major clades (Figure 2). The first clade refers to the O. macintoshi species group, including the Macintoshi and Lactea morpho-groups of Envall and Norenburg (2001). The second clade is composed of the Erneba, Pallida, and Santacruzensis morphological species/groups (see Leasi et al., 2016), the O. parmula species group, and the O. duplex species group (see Kajihara et al., 2018a), but is not supported by high nodal values. A sister relationship between the monophyletic Erneba morphological species/group and O. parmula species group is supported by high nodal values (BP = 97%, PP = 1.00). The O. duplex species group constitutes a monophyletic clade, but the Santacruzensis morphological species/group is not monophyletic (Figure 2).
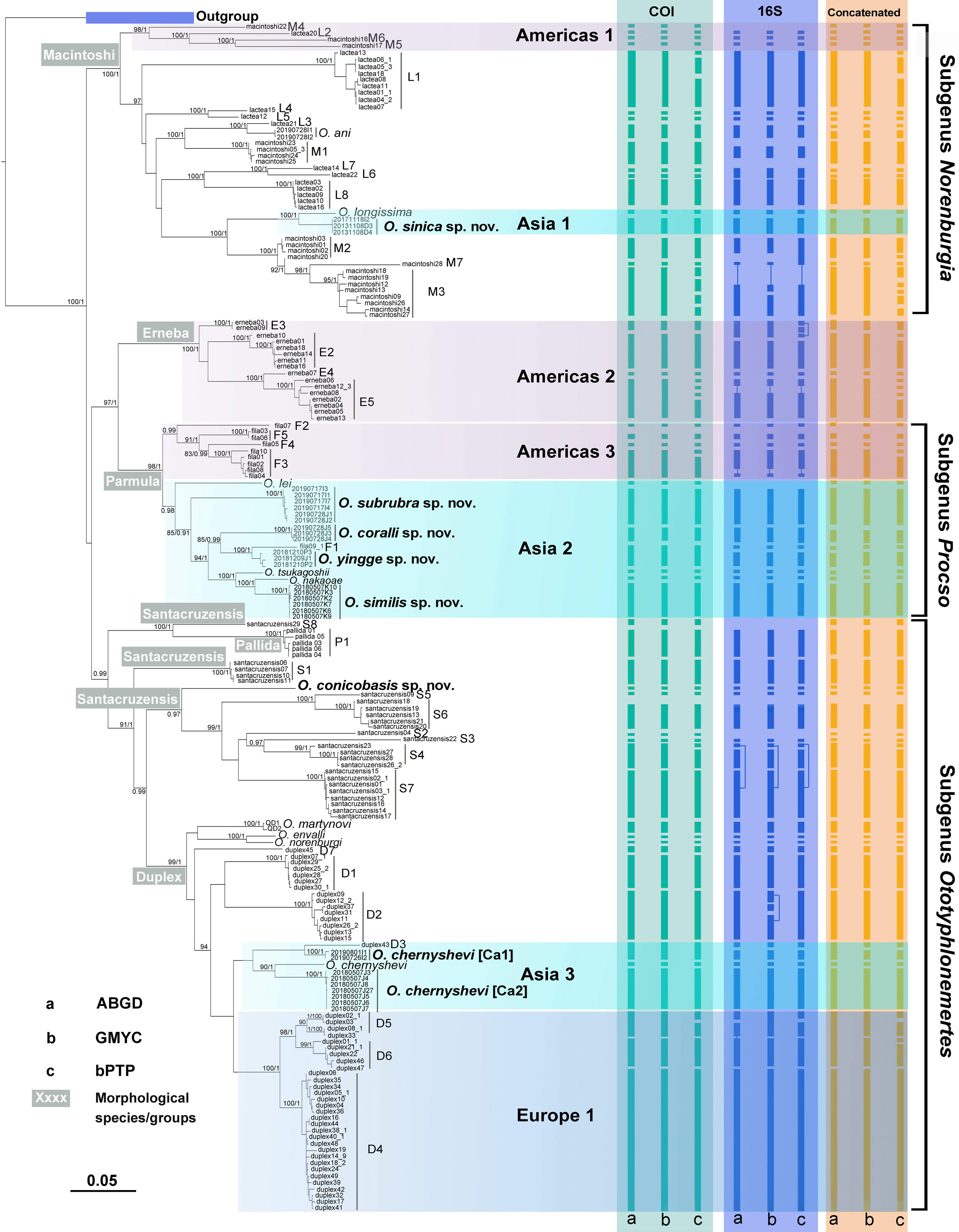
Figure 2 Phylogenetic tree and results of species delimitation analyses. The tree is reconstructed by RAxML analyses based on the concatenated dataset (COI, 16S, 18S and 28S). The Bayesian inference analysis yields almost identical tree topology (see Figure S1). Bootstrap support values >80 of the RAxML analysis (1000 bootstrap replicates) and Bayesian posterior probabilities >0.80 are provided as numbers next to nodes. Results of three species-delimitation analyses, ABGD, GMYC, bPTP, are indicated by the columns of vertical bars for each marker.
Lineages endemic to a particular geographic area are common in the genus. For instance, the clades Americas 1, 2, and 3 are only recorded from Americas, mostly in Panama and Caribbean waters; the clades Asia 1, 2, and 3 are recorded only from the South China Sea and nearby Asian waters (several questionable species/entities are also recorded from islands in the eastern Pacific, see below); the clade Europe 1 is an endemic lineage of European waters (Figure 2).
DNA taxonomy
The GMYC analysis results in 54 entities for COI, 52 for 16S, and 57 for the concatenated dataset; bPTP results in 65 entities for COI, 51 for 16S, and 72 for the concatenated dataset; and ABGD results in 54 entities for COI, 50 for 16S and 55 for the concatenated dataset (Figure 2; Table S2). Present molecular delimitation analyses consistently allocate the specimens collected from the South China Sea to 10 molecular entities, with two of them belonging to Ototyphlonemertes ani Chernyshev, 2007 and Ototyphlonemertes longissima Liu and Sun, 2018, and the other eight being undescribed entities. All the six species (Ototyphlonemertes chernyshevi Kajihara et al., 2018, Ototyphlonemertes envalli Kajihara et al., 2018, Ototyphlonemertes lei Kajihara et al., 2018, Ototyphlonemertes nakaoae Kajihara et al., 2018, Ototyphlonemertes norenburgi Kajihara et al., 2018, and Ototyphlonemertes tsukagoshii Kajihara et al., 2018) described from the Gulf of Thailand (Kajihara et al., 2018a) are well supported by the present multiple marker analyses. However, the results obtained from different datasets are not always consistent. For instance, in analysis based on COI dataset, O. ani and network L3 are classified as one species, Ototyphlonemertes yingge sp. nov. and network F1 as one species, Ototyphlonemertes chernyshevi [Ca1] and network D3 as one species, whereas in analyses with 16S and concatenated datasets, each of them is delimitated as a separate species (more information see Remarks of related species).
The uncorrected p-distances of COI between the entities range from 7.7% to 22.5%, those within entities range from 0% to 3.9%. For 16S, the between-entity distances range from 2.9% to 20.8%, those within entities range from 0% to 2.2%.
Systematic description
Family Ototyphlonemertidae Bürger, 1895
Genus Ototyphlonemertes Diesing, 1863
Remarks: The taxonomy and nomenclature at the infra-generic, supra-specific ranks in Ototyphlonemertes have been in flux (Kajihara et al., 2018a). As mentioned in the Introduction and reviewed by previous authors (Chernyshev, 1993; Kajihara et al., 2018a), several classification strategies have been proposed for genus Ototyphlonemertes, separating it into different genera (Chernyshev, 1993; Envall, 1996), classifying it to different subgenera (Chernyshev, 1993; Chernyshev, 1998), different morpho-groups (polymorphs, morphological species) (Envall and Norenburg, 2001; Leasi et al., 2016) or species groups (Kajihara et al., 2018a). In this study, the taxonomic rank subgenus is adopted for the following reasons. 1) Members of the genus can be segregated into different groups by both morphological and molecular evidences, which are basically supported by each other. 2) Names of morpho-groups/polymorphs were not proposed in a form satisfying the requirement of the International Code of Zoological Nomenclature (ICZN). 3) At least some con-group species can be easily recognized by morphological characters. For instance, in Macintoshi group, Ototyphlonemertes nikolaii Chernyshev, 1998, and Ototyphlonemertes dolichobasis Kajihara, 2007 (Kajihara, 2007a) can be distinguished from the others doubtlessly by the morphology of the stylet and basis. Thus, there may have good morphological species within a ‘group’, though each of them may have cryptic diversity (species). In this case, species group (an infra-subgeneric, supra-specific rank) (Kajihara et al., 2018a) seems to be more appropriate for the collection of such cryptic or sibling species than for group in whole.
The classification of subgenera is adopted by the current version of World Nemertea Database, which lists three subgenera, Duplex Kajihara, Tamura and Tomioka, 2018; Macintoshi Kajihara, Tamura and Tomioka, 2018; and Parmula Kajihara, Tamura and Tomioka, 2018 (Norenburg et al., 2022). However, Kajihara et al. (2018a) proposed these “species group” names for species aggregates, and indicated that the rank was infra-subgeneric and supra-specific, and did not designate any type species. They cannot be considered as generic-group names (ICZN: Articles 6.1, 6.2; Recommendations 6A, 6B). Chernyshev (1993) separated the genus into two genera and established the genus Norenburgia Chernyshev, 1993 for species without cerebral organs (consisting of two subgenera, Norenburgia Chernyshev, 1993, with cephalic cirri; Accirinia Chernyshev, 1993, no cephalic cirri). This proposal was not adopted by other authors because the names were considered to be not formally diagnosed (e.g., Gibson, 1995). To our opinion, the nomenclatural proposal of Chernyshev (1993) was not contradictory to the ICZN, though the original diagnoses were very simple. Moreover, histological characters based on serial sections are no longer considered as indispensable for making “formal” diagnoses of nemertean taxa (e.g., Sundberg and Strand, 2010; Strand and Sundberg, 2011; Sundberg et al., 2016a). Hence, the generic-group name Norenburgia (as well as Accirinia) is considered as an available name in the present paper.
Chernyshev (1998) degraded the rank of Norenburgia from genus to subgenus. However, both of the subgenera Ototyphlonemertes Diesing, 1863 and Norenburgia Chernyshev, 1993 defined by morphological characters (Chernyshev, 1998) are not monophyletic in phylogenetic trees (Figure 2; see also Leasi et al., 2016; Kajihara et al., 2018a). Here, based on morphology and molecular phylogeny, these subgenera are redefined and a new subgenus is established.
Subgenus Ototyphlonemertes Diesing, 1863
Typhlonemertes Du Plessis, 1891: 416
Type species: Oerstedia pallida Keferstein, 1862.
Diagnosis (modified from Chernyshev, 1998): Statolith oligogranular, with 2–5 granules (rarely 6–10). Cerebral organs normal, locating usually close to brain. Proboscis with short diaphragm, bulbous middle chamber, opaque posterior chamber. Stylet smooth, basis thick. Central stylet not longer than two times of basis length. Cirri present at both ends.
Remarks: Chernyshev (1993) proposed to restore the name Typhlonemertes Du Plessis, 1891 as a subgenus. Chernyshev (1998) merged Typhlonemertes into the subgenus Ototyphlonemertes, and classified species with oligogranular statoliths (2–3, rarely 4–9 particles that do not aggregate in a single stone), well developed cerebral organs and smooth stylets into the subgenus Ototyphlonemertes. Ototyphlonemertes erneba Corrêa, 1950 was accordingly included in this subgenus (Chernyshev, 1998). The only morphological difference between the Erneba morphological species/group and the subgenus Ototyphlonemertes is that the ratio of stylet/basis is at least 2:1 in Erneba morphological species/group. However, in phylogenetic trees (Figure 2; see also Leasi et al., 2016), the Erneba morphological species/group is a sister group of Parmula morpho-group, which possesses sculptured stylets (allocated to a new subgenus; see below). Since the morphological similarity is not supported by molecular analyses, here the Erneba morphological species/group is excluded from the subgenus Ototyphlonemertes. The subgenus thus consists of species of the Santacruzensis morphological species/group, Pallida morphological species/group (Leasi et al., 2016), and Duplex morpho-group (Envall and Norenburg, 2001) (= O. duplex species group; Kajihara et al., 2018a). Currently there are 13 named species in this subgenus: Ototyphlonemertes antipai Müller, 1968; Ototyphlonemertes aurantiaca (Du Plessis, 1891); Ototyphlonemertes brunnea Bürger, 1895; O. chernyshevi; Ototyphlonemertes conicobasis sp. nov.; Ototyphlonemertes correae Envall, 1996; Ototyphlonemertes duplex Bürger, 1895; O. envalli; Ototyphlonemertes evelinae Corrêa, 1948; Ototyphlonemertes martynovi Chernyshev, 1993; O. norenburgi; Ototyphlonemertes pallida (Keferstein, 1862); and Ototyphlonemertes santacruzensis Mock and Schmidt, 1975.
Ototyphlonemertes (Ototyphlonemertes) conicobasis sp. nov.
Zoobank registration: urn:lsid:zoobank.org:act:A2E948F4-0EAC-400B-A34E-7C81D93D324E.
Material examined: Holotype: MBM287546, Yinggehai (18.50°N, 108.69°E), Ledong, Hainan, China, coll. Hai-Long Liu, 10 December 2018, immature, full series of transverse sections, deposited at the Marine Biological Museum, Chinese Academy of Sciences (MBMCAS), Qingdao, China. Voucher specimen: 20181210M2, locality same as holotype, coll. Hai-Long Liu, 10 December 2018, immature, extracted DNA from the whole body, deposited at Institute of Evolution and Marine Biodiversity, Ocean University of China (IEMBOUC).
Etymology: The specific name is a compound of the Greek words kōnikos (conical) and basis (basis), referring to the conical basis of this species.
Description (Figure 3): Length 4–8 mm, width 0.3–0.4 mm, uniform. Epidermis translucent whitish or with pale-yellow tinge in intestinal region; slightly reddish in brain region (Figure 3A). Cephalic furrows (posterior cephalic furrows; same below) posterior to brain (Figures 3A, B). Cerebral organs normal (Envall and Norenburg, 2001), locating near antero-lateral border of brain. Cirri present at hind end and cephalic region. Cirrus formula (cf. Norenburg, 1988) in holotype: A=8, B=0, C=7, D=5+5, E=0, tail=6 (Figure 3B). Paired statocysts ovoid in shape, 34–37 µm in diameter; statoliths oligogranular, containing 4 or 5 granules (Figures 3E, F). Rhynchocoel extending to about 2/3 of body length (Figure 3C). Proboscis looped in rhynchocoel (Figure 3C); anterior chamber about 4 mm long, approximately twice posterior chamber length (Figure 3C), papillae with cap-like distal end (Figure 3H); diaphragm short, 106–112 µm long, 141–150 µm wide; middle chamber bulbous, 87–109 µm long, 116–150 µm wide; posterior chamber of opaque type (as defined by Envall and Norenburg, 2001), about 1.8 mm long (Figures 3C, G). Stylets smooth; central stylet 44–46 µm long; basis rounded conical, 40–42 µm long, 22–24 µm wide, length/width ratio (B/b) 1.7–1.8; stylet/basis ratio (S/B) 1.1; accessory stylet pouches 2, each containing 4 stylets, pointing either forward or backward (Figures 3D, G). Intestine with shallow diverticula (Figure 3C).
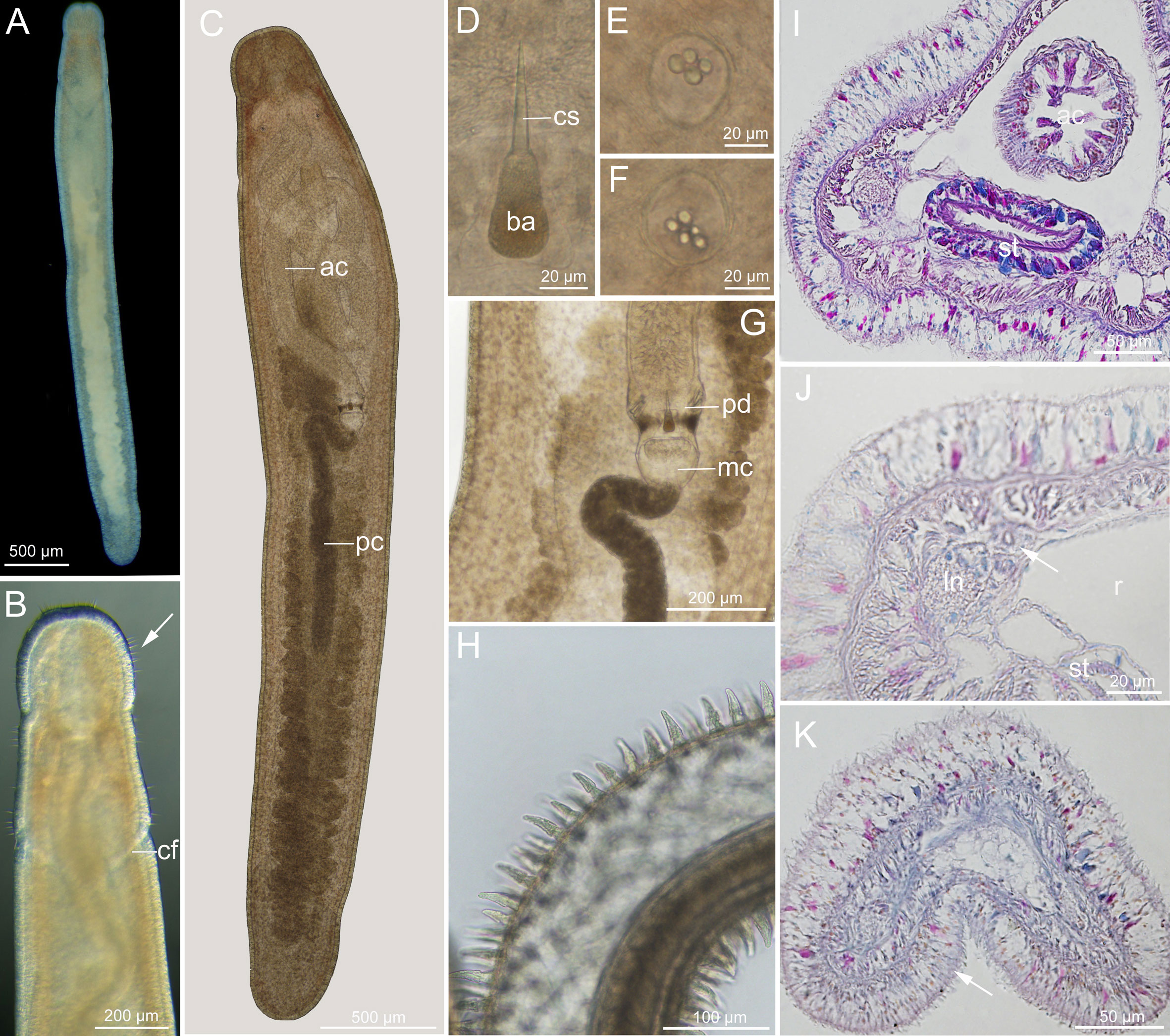
Figure 3 Ototyphlonemertes (Ototyphlonemertes) conicobasis sp. nov. (A) Photograph of a live specimen; (B) dorsal view of head, arrow indicating cirri; (C) composite photomicrograph of a squeezed specimen; (D) central stylet and basis; (E, F) statoliths; (G) photomicrograph of partial proboscis from a squeezed specimen; (H) everted proboscis; (I, J) transverse section through stomach region, nephridia duct is arrowed; (K) transverse section through caudal adhesion plate (arrowed). ac, anterior chamber of proboscis; ba, basis; cf, cephalic furrow; cs, central stylet; ln, lateral nerve; mc, middle chamber of proboscis; pc, posterior chamber of proboscis; pd, proboscis diaphragm; r, rhynchocoel; st, stomach.
Ciliated glandular epidermis 15–32 µm thick. Dermis 1–2 µm thick. Outer circular muscle layer mostly one or two fibers thick. Longitudinal muscle layer 5–15 µm thick. Dorsoventral muscles not found. Rhynchocoel consisting of outer circular and inner longitudinal muscle layers. Esophagus about 140 µm long. Stomach with cilia and gland cells (Figure 3I), approximately 308 µm long, stomach wall barely folding, becoming thinner and less glandular at pylorus region. Intestine with no caecum. Frontal organ not observed. Cephalic glands well developed, extending back to brain region. Dorsal cerebral commissure 12 µm thick, ventral cerebral commissure 25 µm thick; extra ventral commissure not detected. Excretory system containing 1–2 collecting tubules on either side, locating close to lateral nerves, extending from posterior part of stomach region to anterior part of intestinal region (Figure 3J). Caudal adhesion plate present, characterized by subanal aggregation of epidermal gland cells in sections (Figure 3K).
Ecology: Intertidal, coarse-grained sand.
Distribution: So far, the species is only known from the type locality, Yinggehai, Ledong, Hainan, China.
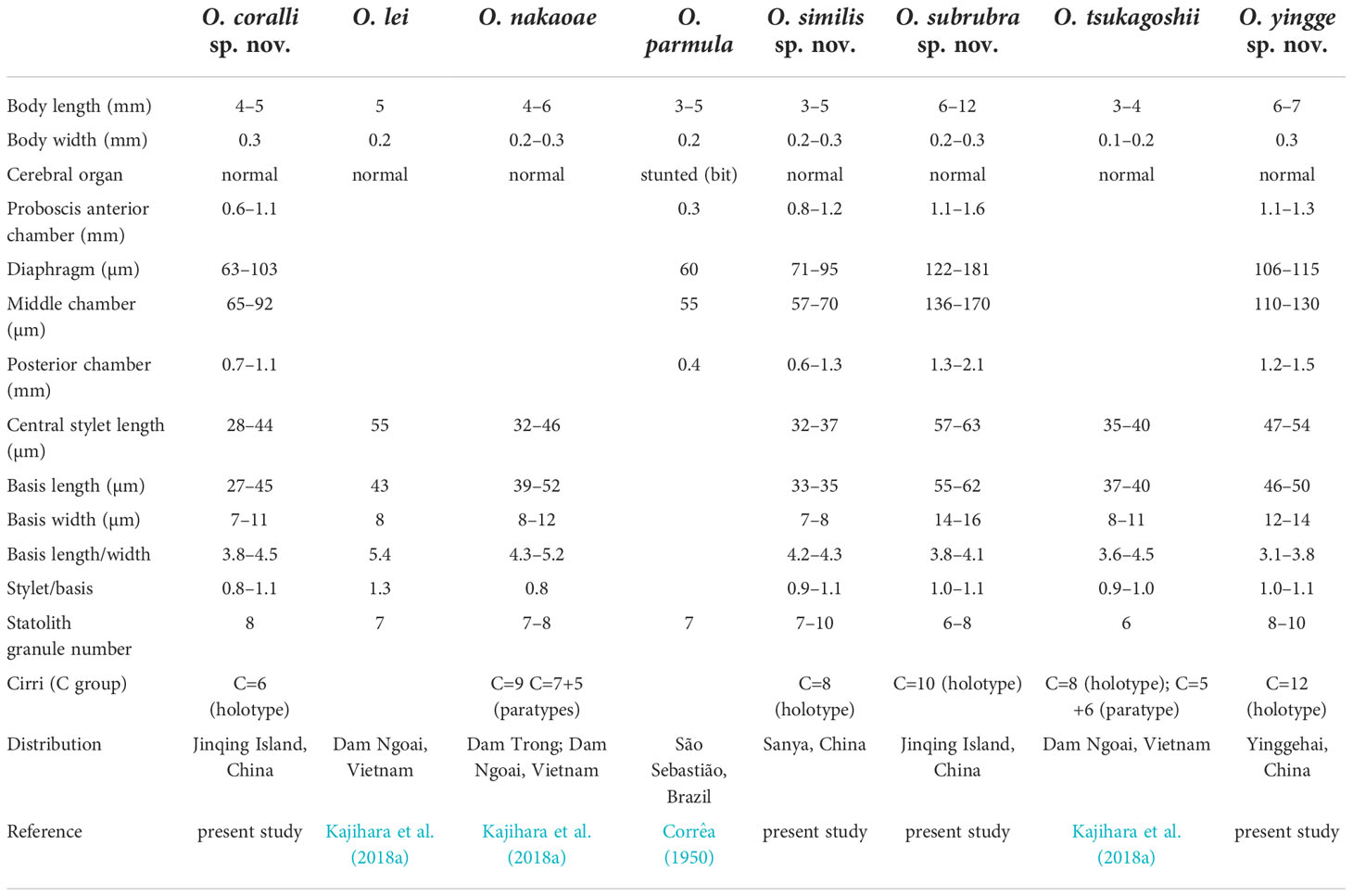
Remarks: Ototyphlonemertes conicobasis sp. nov., which is supported by three species delimitation analyses, is nested within the Santacruzensis morphological species/group in the molecular phylogenetic tree (Figure 2). The new species has a conical-shaped stylet basis with the largest width (22–24 µm) recorded in this genus (13 µm in O. santacruzensis). The central stylet and basis of O. conicobasis sp. nov. are much longer than those of O. santacruzensis (44–46 µm vs. 28µm, 40–42 µm vs. 28 µm, respectively). Besides, in the new species the length of the anterior proboscis chamber reaches twice the length of posterior chamber, and the epidermis of the anterior proboscis chamber bears capped papillae; these characteristics have not been reported in other species of the genus. The uncorrected p-distances between O. conicobasis sp. nov. and the other species/entities in the Santacruzensis morphological species/group range from 0.131 to 0.195 for COI, and 0.109 to 0.151 for 16S.
Ototyphlonemertes (Ototyphlonemertes) chernyshevi [Ca1]
? Ototyphlonemertes duplex (Network D3; not Bürger, 1895): Leasi et al., 2016.
Material examined: 20190726I1, Zhaoshu Island (16.45°N, 111.71°E), Sansha, Hainan, China, coll. Hai-Long Liu, July 26 2019; 20190801I1, Chenhang Island (16.95°N, 112.32°E), Sansha, Hainan, China, coll. Hai-Long Liu, August 1 2019. Both extracted DNA from whole body, deposited at IEMBOUC.
The COI sequence OM836553 (referring to specimen 20190726I1) is designated as the voucher sequence.
Description (Figures 4A–E): Body length 9–10 mm, width 0.3 mm. Epidermis whitish, translucent; tissues around brain reddish; intestine tinged with pale-yellow color. Cephalic furrows posterior to brain (Figure 4A). Cerebral organs normal, locating near antero-lateral border of brain (Figures 4B, C). Cirri present at hind end and cephalic region; cirrus formula: A=0, B=1–2, C=2, D=2+0, E=0, tail=8. Statocyst 32–34 µm in diameter; statolith with two granules (Figure 4D). Rhynchocoel accounting for 1/3 of total body length. Proboscis diaphragm short, 61–102 µm long; middle chamber bulbous, 58–72 µm long; posterior chamber of opaque type (Figure 4B). Central stylet smooth, 30–36 µm long; basis 30–34 µm long, 10–14 µm wide, B/b 2.5–3.1; S/B 1.0 (Figure 4E). Accessory stylet pouches 2, each containing 2–3 stylets, pointing either forward or backward. Caudal adhesion plate present.
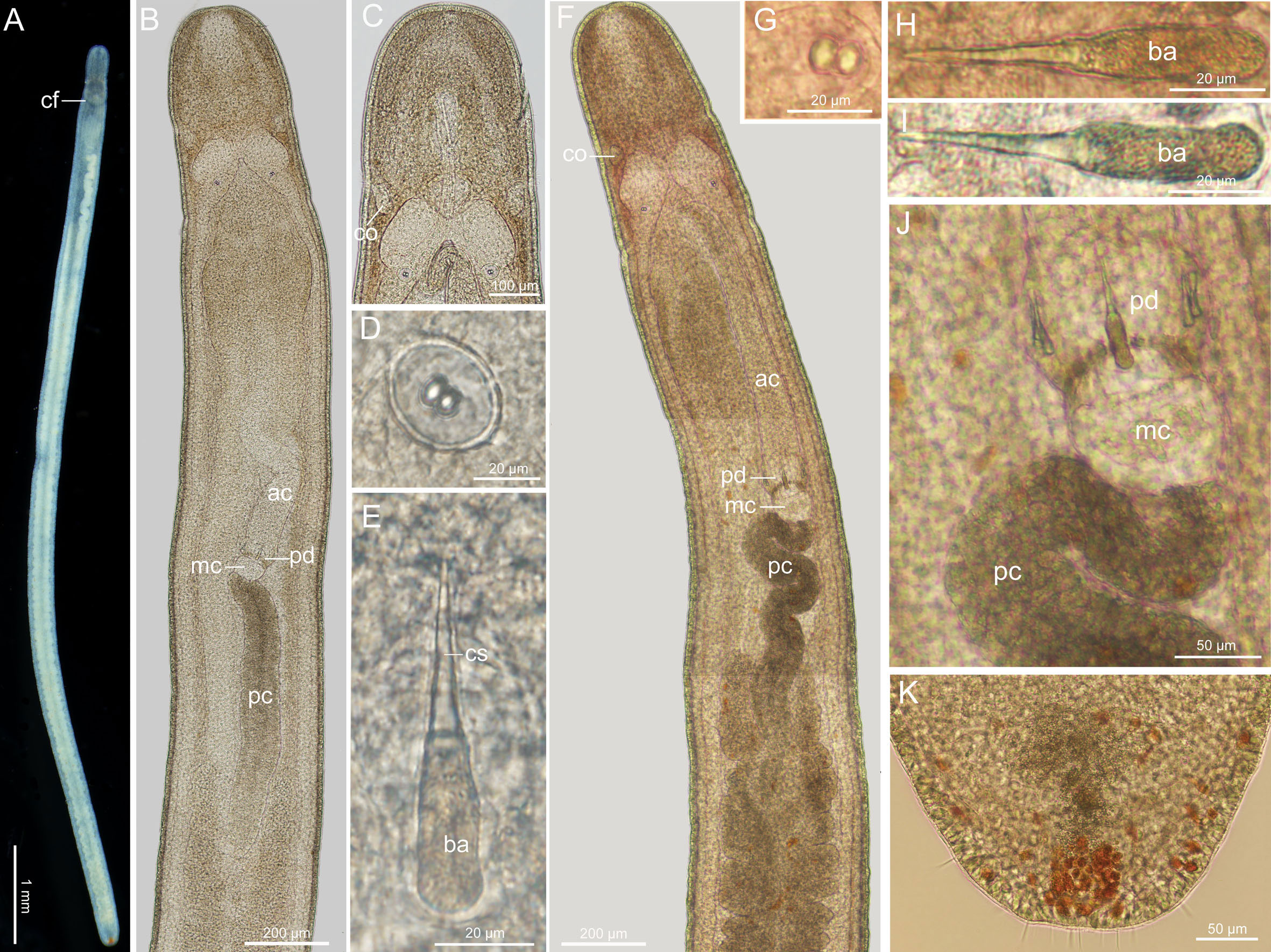
Figure 4 Ototyphlonemertes (Ototyphlonemertes) chernyshevi [Ca1] (A–E) and Ototyphlonemertes (Ototyphlonemertes) chernyshevi [Ca2] (F–K). (A) Photograph of a live specimen; (B, F) composite photomicrographs of squeezed specimens; (C) photomicrograph of anterior region of a squeezed specimen; (D, G) statoliths; (E, H, I) central stylet and basis; (J) photomicrograph of partial proboscis from a squeezed specimen; (K) posterior end of body. ac, anterior chamber of proboscis; ba, basis; cf, cephalic furrow; co, cerebral organ; cs, central stylet; mc, middle chamber of proboscis; pc, posterior chamber of proboscis; pd, proboscis diaphragm.
Ecology: Intertidal, coral sand.
Distribution: So far only known from Zhaoshu Island and Chenhang Island, Sansha, Hainan, China.
Remarks: Obvious morphological difference is not found between present specimens and O. chernyshevi. The uncorrected p-distances between O. chernyshevi [Ca1] and O. chernyshevi are 0.139–0.141 for COI and 0.087 for 16S, those between O. chernyshevi [Ca1] and O. chernyshevi [Ca2] are 0.135–0.139 for COI and 0.075–0.077 for 16S. Following recent studies in other animal groups (e.g., Padial et al., 2010; Cesari et al., 2019), this molecular entity is here treated as a candidate species, and is named by the binomen of the most similar species followed by ‘Ca1’ in square brackets (the first candidate species) (more information see Discussion).
In the ML tree, O. chernyshevi [Ca1] and D3 form a clade, which is sister to the clade of O. chernyshevi + O. chernyshevi [Ca2] (Figure 2). In the BI tree O. chernyshevi [Ca1] is sister to the clade of D2 + D3 (Figure S1). The species delimitation analysis on COI shows that present specimens are conspecific with the Entity/Network D3 (Leasi et al., 2016), but they belong to different species when analyzed with the other datasets (Figure 2). The uncorrected p-distance for COI between O. chernyshevi [Ca1] and the entity D3 (duplex43; Rangiroa, French Polynesia; Leasi et al., 2016) is low (0–0.002). However, they have larger genetic distance in other genes (0.118 for 16S and 0.043 for 28S). The 16S of duplex43 is the most similar (uncorrected p-distance = 0.040) to those of six D1 specimens (from Panama; see Table S1). The 28S of duplex43 is identical to those of eight D2 specimens (from Panama, Belize, Saint Barthélemy; see Table S1). Mitochondrial and nuclear discordance has been reported in various animals, and was generally explained by differential dispersal of males and females, incomplete lineage sorting or introgression (Toews and Brelsford, 2012). For the divergence in mtDNA clades where foreign types of mtDNA haplotypes were found, the haplotypes were considered to be commonly driven by sex-biased asymmetries and/or adaptive introgression (Toews and Brelsford, 2012). In the present case, however, the divergence between COI and 16S clades occurs in the same individual (i.e. duplex 43; Leasi et al., 2016). A possible explanation is that the D3 underwent events of interspecific crosses and paternal leakage (which seems to be relatively common in interspecific crosses; Eyre-Walker, 2000), and is probably followed by mtDNA recombination. Species of the genus Mytilus (Mollusca: Bivalvia) provide examples for such paternal leakage (Rawson et al., 1996) and recombination (Ladoukakis and Zouros, 2001; Burzyński et al., 2003). However, because sequences of D3 are available for only a single specimen, whether this phenomenon is common in individuals of the Rangiroa population awaits to be elucidated by future studies.
Ototyphlonemertes (Ototyphlonemertes) chernyshevi [Ca2]
Material examined: Seven specimens, 20180507J3–8, 20180507J27, Sanya (18.21°N, 109.50°E), Hainan, China, coll. Hai-Long Liu, May 7 2018, all DNA samples from whole body, deposited at IEMBOUC.
The COI sequence OM836528 (referring to specimen 20180507J3) is designated as the voucher sequence.
Description (Figures 4F–K): Body length 5–10 mm, width 0.2–0.3 mm. Epidermis whitish, translucent; tissues around brain slightly reddish; intestine tinged with pale-yellow. Cephalic furrows posterior to brain. Cerebral organs normal, locating near antero-lateral border of brain (Figure 4F). Cirri present at hind end (Figure 4K) and cephalic region; cirrus formula: A=0, B=1–2, C=2–4, D=(2–3)+(1–2), E=0, tail=6–8. Statocyst 27–33 µm in diameter; statolith with two granules (Figure 4G). Rhynchocoel accounting for about 1/3 of total body length. Proboscis diaphragm short, 62–96 µm long; middle chamber bulbous, 58–102 µm long; posterior chamber of opaque type (Figures 4F, J). Central stylet smooth, 31–37 µm long; basis, 30–39 µm long, 10 µm wide, B/b 3.0–3.8; S/B 0.9–1.1 (Figures 4H, I); accessory stylet pouches 2, each containing 2–4 stylets, pointing either forward or backward. Caudal adhesion plate present.
Ecology: Intertidal, sand with coral particles.
Distribution: So far only known from Sanya, Hainan, China.
Remarks: Similar to O. chernyshevi [Ca1], this form is recognized as a putative species by delimitation analyses, but cannot be distinguished from O. chernyshevi by morphology. In the phylogenetic tree, O. chernyshevi [Ca2] is sister to O. chernyshevi (Figure 2). Obvious morphological difference is not found between present specimens and O. chernyshevi, though they have quite large genetic distance (0.134–0.135 for COI and 0.061–0.063 for 16S).
Subgenus Norenburgia Chernyshev, 1993
Norenburgia Chernyshev, 1993: 8.
Accirinia Chernyshev, 1993: 8.
Accirrinia Chernyshev, 1998 (clerical error): 267.
Otohelicophora Envall, 1996: 274.
Type species: Ototyphlonemertes lactea Corrêa, 1953.
Diagnosis (modified from Chernyshev, 1998): Statolith polygranular, with 12–30 granules. Cerebral organs absent or stunted. Proboscis diaphragm long or short, middle chamber long tubular or short (often squarish), posterior chamber vesicular. Stylets sculptured. Cirri usually absent.
Remarks: Chernyshev (1998) included ten species in this subgenus, defining the subgenus as: statocyst containing 7–30 granules aggregated in a single statolith, cerebral organs stunted or absent, and stylets smooth or sculptured. They belong to clades of the O. macintoshi species group (Macintoshi + Lactea morpho-groups), the O. parmula species group (Parmula/Fila Lactea morpho-group), and the Cirrula morpho-group. In phylogenetic trees, the O. macintoshi and O. parmula species groups do not constitute a monophyly (Figure 2; Leasi et al., 2016; Kajihara et al., 2018a), and there is no molecular data for the Cirrula morpho-group. Hence, only species of the Lactea and Macintoshi morph-groups are included in this subgenus in the present paper. We will establish a new subgenus for the Parmula morpho-group (for Cirrula morpho-group, see Discussion). Currently there are 11 named species in the subgenus Norenburgia: Ototyphlonemertes americana Gerner, 1969; O. ani; O. dolichobasis; O. lactea; O. longissima; Ototyphlonemertes macintoshi Bürger, 1895; Ototyphlonemertes nikolaii Chernyshev, 1998; Ototyphlonemertes pellucida Coe, 1943; Ototyphlonemertes sinica sp. nov.; Ototyphlonemertes spiralis Coe, 1940; and Ototyphlonemertes valentinae Chernyshev, 2003.
Ototyphlonemertes (Norenburgia) aniChernyshev, 2007
Ototyphlonemertes (Norenburgia) ani Chernyshev, 2007: 196–199, (figs 1, 2).
? Ototyphlonemertes lactea (Network L3; not Corrêa, 1954): Leasi et al., 2016.
Material examined: 20190728I1, 20190728I2, Jinqing Island (16.46° N, 111.74° E), Sansha, Hainan, China, coll. Hai-Long Liu, 28 July 2019, extracted DNA from the whole body, deposited at IEMBOUC. 20190726I1, 20190726I2, Zhaoshu Island (16.95° N, 112.32° E), Sansha, Hainan, China, coll. Hai-Long Liu, 26 July 2019, preserved in 95% EtOH, deposited at IEMBOUC.
Description (Figure 5): Body length 4–7 mm, width 0.3 mm. Epidermis whitish, translucent; tissues around brain reddish; intestine tinged with pale-yellow (Figure 5A). Cephalic furrows posterior to brain. Cerebral organs undetected. Cirri present at cephalic region (Figure 5B) and hind end, but cephalic cirri only detectable on lateral view. Round statocysts 24–26 µm in diameter; statolith polygranular, containing 18–22 granules (Figure 5D). Proboscis anterior chamber with rhabdoid-like papillae, 0.4–0.7 mm long (Figure 5E); diaphragm long, 195–226 µm long, 92–127 µm wide; middle chamber elongated, 178–190 µm long, 87–122 µm wide; posterior chamber vesicular (Figures 5C, G). Stylets sculptured; central stylet 28–39 µm long; basis thin, 28–31 µm long, 4–6 µm wide, B/b 4.8–6.5; S/B 1.0-1.3 (Figure 5F); accessory stylet pouches 2, each containing 4–6 stylets, pointing either forward or backward. Intestinal diverticula shallow. Caudal adhesion plate present.

Figure 5 Ototyphlonemertes (Norenburgia) ani Chernyshev, 2007. (A) Photograph of a live specimen; (B) head, arrow indicating cirri; (C) composite photomicrograph of a squeezed specimen; (D) statolith; (E) everted proboscis, arrow indicating rhabdoid-like papillae; (F) central stylet and basis; (G) photomicrograph of partial proboscis from squeezed specimen. ac, anterior chamber of proboscis; mc, middle chamber of proboscis; pc, posterior chamber of proboscis; pd, proboscis diaphragm.
Ecology: Intertidal, coral sand.
Distribution: Jinqing Island and Zhaoshu Island, Sansha, Hainan, China (present study); Tche Bay (type locality), Hong-Long Island, Van Phong Bay, Vietnam (Chernyshev, 2007); Dam Ngoai, Vietnam (Kajihara et al., 2018a); Gesashi and Seto, Japan (Leasi et al., 2016) (but see below).
Remarks: The morphology of present specimens is basically consistent with the original description (Chernyshev, 2007) and later report (Kajihara et al., 2018a), but cephalic tactile cirri are detected in present specimens (Figure 5B). Besides, The histological work of Chernyshev (2007) shows that cerebral organs are present in this species. The uncorrected p-distances between present specimens and those from Dam Ngoai (Kajihara et al., 2018a) are 0.0046–0.0061 in COI and 0 in 16S, 18S and 28S. This is the first record of O. ani from China.
The species delimitation analyses on COI and 16S show the present specimens are conspecific with the Entity/Network L3 (Leasi et al., 2016), but they belong to different species when analyzed with the concatenated dataset (Figure 2). The uncorrected p-distances between the present specimens and L3 (lactea 139, 180, 160, Gesashi, Japan; Leasi et al., 2016) are 0.0015–0.0046 for COI, and 0 for 16S. They have larger genetic distance for 28S (0.062). However, the 28S of lactea 160 and Network L4 (Leasi et al., 2016) share the same haplotype. Thus, they exhibit discordance between mitochondrial and nuclear markers (see remarks for O. chernyshevi [Ca1]).
Ototyphlonemertes (Norenburgia) sinica sp. nov.
Zoobank registration: urn:lsid:zoobank.org:act:1C5FB285-3200-4015-915E-FEAD6014609D.
Material examined: Holotype: MBM287547, Fangchenggang (21.51°N, 108.23°E), Guangxi, China, coll. Hai-Long Liu, 18 November 2017, mature, female, series of transverse sections of anterior body region and caudal region, deposited at MBMCAS, remained tissues extracted DNA. Voucher specimens: 20131108D1, 20131108D3, 20131108D6, Naozhou Island (20.92°N, 110.57°E), Zhanjiang, Guangdong, China, coll. Shi-Chun Sun, 8 November 2013, D1 fixed with 10% formalin seawater, preserved in 70% ethanol, D3 and D6 extracted DNA from whole body, deposited at IEMBOUC.
Etymology: The specific name is an adjective, from the Latin word sinicus to indicate it is found in Chinese waters.
Description (Figure 6): Length 30–51 mm, width 0.3–0.5 mm. Epidermis translucent with white spots, distinctly reddish in brain region, and with orange tinge in intestinal region (Figures 6A-C). Cephalic furrows posterior to brain, V-shaped on dorsal side and inverted V-shaped on ventral side (Figures 6B, C). Cerebral organs absent. Sensory cirri absent. Round statocysts 22–27 µm in diameter; statoliths polygranular, consisting of 16–20 granules (Figure 6E). Proboscis anterior chamber 10.7 mm long; diaphragm slightly elongated, 306 µm long and 200 µm wide; middle chamber tubular, 543 µm long and 199 µm wide; posterior chamber of vesicular type, 12.2 mm long, folding more obviously than anterior chamber (Figures 6D, F). Stylets spiral; central stylet 60–70 µm long (Figure 6G); basis thin, cylindrical, 40–50 µm long, 11 µm wide, B/b 4.1 (Figure 6G); S/B 1.5; accessory stylet pouches 2, each containing 2–3 stylets, pointing either forward or backward (Figure 6D). Intestinal diverticula shallow (Figure 6F). Ovaries visible at intestinal region in specimens collected in November (Figure 6H). Caudal adhesive plate not detected.
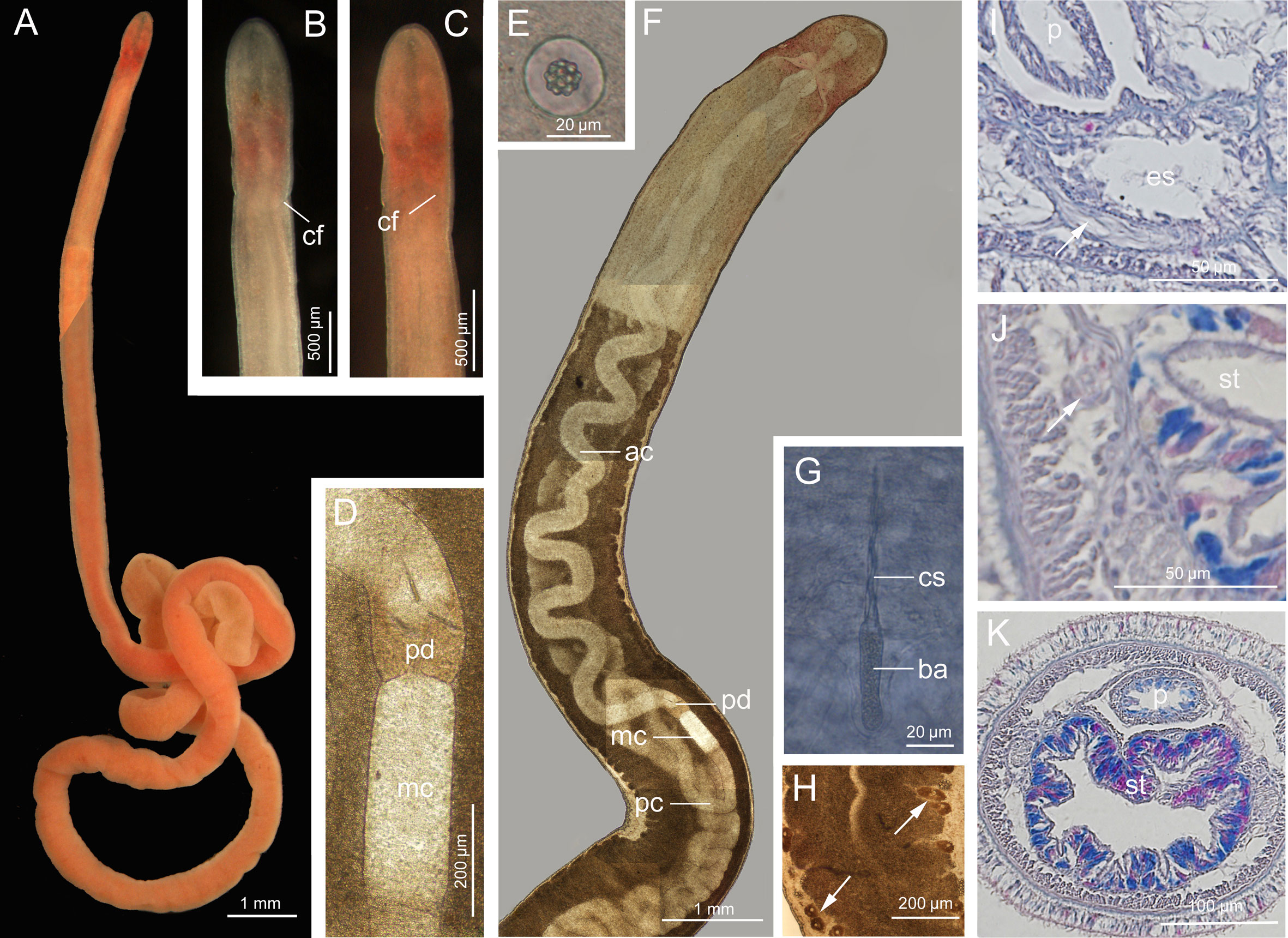
Figure 6 Ototyphlonemertes (Norenburgia) sinica sp. nov. (A) Photograph of a live specimen; (B) dorsal view of head; (C) ventral view of head; (D) photomicrograph of partial proboscis from squeezed specimen; (E) statolith; (F) composite photomicrograph of a squeezed specimen; (G) central stylet and basis; (H) intestinal region a squeezed specimen, showing ovaries (arrowed); (I) transverse section through foregut region, showing extra ventral commissure (arrowed); (J, K) transverse section through stomach region, nephridia indicated by arrow. ac, anterior chamber of proboscis; ba, basis; cf, cephalic furrow; cs, central stylet; mc, middle chamber of proboscis; es, esophagus; p, proboscis; pc, posterior chamber of proboscis; pd, proboscis diaphragm; st, stomach.
Ciliated glandular epidermis 15–30 µm thick. Dermis about 1–2 µm thick. Outer circular muscle layer mostly one or two muscle fibers thick. Longitudinal muscle layer 6–17 µm thick. Dorsoventral muscles not found. Esophagus about 380 µm long. Folding stomach marked from esophagus by having cilia and glands (Figure 6K), approximately 2.5 mm long. Intestine with no caecum. Frontal organ present. Cephalic glands extending back to foregut region. Dorsal cerebral commissure 10 µm thick, ventral commissure 28 µm thick. Extra ventral commissure present (Figure 6I). Lateral nerves about 25 µm in diameter, accessory lateral nerves unobserved. Excretory system extending from posterior part of stomach region to anterior part of intestinal region, with 1–2 collecting tubules, locating dorsal to lateral nerve at either side (Figure 6J).
Ecology: Intertidal, coarse-grained sands.
Distribution: Fangchenggang, Guangxi, China, and Naozhou Island, Zhanjiang, Guangdong, China.
Remarks: Ototyphlonemertes sinica sp. nov. is supported by three species delimitation analyses, and is sister to O. longissima with high support values (Figure 2). The two species are similar in external appearance, but have the following morphological differences: the body length of O. sinica sp. nov. is much shorter than that of O. longissima (30–51 mm vs. 138–158 mm); the statolith of O. sinica sp. nov. has more granules than that of O. longissima (about 20 vs. 12); the S/B value in O. sinica is greater than in O. longissima (about 1.5 vs 1.1) (see Liu and Sun, 2018). The uncorrected p-distances between O. sinica sp. nov. and O. longissima are 0.109–0.111 in COI, and 0.052 in 16S. Besides, O. sinica sp. nov. is only found from sand, while O. longissima lives both in sand and rock crevices.
Ototyphlonemertes (Norenburgia) longissima Liu and Sun, 2018
Material examined: 20180509I5, Guangcun (19.87°N, 109.44°E), Danzhou, Hainan, China (see Liu and Sun, 2018). Present study sequenced its 16S, 18S and 28S.
Distribution: Lingao and Danzhou, Hainan, China; Fangchenggang, Guangxi, China (Liu and Sun, 2018).
Remarks: Description see Liu and Sun (2018). This species has the longest body (up to 158 mm) recorded for Ototyphlonemertes. In addition to coarse sand, the species lives also in rock crevices.
Subgenus Procso subgen. nov.
Type species: Ototyphlonemertes subrubra sp. nov.
Etymology: The name Procso is combination of the prefix pro- (before in place or position) and the abbreviation CSO (cerebral sensory organ), referring that the cerebral organs of this subgenus are located more anteriorly than the congeners in other subgenera. The gender of the subgenus name is feminine.
Diagnosis: Statolith polygranular, with 6–12 granules aggregating in single statolith. Cerebral organs normal or stunted (rare), posteriorly not reaching anterior border of brain. Proboscis diaphragm short, middle chamber bulbous, posterior chamber with a specialized anterior region. Stylets sculptured. Cirri present at both ends.
Remarks: The subgenus refers to the clade of the O. parmula species group (Parmula/Fila morpho-group) (Figure 2), which was assigned to the subgenus Norenburgia in Chernyshev (1998). The new subgenus is morphologically different from the subgenus Ototyphlonemertes by having sculptured stylets, a posterior proboscis chamber with a specialized anterior region, and a larger number of granules aggregating in a single statolith. It differs from the subgenus Norenburgia by having cirri at both anterior and posterior ends, statoliths with fewer granules, well-developed cerebral organs, and a posterior proboscis chamber with a specialized anterior region. Besides, observations on Ototyphlonemertes specimens available to us show that the cerebral organs of Procso subgen. nov. are always located in front of the brain (posteriorly not reaching brain), while those of subgenera Ototyphlonemertes and Norenburgia are located at the antero-lateral border of the brain (posteriorly reaching brain) (see Discussion). Currently there are eight named species: Ototyphlonemertes coralli sp. nov.; O. lei; O. nakaoae; O. parmula; Ototyphlonemertes similis sp. nov.; Ototyphlonemertes subrubra sp. nov.; O. tsukagoshii; and Ototyphlonemertes yingge sp. nov.
Ototyphlonemertes (Procso) subrubra sp. nov.
Zoobank registration: urn:lsid:zoobank.org:act:E193AF5C-1EFD-4E17-A9C7-786D23AB2497.
Material examined: Holotype: MBM287548, Sanya (18.21° N, 109.50°E), Hainan, China, coll. Hai-Long Liu and Xiao-Qi Zeng, 17 July 2019, mature, series of transverse sections of anterior body region, deposited at MBMCAS, remained tissues extracted DNA. Voucher specimens: 20190717I1, 20190717I3, 20190717I4, DNA samples, 20190717I5, 20190717I6, 20190717I8, 20190717I9, same locality as holotype, coll. Hai-Long Liu and Xiao-Qi Zeng, 17 July 2019, preserved in 95% EtOH; 20190728J1, 20190728J2, Jinqing Island (16.46°N, 111.74°E), Sansha, Hainan, China, coll. Hai-Long Liu, 28 July 2019, DNA samples, deposited at IEMBOUC.
Etymology: The specific name is an adjective from the Latin word subruber (reddish), referring to the color of living worms.
Description (Figure 7): Body length 6–12 mm, width 0.2–0.3 mm. Epidermis translucent; tissues around brain reddish; intestine reddish in newly collected specimens, becoming tangerine-colored after several days of starvation (Figure 7A). Cephalic furrows posterior to brain (Figure 7A). Cerebral organs normal, anterior to brain (Figures 7B, J). Cirri present at hind end and cephalic region, cirrus formula in holotype: A=3, B=0, C=10, D=6+4, E=0, tail=12. Ovoid statocyst, 21–28 µm in diameter; statolith polygranular, containing 6–8 granules (Figure 7C). Rhynchocoel about 1/3 of total body length (Figure 7B). Proboscis anterior chamber with flap-like protrusions (Figure 7G), 1.1–1.6 mm long; diaphragm short, 122–181 µm long, 161–202 µm wide; middle chamber bulbous, 136–170 µm long, 136–183 µm wide; posterior chamber of opaque type with specialized anterior part, 1.3–2.1 mm long (Figures 7B, E, G). Stylets spiral; central stylet 57–63 µm long; basis thin, cylindrical (hind region slightly thicker) (Figure 7D), 55–62 µm long, 14–16 µm wide, B/b 3.8–4.1; S/B 1.0–1.1; accessory stylet pouches 2, each containing 3–6 stylets, pointing either forward or backward. Testes present in posterior 2/3 of intestinal region (Figures 7A, B, H, L). Intestinal diverticula deep. Caudal adhesion plate present.
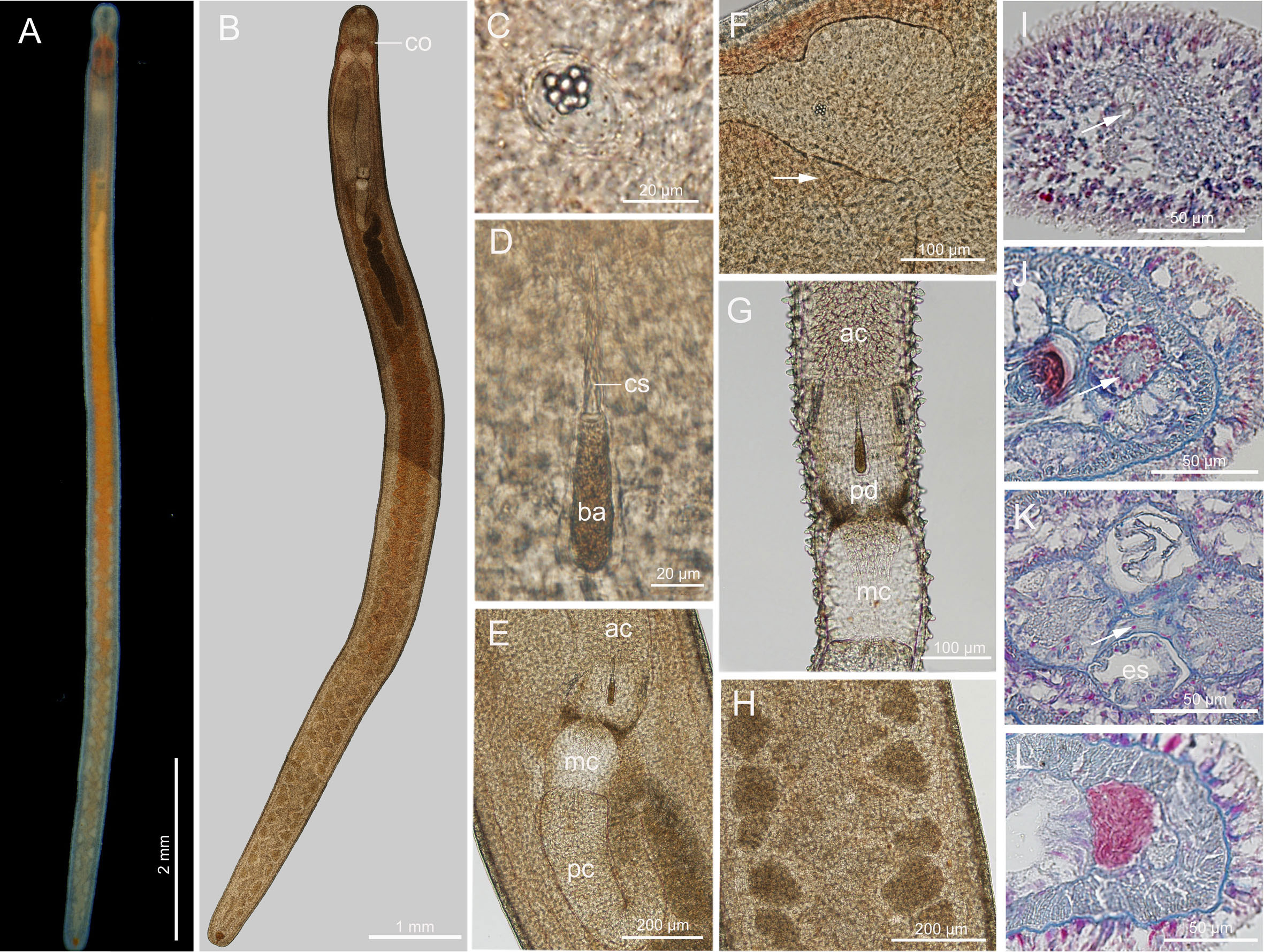
Figure 7 Ototyphlonemertes (Procso) subrubra sp. nov. (A) Photograph of a live specimen; (B) composite photomicrograph of a squeezed specimen; (C) statolith; (D) central stylet and basis; (E) photomicrograph of partial proboscis from a squeezed specimen; (F) photomicrograph of partial brain from a squeezed specimen, showing the extra ventral commissure (arrowed); (G) everted proboscis; (H, L) testes; (I) transverse section through frontal organ (arrowed); (J) transverse section through a cerebral organ (arrowed); (K) transverse section through cerebral region, arrow pointing to extra ventral commissure. ac, anterior chamber of proboscis; ba, basis; co, cerebral organ; cs, central stylet; es, esophagus; mc, middle chamber of proboscis; pc, posterior chamber of proboscis; pd, proboscis diaphragm.
Ciliated glandular epidermis 18–25 µm thick. Dermis 1–2 µm thick. Outer circular muscle layer mostly one or two fibers thick. Longitudinal muscle layer 5–18 µm thick. Dorsoventral muscles not found. Esophagus about 280 µm long. Stomach about 714 µm in length. No intestinal caecum. Frontal organ present (Figure 7I). Cephalic glands well developed, extending back to foregut region. Dorsal cerebral commissure about 12 µm thick, ventral commissure about 26 µm thick. Extra ventral commissure present, passing above esophagus at posterior part of cerebral region (Figures 7F, K). Excretory system with 1–2 collecting tubules on either side.
Ecology: Intertidal, coral sands.
Distribution: So far only known from Sanya and Jinqing Island, Hainan, China.
Remarks: Ototyphlonemertes subrubra sp. nov. has the longest central stylet and the largest basis recorded in this subgenus (Table 1). In comparison with the other species studied, the reddish appearance of live worms brought a deep impression to the collector. This new species is supported by three molecular species delimitation analyses. In the phylogenetic tree, O. subrubra sp. nov. is sister to a clade that is composed of O. coralli sp. nov., O. nakaoae, O. similis sp. nov., O. tsukagoshii, and O. yingge sp. nov. (Figure 2). Its intraspecific uncorrected p-distances are 0.002–0.011 in COI and 0.00 in 16S. The interspecific uncorrected p-distances between O. subrubra sp. nov. and the aforementioned five species are 0.127–0.150 in COI and 0.106–0.124 in 16S.
Ototyphlonemertes (Procso) similis sp. nov.
Zoobank registration: urn:lsid:zoobank.org:act:A01F972C-CB3B-4753-9317-E9B6534F892A
Material examined: Holotype: MBM287549, Sanya (18.21° N, 109.50°E), Hainan, China, coll. Hai-Long Liu, 7 May 2018, mature, female, full series of transverse sections, deposited at MBMCAS. Voucher specimens: 20180507K2–3, 20180507K6–7, 20180507K9–10, locality same as holotype, coll. Hai-Long Liu, 7 May 2018, extracted DNA from whole body, deposited at IEMBOUC.
Etymology: The specific name silimis (similar) is a Latin adjective, referring to that the new species is morphologically similar to O. nakaoae.
Description (Figure 8): Length 3–5 mm, width 0.2–0.3 mm. Epidermis translucent whitish, tissues around brain slightly reddish, intestinal region tinged with pale-yellow. Cephalic furrows posterior to brain. Cerebral organs normal, anterior to brain (Figures 8A, F). Cirri present at hind end and cephalic region. Cirrus formula in holotype: A=2, B=0, C=8, D=5+5, E=0, tail=10 (Figure 8C). Statocyst round, 20–24 µm in diameter; statolith polygranular, about 9 µm in diameter, consisting of 7–10 granules (Figure 8B). Rhynchocoel extending to about 1/3 of total body length (Figure 8A). Proboscis anterior chamber 0.8–1.2 mm long; diaphragm short, 71–95 µm long, 60–81 µm wide; middle chamber bulbous, 57–70 µm long, 69–80 µm wide; posterior chamber of opaque type, with a specialized anterior part, 0.6–1.3 mm long (Figures 8A, D). Stylets spiral; central stylet 32–37 µm long; basis cylindrical, with narrower posterior region in some specimens (Figure 8E), 33–35 µm long, 7.8–8.3 µm wide; B/b 4.2–4.3; S/B 0.9–1.1; accessory stylet pouches 2, each containing 3–4 spiral stylets pointing either forward or backward (Figure 8D). Ovaries present in posterior 2/3 of intestinal region (Figures 8A, H). Intestinal diverticula deep.
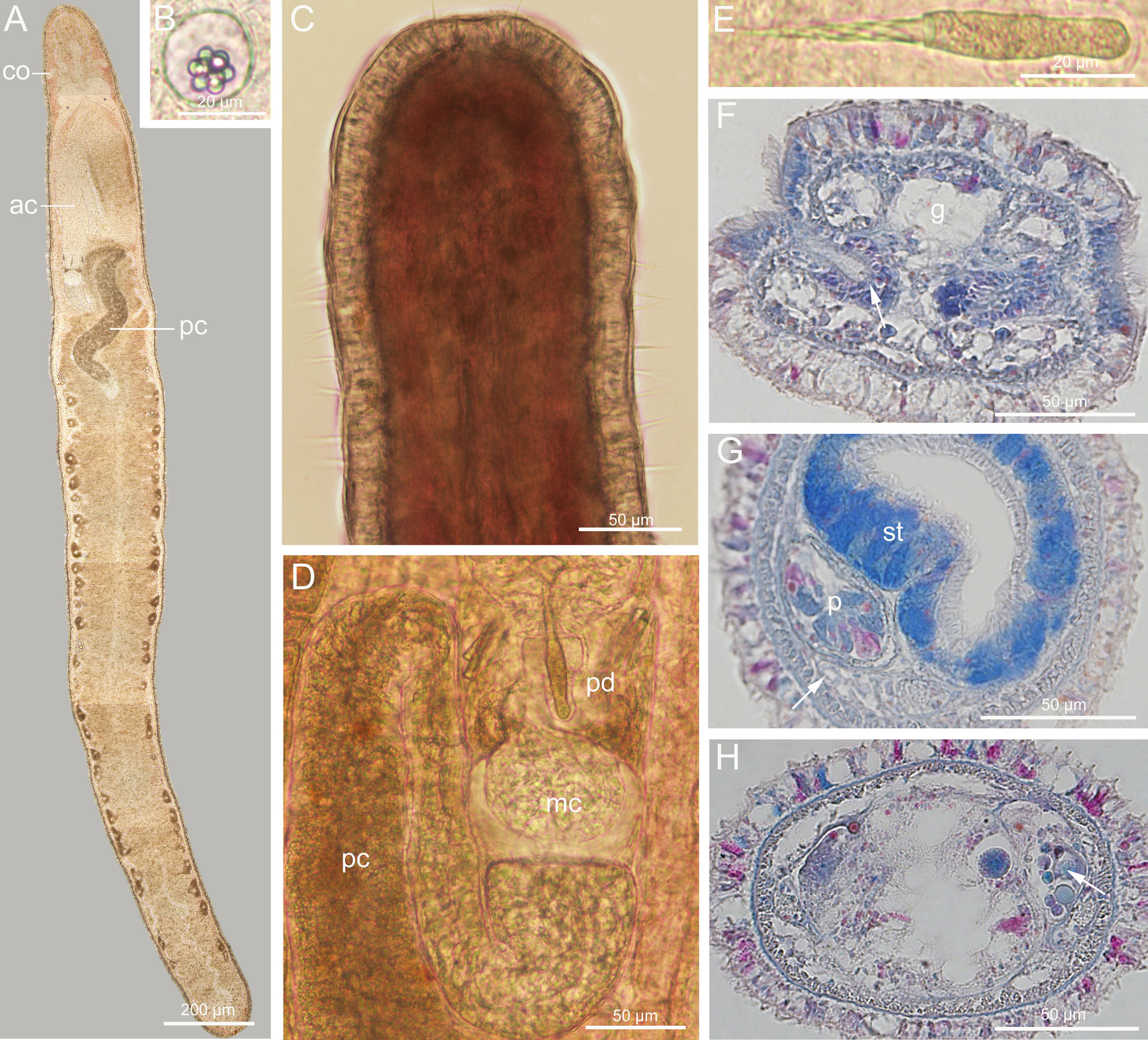
Figure 8 Ototyphlonemertes (Procso) similis sp. nov. (A) Composite photomicrograph of a squeezed specimen; (B) statolith; (C) dorsal view of head; (D) photomicrograph of partial proboscis from squeezed specimen; (E) central stylet and basis; (F) transverse section through a cerebral organ (arrowed); (G) transverse section through stomach region, nephridia indicated by arrow; (H) transverse section through ovaries (arrowed). ac, anterior chamber of proboscis; co, cerebral organ; g, cephalic glands; mc, middle chamber of proboscis; p, proboscis; pc, posterior chamber of proboscis; pd, proboscis diaphragm; st, stomach.
Ciliated glandular epidermis mostly about 20 µm thick. Dermis and circular muscle layers thin, 1–2 µm thick. Longitudinal muscle layer 5–18 µm thick. Dorsoventral muscles not found. Esophagus about 250 µm in length. Stomach about 245 µm long. No intestinal caecum. Dorsal cerebral commissure 11 µm thick, ventral cerebral commissure 24 µm thick. Extra ventral commissure present, passing below esophagus in posterior part of cerebral region. Lateral nerves about 20 µm in diameter; accessory lateral nerves unobserved. Frontal organ present. Cephalic glands extending back to foregut region. Excretory system with 1–2 collecting tubules at either side, positioned dorsal to lateral nerve, extending from posterior part of stomach region to anterior part of intestinal region (Figure 8G). Caudal adhesion plate present, exhibiting as subanal aggregation of epidermal gland cells in sections.
Ecology: Intertidal, coarse sand consisting of coral particles.
Distribution: So far only known from the type locality, Sanya, Hainan, China.
Remarks: This new species is supported by three species delimitation analyses. In the phylogenetic tree, O. similis sp. nov. is sister to O. nakaoae with high support values (BP = 100%, PP = 1) (Figure 2). The two species are similar in morphology, but O. similis sp. nov. has a smaller stylet basis (33−35 µm vs 39−52 µm) and a larger S/B value (0.9−1.1 vs 0.76/holotype) (Table 1; Kajihara et al., 2018a). Their reproductive season may be different. Mature specimens of O. nakaoae were collected in December (Kajihara et al., 2018a), while mature specimens of O. similis sp. nov. were collected in May. The intraspecific uncorrected p-distances of O. similis sp. nov. are 0–0.005 in COI and 0–0.005 in 16S. The uncorrected p-distances between O. similis sp. nov. and O. nakaoae are 0.107–0.109 in COI and 0.047–0.049 in 16S.
Ototyphlonemertes (Procso) yingge sp. nov.
?Ototyphlonemertes fila (Network F1; not Corrêa, 1953): Leasi et al., 2016.
Zoobank registration: urn:lsid:zoobank.org:act:5FBFB876-285E-4643-89EC-678815D2B363
Material examined: Holotype: MBM287550, Yinggehai (18.50° N, 108.69° E), Ledong, Hainan, China, coll. Hai-long Liu, 10 December 2018, mature, full series of transverse sections, deposited at MBMCAS. Voucher specimens: 20181210P2, 20181210P3, same locality as holotype, coll. Hai-Long Liu, 10 December 2018; 20181209J1, Danzhou (19.87°N, 109.44°E), Hainan, China, coll. Hai-Long Liu, 9 December 2018, extracted DNA from the whole body; deposited at IEMBOUC.
Etymology: The specific name yingge is a Chinese word (yīng gē). It was said to be a kind of bird living at the type locality, and “Yinggehai” (meaning Yingge Sea) was probably named after this bird. A more well-known and poetic meaning of “yīng gē” is songs of the oriole.
Description (Figure 9): Body length 6–7 mm, width 0.3 mm. Epidermis whitish, translucent; tissues around brain reddish; intestine tinged with yellow (Figure 9A). Cephalic furrows posterior to brain (Figures 9A, D). Cerebral organs normal, anterior to brain (Figure 9B, H). Cirri present at hind end and cephalic region (Figures 9D, E); cirrus formula in holotype: A=2, B=0, C=12, D=6+3, E=0, tail=12. Ovoid statocyst 21–25 µm in diameter; statolith polygranular, containing 8–10 granules (Figure 9C). Rhynchocoel extending to about 1/3 of total body length. Proboscis anterior chamber 1.1–1.3 mm long; diaphragm short, 106–115 µm long, 128–144 µm wide; middle chamber bulbous, 110–130 µm long, 130–145 µm wide; posterior chamber of opaque type, with a specialized anterior part, 1.2–1.5 mm long (Figures 9B, G). Stylets spiral; central stylet 47–54 µm long; basis thin, cylindrical, 46–50 µm long, 12–14 µm wide, B/b 3.1–3.8; S/B 1.0–1.1 (Figure 9F); accessory stylet pouches 2, each containing 3–5 stylets, pointing either forward or backward (Figure 9G). Intestinal diverticula deep (Figure 9B).
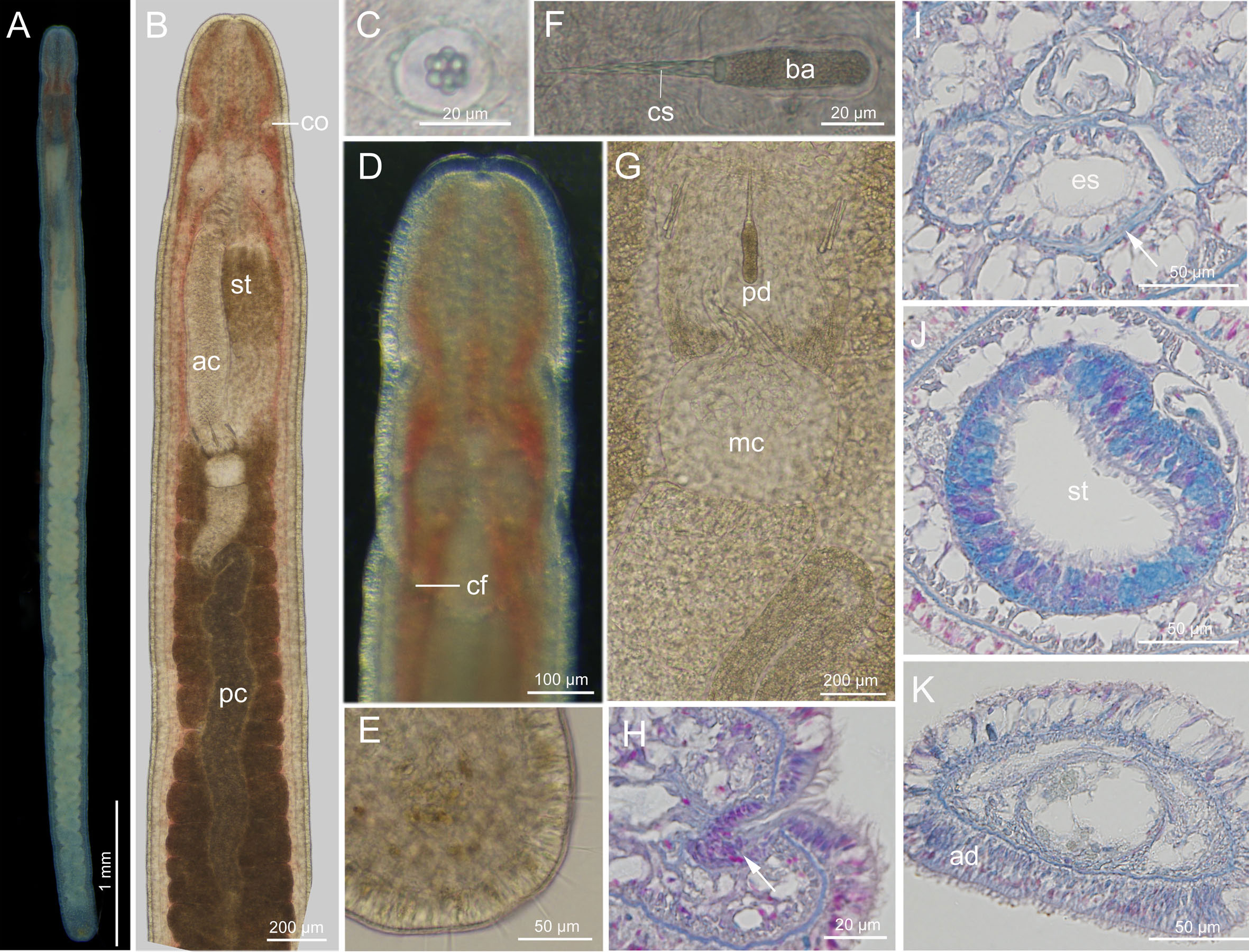
Figure 9 Ototyphlonemertes (Procso) yingge sp. nov. (A) Photograph of a live specimen; (B) composite photomicrograph of a squeezed specimen;(C) statolith; (D) dorsal view of head; (E) posterior end of body; (F) central stylet and basis; (G) part of proboscis; (H) transverse section through a cerebral organ (arrowed); (I) transverse section through the posterior part of cerebral region, showing the extra ventral commissure (arrowed); (J) transverse section through stomach region; (K) transverse section near caudal end showing the epidermis of caudal adhesion. ac, anterior chamber of proboscis; ad, adhesion plate; ba, basis; cf, cephalic furrow; co, cerebral organ; cs, central stylet; es, esophagus; mc, middle chamber of proboscis; pc, posterior chamber of proboscis; pd, proboscis diaphragm; st, stomach.
Ciliated glandular epidermis 10–25 µm thick. Dermis 1–2 µm thick. Outer circular muscle layer one or two fibers thick. Longitudinal muscle layer 5–15 µm thick. Dorsoventral muscles not found. Esophagus approximately 315 µm long. Length of stomach about 546 µm. Stomach marked by cilia and gland cells (Figure 9J). Pylorus less glandular, about 1/7 of total stomach length. Intestinal caecum unobserved. Frontal organ present. Cephalic glands well developed, extending back to foregut region. Dorsal cerebral commissure about 7 µm thick, ventral commissure about 26 µm thick. Extra ventral commissure present, passing below esophagus at posterior part of cerebral region (Figure 9I). Lateral nerves about 20 µm in diameter; accessory lateral nerves unobserved. Excretory system with 1–2 collecting tubules at either side, positioned dorsally to lateral nerves, extending from posterior part of stomach region to anterior part of intestinal region. Caudal adhesion plate present, exhibiting as subanal aggregation of epidermal gland cells in sections (Figure 9K).
Ecology: Intertidal, coarse-grained sands.
Distribution: Yinggehai and Danzhou, Hainan, China. It may also be distributed in Seto and Okinawa, Japan (see below).
Remarks: The size of O. yingge sp. nov. is larger than consubgeners except O. subrubra sp. nov. (Table 1). This new species has a greater number of statolith granules (8–10) than O. coralli sp. nov., O. lei, O. nakaoae, O. subrubra sp. nov., and O. tsukagoshii (6–8; Table 1). Its central stylet and basis are larger than O. coralli sp. nov., O. nakaoae, O. similis sp. nov., and O. tsukagoshii, but smaller than O. subrubra sp. nov. The basis is usually stouter (with a smaller B/b) than the other species of the subgenus (Table 1). Ototyphlonemertes yingge sp. nov. possesses more C cirri (12) than O. coralli sp. nov. (6).
In the phylogenetic tree, O. yingge sp. nov. is sister to F1 (Seto and Okinawa, Japan; lacking morphological description; Leasi et al., 2016) with high support values (BP = 100%, PP = 1). The uncorrected p-distance of COI between them is low (0.018), and DNA taxonomy assigns them to the same species (Figure 2). However, they have larger genetic distance in other genes (0.077 for 16S and 0.029-0.032 for 28S), and they are delimitated as two species by 16S and concatenated datasets (Figure 2). This is similar to the result regarding O. chernyshevi [Ca1] and D3 (see above). Their relationship remains unresolved.
Ototyphlonemertes (Procso) coralli sp. nov.
?Ototyphlonemertes erneba (Network E1; not Corrêa, 1950): Leasi et al., 2016.
Zoobank registration: urn:lsid:zoobank.org:act:6473D51C-D5F2-440B-896C-B6111F930C15
Material examined: Holotype: MBM287551, Jinqing Island (16.46°N, 111.74°E), Sansha, Hainan, China, coll. Hai-Long Liu, 28 July 2019, immature, fixed with 95% EtOH, deposited at MBMCAS. Voucher specimens: 20190728J3, 20190728J4, 20190728J5, same locality as holotype, coll. Hai-Long Liu, 28 July 2019, extracted DNA from whole body, deposited at IEMBOUC.
Etymology: The specific name is a noun in the genitive case, from the Latin word corallum (coral), referring to that the species was collected from coral sand at a coral reef.
Description (Figure 10): Body length 4–5 mm, width 0.3 mm. Epidermis whitish, translucent; tissues around brain reddish; intestine tinged with yellowish brown (Figure 10A). Cephalic furrows posterior to brain (Figure 10A). Cerebral organs normal, anterior to brain (Figure 10B). Cirri present at hind end and cephalic region; cirrus formula in holotype: A=2, B=0, C=6, D=6+4, E=0, tail=8. Statocyst ovoid, 22–28 µm in diameter; statolith polygranular, containing 8–10 granules (Figure 10C). Rhynchocoel about 1/3 of total body length. Proboscis anterior chamber 0.6–1.1 mm long; diaphragm short, 63–103 µm long, 82–130 µm wide; middle chamber bulbous, 65–92 µm long, 65–100 µm wide; posterior chamber of opaque type, with a specialized anterior part, 0.7–1.1 mm long (Figures 10B, F). Stylets spiral; central stylet 28–44 µm long; basis thin, variable in shape, 27–45 µm long, 7–11 µm wide, B/b 3.8–4.5; S/B 0.8–1.1 (Figures 10D, E); accessory stylet pouches 2, each containing 2–4 stylets, pointing either forward or backward. Intestinal diverticula deep. Caudal adhesion plate present.
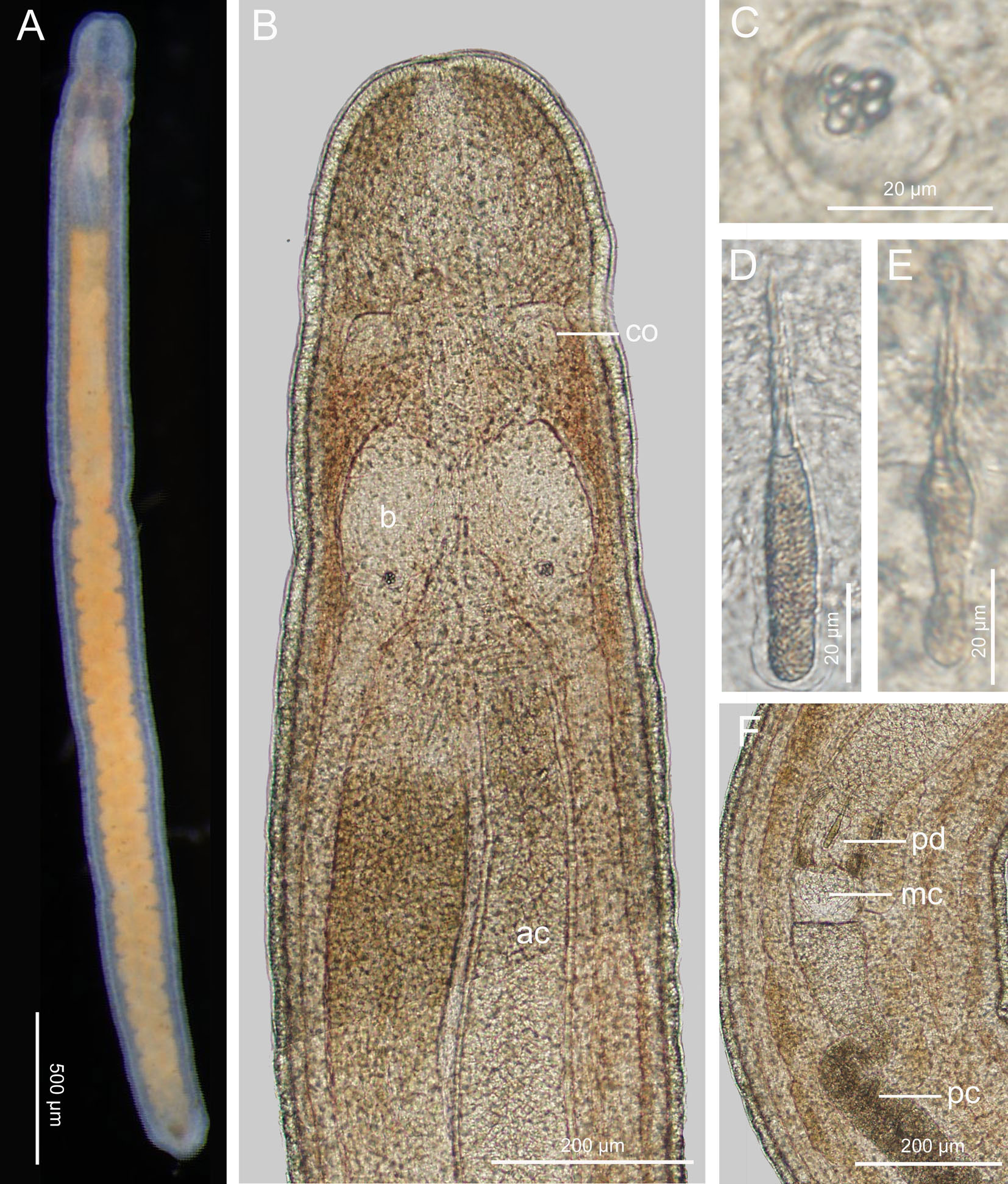
Figure 10 Ototyphlonemertes (Procso) coralli sp. nov. (A) Photograph of a live specimen; (B) composite photomicrograph of a squeezed specimen; (C) statolith; (D, E) central stylet and basis; (F) photomicrograph of partial proboscis from a squeezed specimen. ac, anterior chamber of proboscis; b, brain; co, cerebral organ; mc; middle chamber of proboscis; pc, posterior chamber of proboscis; pd; proboscis diaphragm.
Histology not studied.
Ecology: Intertidal, coral sand.
Distribution: Jinqing Island, Sansha, Hainan, China. It may also be distributed in Okinawa (Japan), Hawaii (USA) and French Polynesia (see below).
Remarks: Ototyphlonemertes coralli sp. nov. is supported by three molecular species delimitation analyses. In the phylogenetic tree, O. coralli sp. nov. is sister to the clade of O. yingge sp. nov. + F1 (Leasi et al., 2016) (Figure 2). This new species possesses the fewest C cirri (6; n=4) in this subgenus (Table 1). The basis and central stylet of the new species are smaller than those of O. yingge sp. nov. (27–45 vs. 46–50 μm and 28–44 vs. 47–54 μm, respectively). The intraspecific uncorrected p-distances of O. coralli sp. nov. are 0–0.004 in COI and 0.000 in16S. The uncorrected p-distances between O. coralli sp. nov. and O. yingge sp. nov. are 0.132–0.137 in COI and 0.077–0.082 in 16S.
The uncorrected p-distance for COI between O. coralli sp. nov. and the entity E1 (erneba 150, Japan and French Polynesia; Leasi et al., 2016) is 0.0052, that for 16S is 0.0146, suggesting they may belong to the same species. However, the 28S of erneba 150 is closer to Network E2 (Leasi et al., 2016) (0.0011) than to O. coralli sp. nov. (0.0731). Thus, there might be mistakes with sequences of E1. The COI distance between O. coralli sp. nov. and erneba 111 (Hawaii; another individual of entity E1) is 0.0419.
Discussion
Taxonomy
The morphology of meiofaunal organisms is characterized by extensive parallelism and convergent adaptations to the mesopsammic environment (Swedmark, 1964; Swedmark, 1968), which may result in low interspecific morphological variability. As a typical meiofaunal/interstitial taxon, the genus Ototyphlonemertes is well known for its low morphological divergence and presence of cryptic/sibling species (Andrade et al., 2011; Tulchinsky et al., 2012; Leasi and Norenburg, 2014; Leasi et al., 2016; Kajihara et al., 2018a; Mendes et al., 2018). Our species delimitation analyses on Ototyphlonemertes from the South China Sea recognized 10 distinct entities. Two of them represent O. ani and O. longissima that were originally described from the Vietnamese and Chinese coasts, respectively (Chernyshev, 2007; Liu and Sun, 2018). In conjugation with morphological studies, six entities are described as new species. Among them, four species belong to the Parmula morpho-group (or O. parmula species group or Procso subgen. nov.). They can only be distinguished from each other and from the four previously described species of this morpho-group by several subtle morphological differences, e.g., body size, color, basis shape and size, cirrial formula, stylet/basis ratio, number of statolith granules (see Table 1 and Remarks of each species). The other two entities are morphologically indistinguishable from O. chernyshevi though they have large genetic distance (see above). In Ototyphlonemertes, a lot of genealogical units (with large genetic variation but little morphological difference or lacking morphological data) have been discovered (e.g., Andrade et al., 2011; Tulchinsky et al., 2012; Leasi and Norenburg, 2014; Leasi et al., 2016; Mendes et al., 2018). Most of them are neither described nor named following the Linnaean system of taxonomy and nomenclature, and are mentioned as, e.g., Clade A, Clade B (Andrade et al., 2011), Entity/Network D1, D2 (Leasi et al., 2016). The bacteriological concept of “candidate species” has recently been explored and applied to animals for such units (Fouquet et al., 2007; Vieites et al., 2009). Candidate species are further classified into three sub-categories: unconfirmed candidate species (UCS; individuals within nominal species showing large genetic distances, but without further information); deep conspecific lineages (DCL; additional data indicating that the genealogical units are not differentiated at the species level); confirmed candidate species (CCS; deep genealogical lineages that can be considered good species following standards of divergence for the group under study but that have not yet been formally described and named) (Vieites et al., 2009; Padial et al., 2010). To standardize the nomenclature of such units, Padial et al. (2010) proposed to designate candidate species through the combination of the binomial species name of the most similar or closely related nominal species, followed (in square brackets) by the abbreviation “Ca” (for candidate) and a numerical code. Such naming scheme for candidate species has been used in tardigrades (Cesari et al., 2019). Following this scheme, the two entities close to O. chernyshevi are here named as O. chernyshevi [Ca1] and O. chernyshevi [Ca2]. Since no morphological differences are found among them, the two candidate species are currently in accordance with the definition of DCL. However, their genetic distance is high, and the monophyly of O. chernyshevi [Ca1] with the other two is not highly supported (Figure 2). To elucidate the relationship and geographic distribution pattern of this complex, population genetic study based on intensive sampling is needed.
Following Chernyshev (1998); Chernyshev (2007); Chernyshev (2016), the taxonomic rank subgenus is adopted in the present study. Based mostly on results of phylogenetic analyses, we redefine the two subgenera proposed by Chernyshev (1998), and establish the new subgenus Procso subgen. nov. Except for the characters used previously (e.g., number of granules in a statolith, development of cerebral organs, type of proboscis chambers and type of stylets), the position of cerebral organs is considered a useful character in the classification of the genus. In all Ototyphlonemertes specimens available to us and photographic images available in literature (Kirsteuer, 1977; Envall and Norenburg, 2001; Herrera-Bachiller and Junoy, 2014; Kajihara et al., 2018a; Kajihara et al., 2018b; Sun and Xu, 2018), the cerebral organs are located in front of the brain in members of the subgenus Procso subgen. nov., while they are located at the antero-lateral border of the brain in the other species of the genus. However, cerebral organs situated in front of the brain are also recorded for some non-Prosco species. In those species the position of cerebral organs was either determined by observing sections of fixed specimens (e.g., O. ani) (Chernyshev, 2007), or illustrated by hand sketching (e.g., O. evelinae, O. erneba, O. pallida) (Corrêa, 1948; Mock and Schmidt, 1975; Mock, 1978). In the latter case, differences in the position of cerebral organs have been reported for the same species, suggesting that some kind of artifact might exist. For example, the cerebral organs of O. erneba were close to the brain in Corrêa (1950: fig. 21), but were located for some distance before the brain in Mock and Schmidt (1975: fig. 8A); the cerebral organs of O. pallida were in front of the brain in Mock (1978: fig. 1), while they were close to the brain in a photomicrograph taken by Jon Norenburg (available at: https://www.herrerabachiller.com/nemertea/hoplonemertea/monostilifera/ototyphlonemertes/ototyphlonemertes-pallida).
In this study, no species are identified by histological characters. As far as we are aware, the only species that can be doubtlessly identified by histological characters is O. valentinae, which has a unique bilateral construction in the anterior proboscis chamber (Chernyshev, 2003). However, histological work is still useful in the taxonomic study of Ototyphlonemertes. For instance, the cerebral organs of O. ani are not distinguishable in vivo, but can be confirmed on histological sections (Chernyshev, 2007). The caudal adhesion plate, whose recognition in living worms is to some extent dependent on observing the behavior (Envall and Norenburg, 2001), can also be confirmed by histological study.
Two morphological groups, Cirrula and Erneba, are not assigned to any subgenus. The Cirrula morpho-group, including Ototyphlonemertes cirrula Mock and Schmidt, 1975 and Ototyphlonemertes brevis Corrêa, 1948, is similar to the subgenera Norenburgia and Procso by having polygranular statoliths. Their cerebral organs are either absent or tiny (Corrêa, 1948; Mock and Schmidt, 1975), which is the state of the subgenus Norenburgia. However, Cirrula is close to the subgenus Ototyphlonemertes in having smooth stylets. As mentioned earlier, there is only slight morphological difference between the Erneba morpho-group and the subgenus Ototyphlonemertes. Because molecular phylogenetic analyses are currently unavailable for Cirrula group, and results of morphological and molecular analyses regarding Erneba morpho-group are contradictory (see above), here we leave the subgeneric assignment of these two groups to be settled by future studies.
Diversity and biogeography
Based on literature and present results, a total of 101 species/entities of Ototyphlonemertes can be recognized (checklist see Table S3). Their distribution and ascription of marine biogeographic provinces, as realigned by Briggs and Bowen (2012), are summarized in Table 2 and Figure 11, which show that Ototyphlonemertes are currently known from 18 provinces, mainly distributed in the tropical and warm-temperate provinces as having been indicated by previous authors (Envall and Norenburg, 2001). The maximum species/entity diversity is recorded from the Indo-Polynesian Province (22 species/entities), followed by the Caribbean Province (17 species/entities), they together account for 38.6% of the world total number of species/entities. While most species/entities (89, 88.1%) are endemic to a single province, there are 13 species/entities distributed in two or three biogeographic provinces. Among them, Entities D2, D4, E3, and F2 range across tropical and cold temperate waters, with E3 being recorded from Brazilian Province, Caribbean Province, and West Atlantic Region. In Asia, O. martynovi is widely distributed in Oriental Province, Kurile Province, and Sino-Japanese Province. All these taxa are found along the same side of a continent and/or in archipelagic sea areas. The scattered suitable habitats along the coastline of the continent and islands could be “stepping-stones”, as suggested by Mendes et al. (2018). Ototyphlonemertes could finally expand their distribution scope by jumping between these “stepping-stones”.
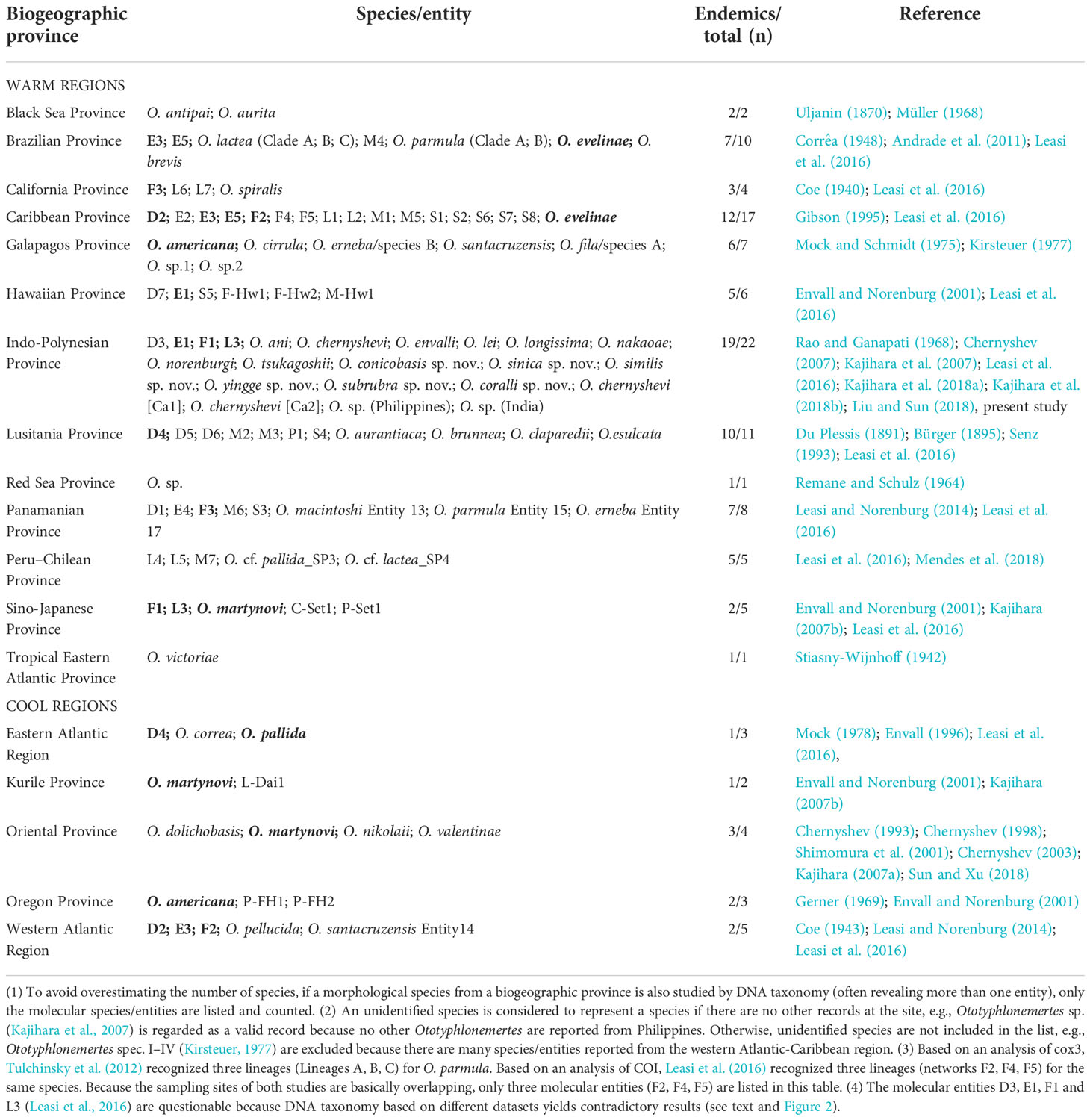
Table 2 Distribution of Ototyphlonemertes in marine biogeographic provinces. Species/entities occurring in two or more provinces are shown in bold.
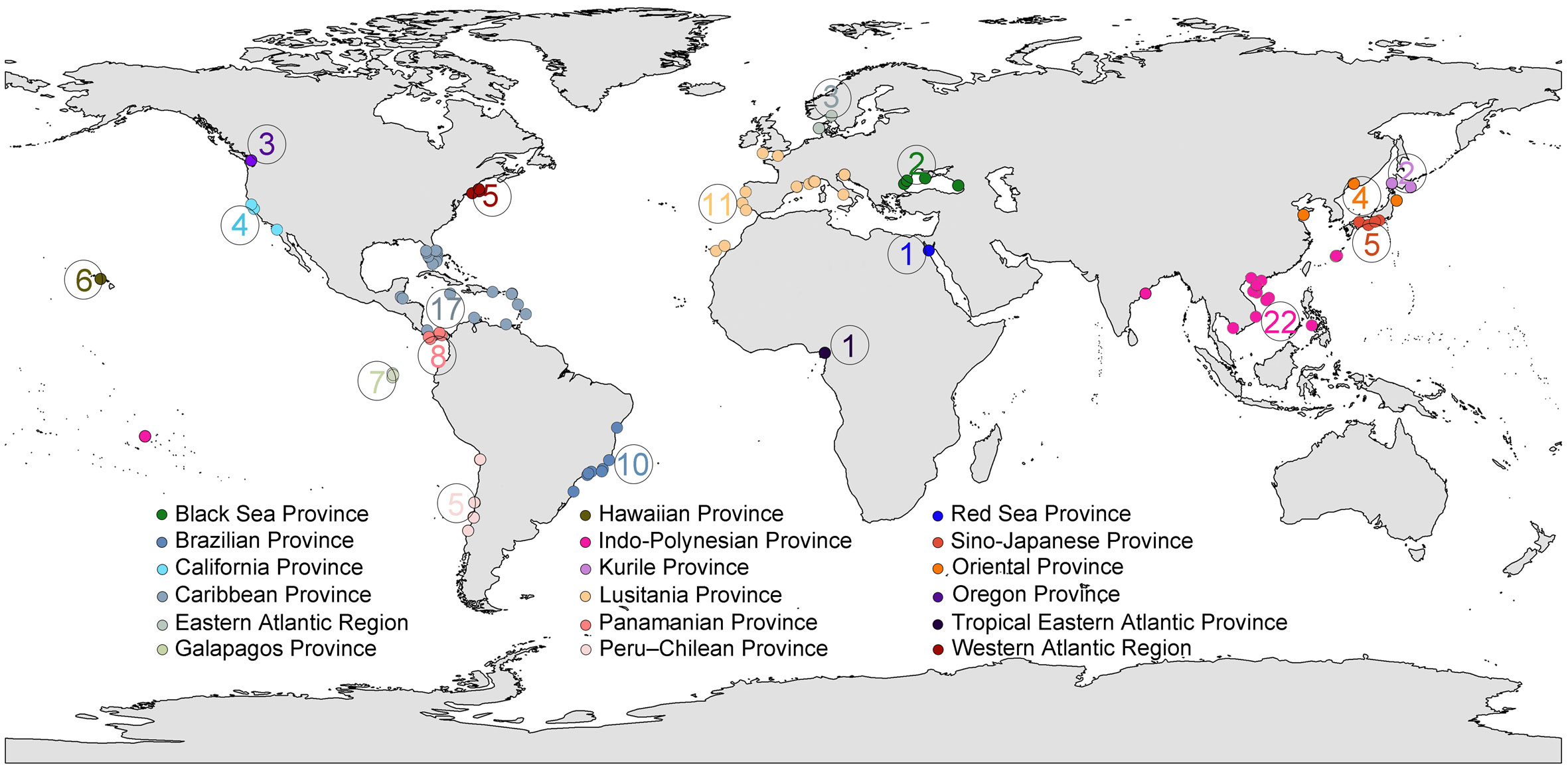
Figure 11 Global distribution of Ototyphlonemertes. Color codes represent different marine biogeographic provinces as defined by Briggs and Bowen (2012). The circled number is the number of species/entities in each biogeographic province.
Endemism of Ototyphlonemertes occurs not only at species/entity level, but also is common for lineages of species/entity aggregation, e.g., the distributions of clades Americas 1–3, Asia 1–3, and Europe 1 are confined to Americas, eastern Asia, and Europe, respectively (see Results and Figure 2). Because of the isolation of suitable habitats and low dispersal capability (Leasi et al., 2016), when a small number of individuals happen to arrive at a new suitable site, the founder effect may quickly result in genetic divergence, even though the new site is not far away from their original distribution area. Thus, evolutionary lineages endemic to a geographic region could be formed by such kind of allopatric speciation. The existence of regional evolutionary lineages found in Ototyphlonemertes is not a unique case in phylum Nemertea. Other examples can be seen in the high-diversity and relatively well-studied genera, Tetrastemma Ehrenberg, 1828 (Hoplonemertea) and Cephalothrix Örsted, 1843 (Palaeonemertea). In the former genus, Chernyshev et al. (2021) recognized four geographically cohesive subclades (North Pacific, American Atlantic, North Atlantic, and Asian-Australian subclades). In the latter genus, the clade of Cephalothrix simula/hongkongiensis species complex (containing at least four species/networks) is confined to the Northeast Asia (occurrences in other areas are thought to be recent anthropogenic introduction), the clade of Networks 1, 10, 16, and 24 is only recorded from European waters (see Chen et al., 2010; Sagorny et al., 2019).
Our results suggest that Ototyphlonemertes my have a high species diversity at a small sampling location, provided there is a suitable habitat. We collected three species at a small beach of Sanya (O. chernyshevi [Ca2], O. similis sp. nov., O. subrubra sp. nov.) and Jinqing Island (O. ani, O. coralli sp. nov., O. subrubra sp. nov.), respectively. High species diversity of Ototyphlonemertes in a small geographic scale was also reported in other well-sampled areas. For example, seven species were found from two, small, uninhabited islands in southern Vietnam (Kajihara et al., 2018a); ten independent entities were discovered at Carrie Bow Cay, Belize (Leasi and Norenburg, 2014; Leasi et al., 2016). Leasi et al. (2016) explained that local extinction combined with ‘island’ effect might play a major role in generating genetic diversification, and the co-occurrence of cryptic species might be a consequence of multiple colonization events from different locations and/or different genetic clusters.
To date, Ototyphlonemertes have been sampled from only handful sites in the Indo-Polynesian Province yet maximum species diversity has been found in this province. The majority of species were recorded from Chinese and Vietnamese waters, merely because studies have targeted Ototyphlonemertes from these areas. Only a single unidentified species was reported from the Coral Triangle (Philippines; Kajihara et al., 2007), which is a center of the highest marine biodiversity (Hoeksema, 2007; Veron et al., 2011). Even in the South China Sea, a large number of islands and coral reefs, e.g., the Nansha Islands (Spratly Islands), have never been investigated with respect to these nemerteans. It can be expected that a great number of uncovered species will be found by future studies.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
H-LL and S-CS conceived the study and participated in the design of the study. H-LL and S-CS collected and analyzed the morphology data. H-LL collected and analyzed the genetics data. All authors took part in the collection of samples and field data used in this study. H-LL drafted the manuscript, which was revised and improved by HK and S-CS. All authors gave final approval for submission and publication.
Funding
The study was financially supported by the National Natural Science Foundation of China (No. 31471957), the Fundamental Research Funds for the Central Universities (No. 201762017), and Japan Society for the Promotion of Science (No. 20K06780).
Acknowledgments
We are grateful to Professor Xiao-Qi Zeng for help with the fieldwork, and to Dr Yang Zhang for material transfer.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.1009536/full#supplementary-material
References
Andrade S. C. S., Norenburg J. L., Solferini V. N. (2011). Worms without borders: genetic diversity patterns in four Brazilian Ototyphlonemertes species (Nemertea, Hoplonemertea). Mar. Biol. 158, 2109–2124. doi: 10.1007/s00227-011-1718-3
Briggs J. C., Bowen B. W. (2012). A realignment of marine biogeographic provinces with particular reference to fish distributions. J. Biogeogr. 39, 12–30. doi: 10.1111/j.1365-2699.2011.02613.x
Bürger O. (1895). “Die nemertinen des Golfes von Neapel und der angrenzenden meeres-abschnitte,” in Fauna und flora des Golfes von Neapel und der angrenzenden meeres-abschnitte, vol. 22. (Berlin: R. Friedländer & Sohn).
Burzyński A., Zbawicka M., Skibinski D. O., Wenne R. (2003). Evidence for recombination of mtDNA in the marine mussel Mytilus trossulus from the Baltic. Mol. Biol. Evol. 20, 388–392. doi: 10.1093/molbev/msg058
Castresana J. (2000). Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 17, 540–552. doi: 10.1093/oxfordjournals.molbev.a026334
Casu M., Lai T., Sanna D., Cossu P., Galletti M. C. (2009). An integrative approach to the taxonomy of the pigmented European Pseudomonocelis Meixner, 1943 (Platyhelminthes: Proseriata). Biol. J. Linn. Soc 98, 907–922. doi: 10.1111/j.1095-8312.2009.01316.x
Cesari M., Montanari M., Kristensen R. M., Bertolani R., Guidetti R., Rebecchi L. (2019). An integrated study of the biodiversity within the Pseudechiniscus suillus–facettalis group (Heterotardigrada: Echiniscidae). Zool. J. Linn. Soc 20, 1–16. doi: 10.1093/zoolinnean/zlz045
Chen H., Strand M., Norenburg J. L., Sun S. C., Kajihara H., Chernyshev A. V., et al. (2010). Statistical parsimony networks and species assemblages in cephalotrichid nemerteans (Nemertea). PloS One 5, e12885. doi: 10.1371/journal.pone.0012885
Chernyshev A. V. (1993). Ototyphlonemertes martynovi sp. n. (Enopla, Ototyphlonemertidae): a new interstitial nemertean from the Sea of Japan. Zool. Zh. 72, 5–8.
Chernyshev A. V. (1998). New data on interstitial nemerteans of the family Ototyphlonemertidae (Enopla, Monostilifera) from the Sea of Japan. Zool. Zh. 77, 266–269.
Chernyshev A. V. (2003). Ototyphlonemertes valentinae sp. n. (Enopla, Ototyphlonemertidae) is an interstitial nemertean from the Sea of Japan. Zool. Zh. 82, 868–871.
Chernyshev A. V. (2007). Nemerteans of the genus Ototyphlonemertes (Enopla: Ototyphlonemertidae) from Van Phong Bay (South Vietnam). Russ. J. Mar. Biol. 33, 196–199. doi: 10.1134/S1063074007030091
Chernyshev A. V. (2016). “Nemerteans of the coastal waters of Vietnam,” in Biodiversity of the western part of the South China Sea, vol. 279–314 . Eds. Adrianov A. V., Lutaenko K. A. (Vladivostok: Dalnauka).
Chernyshev A. V., Polyakova N. E., Norenburg J. L., Kajihara H. (2021). A molecular phylogeny of Tetrastemma and its allies (Nemertea, Monostilifera). Zool. Scr. 50, 824–836. doi: 10.1111/zsc.12511
Chernyshev A. V., Polyakova N. E., Turanov S. V., Kajihara H. (2018). Taxonomy and phylogeny of Lineus torquatus and allies (Nemertea, Lineidae) with descriptions of a new genus and a new cryptic species. Syst. Biodivers. 16, 55–68. doi: 10.1080/14772000.2017.1317672
Coe W. R. (1901). Papers from the Harriman Alaska expedition. XX. the nemerteans. Proc. Wash. Acad. Sci. 3, 1–84. doi: 10.5962/bhl.title.1985
Coe W. R. (1940). Revision of the nemertean fauna of the pacific coasts of North, Central and northern South America. Reps Allan Hancock Pacif. Exped. 2, 247–322. doi: 10.25549/hancock-c82-14439
Coe W. R. (1943). Biology of the nemerteans of the Atlantic coast of North America. Trans. Conn. Acad. Arts Sci. 35, 129–328.
Corrêa D. D. (1948). Ototyphlonemertes from the Brazilian coast. Comun. Zool. Mus. Hist. Nat. Montev. 2, 1–12.
Corrêa D. D. (1950). Sôbre Ototyphlonemertes do Brasil. Bolm Fac. Filo, Ciênc. Univ. S. Paulo 15, 203–234. doi: 10.11606/issn.2526-4877.bsffclzoologia.1950.125194
Corrêa D. D. (1953). Sôbre a neurofisiologia locomotora de hoplonemertinos e a taxonomia de Ototyphlonemertes. Anais Acad. Bras. Ciénc. 25, 545–555.
Corrêa D. D. (1954). Nemertinos do litoral Brasileiro. Bolm. Fac. Filo Ciênc. Univ. S. Paulo 19, 1–90. doi: 10.11606/issn.2526-3382.bffclzoologia.1954.120084
Diesing K. M. (1863). Nachträge zur revision der turbellarien. S. B. Math. Nat. Kl. Akad. Wiss. Wien 46, 173–188.
Du Plessis G. (1891). Sur une nouvelle Oerstedia aveugle mais portant une paire vesicules auditives (otocystes). Zool. Anz. 14, 413–416.
Ehrenberg C. G. (1828). “Phytozoa turbellaria Africana et Asiatica in phytozoorum tabula IV. et V. delineate,” in Symbolae physicae, seu icones et descriptions corporum naturalium novorum aut minus cognitorum quae ex itineribus per libyam, aegyptium, nubiam, dongalam, syriam, arabiam et habessiniam, pars zoologica II, animalia evertebrata exclusis insectis. Eds. Hemprich F. G., Ehrenberg C. G. (Berolina: Officina Academica), IV–V.
Envall M. (1996). Ototyphlonemertes correae sp. nov. and a redescription of O. duplex (Nemertea: Monostilifera: Ototyphlonemertidae), with a phylogenetic consideration of the genus. J. Zool. 238, 253–277. doi: 10.1111/j.1469-7998.1996.tb05393.x
Envall M., Norenburg J. L. (2001). Morphology and systematics in mesopsammic nemerteans of the genus Ototyphlonemertes (Nemertea, Hoplonemertea, Ototyphlonemertidae). Hydrobiologia 456, 145–163. doi: 10.1023/A:1013029310452
Eyre-Walker A. (2000). Do mitochondria recombine in humans? Phil. Trans. R. Soc Lond. B 355, 1573–1580. doi: 10.1098/rstb.2000.0718
Fernández-Álvarez F. Á., Machordom A. (2013). DNA Barcoding reveals a cryptic nemertean invasion in Atlantic and Mediterranean waters. Helgoland Mar. Res. 67, 599–605. doi: 10.1007/s10152-013-0346-3
Folmer O., Black M., Hoeh W., Lutz R., Vrijenhoek R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 3, 294–299. doi: 10.4028/www.scientific.net/DDF.7.460
Fouquet A., Gilles A., Vences M., Marty C., Blanc M., Gemmell N. J. (2007). Underestimation of species richness in Neotropical frogs revealed by mtDNA analyses. PloS One 2, e1109. doi: 10.1371/journal.pone.0001109
Gerner L. (1969). Nemertinen der gattungen Cephalothrix und Ototyphlonemertes aus dem marinen mesopsammal. Helgol. Wiss. Meeresunters. 19, 68–110. doi: 10.1007/BF01625860
Gibson R. (1995). Nemertean genera and species of the world: an annotated checklist of original names and description citations, synonyms, current taxonomic status, habitats and recorded zoogeographic distribution. J. Nat. Hist. 29, 271–561. doi: 10.1080/00222939500770161
Herrera-Bachiller A., Junoy J. (2014). Occurrence of the interstitial nemertean Ototyphlonemertes duplex (Nemertea: Hoplonemertea) in the cabo de gata natural park (Mediterranean, south-east Spain). Mar. Biodivers. Records 7, e126. doi: 10.1017/S1755267214001225
Hiebert T. C., Maslakova S. (2015). Integrative taxonomy of the Micrura alaskensis Coe, 1901 species complex (Nemertea: Heteronemertea), with descriptions of a new genus Maculaura gen. nov. and four new species from the NE Pacific. Zool. Sci. 32, 615–637. doi: 10.2108/zs150011
Hoeksema B. W. (2007). “Delineation of the Indo-Malayan centre of maximum marine biodiversity: The Coral Triagle,” in Biogeography, time, and place: Distributions, barriers, and islands. Ed. Renema W. (London: Springer), 117–178.
Jörger K. M., Norenburg J. L., Wilson N. G., Schrödl M. (2012). Barcoding against a paradox? Combined molecular species delineations reveal multiple cryptic lineages in elusive meiofaunal sea slugs. BMC Evol. Biol. 12, 245. doi: 10.1186/1471-2148-12-245
Jörger K. M., Schrödl M. (2013). How to describe a cryptic species? Practical challenges of molecular taxonomy. Front. Zool. 10, 59. doi: 10.1186/1742-9994-10-59
Kajihara H. (2007a). Ototyphlonemertes dolichobasis sp. nov. (Nemertea: Hoplonemertea: Monostilifera: Ototyphlonemertidae), a new species of interstitial nemertean from Japan. Species Diversity 12, 57–66. doi: 10.12782/specdiv.12.57
Kajihara H. (2007b). A taxonomic catalogue of Japanese nemerteans (phylum Nemertea). Zool. Sci. 24, 287–326. doi: 10.2108/zsj.24.287
Kajihara H., Olympian M., Yap E. S., Gomez-Delan G., Quilantang M. B., Shimomura M., et al. (2007). Preliminary report on the nemertean fauna (phylum Nemertea) in the Philippines. UPV J. Nat. Sci. 12, 123–128.
Kajihara H., Tamura K., Tomioka S. (2018a). Histology-free descriptions for seven species of interstitial ribbon worms in the genus Ototyphlonemertes (Nemertea: Monostilifera) from Vietnam. Species Diversity 23, 13–37. doi: 10.12782/specdiv.23.13
Kajihara H., Tamura K., Yamasaki H. (2018b). Interstitial hoplonemertean Ototyphlonemertes norenburgi (Nemertea: Monostilifera) from Okinawa, Japan. Fauna Ryukyuana 46, 1–3.
Kang X. X., Fernández-Ávarez F. Á., Alfaya J. E. F., Machordom A., Strand M., Sundberg P., et al. (2015). Species diversity of Ramphogordius sanguineus/Lineus ruber-like nemerteans (Nemertea: Heteronemertea) and geographic distribution of R. sanguineus. Zool. Sci. 32, 579–589. doi: 10.2108/zs150064
Katoh K., Asimenos G., Toh H. (2009). Multiple alignment of DNA sequences with MAFFT. Methods Mol. Biol. 537, 39–64. doi: 10.1007/978-1-59745-251-9_3
Katoh K., Standley D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
Keferstein W. (1862). Untersuchungen über niedere seethiere. Z. Wiss. Zool. 12, 1–147. doi: 10.5962/bhl.title.47031
Kirsteuer E. (1977). Remarks on taxonomy and geographic distribution of the genus Ototyphlonemertes diesing (Nemertina, Monostilifera). Mikrofauna Meeresboden 61, 167–181.
Krämer D., Schmidt C., Podsiadlowski L., Beckers P., Horn L., Döhren J. (2017). Unravelling the Lineus ruber/viridis species complex (Nemertea, Heteronemertea). Zool. Scr. 46, 111–126. doi: 10.1111/zsc.12185
Kumar S., Stecher G., Li M., Knyaz C., Tamura K. (2018). MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. doi: 10.1093/molbev/msy096
Ladoukakis E. D., Zouros E. (2001). Direct evidence for homologous recombination in mussel (Mytilus galloprovincialis) mitochondrial DNA. Mol. Biol. Evol. 18 (7), 1168–1175. doi: 10.1093/oxfordjournals.molbev.a003904
Leasi F., Andrade S. C. S., Norenburg J. (2016). At least some meiofaunal species are not everywhere. indication of geographic, ecological and geological barriers affecting the dispersion of species of Ototyphlonemertes (Nemertea, Hoplonemertea). Mol. Ecol. 25, 1381–1397. doi: 10.1111/mec.13568
Leasi F., Norenburg J. L. (2014). The necessity of DNA taxonomy to reveal cryptic diversity and spatial distribution of meiofauna, with a focus on Nemertea. PloS One 9, e104385. doi: 10.1371/journal.pone.0104385
Librado P., Rozas J. (2009). DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452. doi: 10.1093/bioinformatics/btp187
Littlewood D. T. J. (1994). Molecular phylogenetics of cupped oysters based on partial 28S rRNA gene sequences. Mol. Phylogenet. Evol. 3, 221–229. doi: 10.1006/mpev.1994.1024
Liu H. L., Sun S. C. (2018). Ototyphlonemertes longissima sp. nov. (Hoplonemertea: Monostilifera: Ototyphlonemertidae), a new interstitial nemertean from the south China Sea. Zootaxa 4527, 581–587. doi: 10.11646/zootaxa.4527.4.9
Mendes C. B., Norenburg J. L., Solferini V. N., Andrade S. C. S. (2018). Hidden diversity: phylogeography of genus Ototyphlonemertes Diesing, 1863 (Ototyphlonemertidae: Hoplonemertea) reveals cryptic species and high diversity in Chilean populations. PloS One 13, e0195833. doi: 10.1371/journal.pone.0195833
Mock H. (1978). Ototyphlonemertes pallida (Keferstein, 1862) (Hoplonemertini, Monostilifera). Mikrofauna Des. Meeresbodens 67, 559–570.
Mock H., Schmidt P. (1975). Interstitielle fauna von Galapagos XIII. Ototyphlonemertes Diesing (Nemertini, Hoplonemertini). Mikrofauna Des. Meeresbodens 51, 1–40.
Monaghan M. T., Wild R., Elliot M., Fujisawa T., Balke M., Inward. D. J., et al. (2009). Accelerated species inventory on Madagascar using coalescent-based models of species delineation. Syst. Biol. 58, 298–311. doi: 10.1093/sysbio/syp027
Müller G. J. (1968). “Ototyphlonemertes antipai n. sp., ein neues mitglied des mediolitoralen mesopsammals des Schwarzen Meeres. Trav. Mus. Hist. Nat. ‘Grigore Airtipa’ 8, 343–348.
Norenburg J. L. (1988). “Nemertina,” in Introduction to the study of meiofauna. Eds. Higgins R. P., Thiel H. (Washington DC: Smithsonian Institution Press), 287–292.
Norenburg J., Gibson R., Herrera Bachiller A., Strand M. (2022)World nemertea database. Ototyphlonemertes Diesing, 1863. In: World register of marine species. Available at: https://www.marinespecies.org/aphia.php?p=taxdetails&id=122408 (Accessed 2022-05-1).
Örsted A. S. (1843). Forsog til en ny classification af planarierne (Planariea Duges) grundet paa mikroskopisk-anatomiske undersogelser. Naturhist Tidsskr 4, 519–581.
Padial J. M., Miralles A., Riva I. D. L., Vences M. (2010). The integrative future of taxonomy. Front. Zool. 7, 16. doi: 10.1186/1742-9994-7-16
Palumbi S., Martin A., Romano S., McMillan W. O., Stice L., Grabowski G. (1991). The simple fool’s guide to PCR, ver. 2.0 (Honolulu: Department of Zoology and Kewalo Marine Laboratory, University of Hawaii).
Pons J., Barraclough T. G., Gomez-Zurita J., Cardoso A., Duran D. P., Hazell S., et al. (2006). Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Syst. Biol. 55, 595–609. doi: 10.1080/10635150600852011
Puillandre N., Lambert A., Brouillet S., Achaz G. (2012). ABGD, automatic barcode gap discovery for primary species delimitation. Mol. Ecol. 21, 1864–1877. doi: 10.1111/j.1365-294x.2011.05239.x
Rambaut A., Drummond A. J., Xie D., Baele G., Suchard M. A. (2018). Posterior summarisation in Bayesian phylogenetics using tracer 1.7. Syst. Biol. 67, 901–904. doi: 10.1093/sysbio/syy032
Rao G. C., Ganapati P. N. (1968). The interstitial fauna inhabiting the beach sands of Waltair coast. Proc. Natl. India 34, 82–125.
Rawson P. D., Secor C. L., Hilbish T. J. (1996). The effects of natural hybridization on the regulation of doubly uniparental inheritance in blue mussels (Mytilus spp.). Genetics 144, 241–248. doi: 10.1093/genetics/144.1.241
R Core Team (2018). R: A language and environment for statistical computing (Vienna: R Foundation for Statistical Computing). Available at: https://www.R-project.org/.
Remane A., Schulz E. (1964). Die strandzonen des Roten Meeres und ihre tierwelt. Kieler Meeresforsch 20, 5–17.
Ronquist F., Teslenko M., van der Mark P., Ayres D. L., Darling A., Höhna S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Sagorny C., Wesseler C., Krämer D., Döhren J. (2019). Assessing the diversity and distribution of Cephalothrix species (Nemertea: Palaeonemertea) in European waters by comparing different species delimitation methods. J. Zool. Syst. Evol. Res. 57, 497–519. doi: 10.1111/jzs.12266
Sands C. J., Convey P., Linse K., McInnes S. J. (2008). Assessing meiofaunal variation among individuals utilising morphological and molecular approaches: an example using the Tardigrada. BMC Ecol. 8, 7. doi: 10.1186/1472-6785-8-7
Senz W. (1993). Nemertinen europäischer küstenbereiche (Nebst erganzenden angaben zur anatomie von Apatronemertes albimaculosa Wilfert & Gibson 1974). Ann. Nat. Mus. Wien 94/95, 47–145.
Shimomura M., Kato T., Kajihara H. (2001). Records of some marine invertebrates (nemerteans, asellotes and phyllodocids) from the coast around Otsuchi Bay. Otsuchi Mar. Sci. 26, 46–50. doi: 10.15083/00038871
Sluys R., Solà E., Gritzalis K., Farré M. V., Mateos E., Riutort M. (2013). Integrative delineation of species of Mediterranean freshwater planarians (Platyhelminthes: Tricladida: Dugesiidae). Zool. J. Linn. Soc 169, 523–547. doi: 10.1111/zoj.12077
Stamatakis A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Stec D., Arakawa K., Michalczyk Ł. (2018). An integrative description of Macrobiotus shonaicus sp. nov. (Tardigrada: Macrobiotidae) from Japan with notes on its phylogenetic position within the hufelandi group. PloS One 13, e0192210. doi: 10.1371/journal.pone.0192210
Stiasny-Wijnhoff G. (1942). Nemertinen der Westafrikanischen küste. Zool. Jahrbücher Abteilung für Syst. Ökol. und Geogr. der Tiere 75, 121–194.
Strand M., Herrera-Bachiller A., Nygren A., Kånneby T. (2014). A new nemertean species: What are the useful characters for ribbon worm descriptions? J. Mar. Biol. Assoc. U.K. 94, 317–330. doi: 10.1017/S002531541300146X
Strand M., Sundberg P. (2005). Delimiting species in the hoplonemertean genus Tetrastemma (phylum Nemertea): Morphology is not concordant with phylogeny as evidenced from mtDNA sequences. Biol. J. Linn. Soc 86, 201–212. doi: 10.1111/j.1095-8312.2005.00535.x
Strand M., Sundberg P. (2011). A DNA-based description of a new nemertean (phylum Nemertea) species. Mar. Biol. Res. 7, 63–70. doi: 10.1080/17451001003713563
Suchard M. A., Lemey P., Baele G., Ayres D. L., Drummond A. J., Rambaut A. (2018). Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 4, vey016. doi: 10.1093/ve/vey016
Sundberg P., Andrade S. C. S., Bartolomaeus T., Beckers P., Döhren J., Krämer D., et al. (2016a). The future of nemertean taxonomy (phylum Nemertea) — a proposal. Zool. Scr. 45, 579–582. doi: 10.1111/zsc.12182
Sundberg P., Chernyshev A. V., Kajihara H., Kånneby T., Strand M. (2009a). Character-matrix based descriptions of two new nemertean (Nemertea) species. Zool. J. Linn. Soc 157, 264–294. doi: 10.1111/j.1096-3642.2008.00514.x
Sundberg P., Kvist S., Strand M. (2016b). Evaluating the utility of single-locus DNA barcoding for the identification of ribbon worms (phylum Nemertea). PloS One 11, e0155541. doi: 10.1371/journal.pone.0155541
Sundberg P., Strand M. (2010). Nemertean taxonomy – time to change lane? J. Zool. Syst. Evol. Res. 48, 283–284. doi: 10.1111/j.1439-0469.2010.00568.x
Sundberg P., Vodoti E. T., Strand M. (2009b). DNA Barcoding should accompany taxonomy — the case of Cerebratulus spp. (Nemertea). Mol. Ecol. Resour. 10, 274–281. doi: 10.1111/j.1755-0998.2009.02774.x
Sun S.-C., Xu P. (2018). First record of interstitial nemerteans of the genus Ototyphlonemertes (Enopla: Monostilifera: Ototyphlonemertidae) from China. Chin. J. Zool. 53, 249–254. doi: 10.13859/j.cjz.201802010
Swedmark B. (1964). The interstitial fauna of marine sand. Biol. Rev. 39, 1–42. doi: 10.1111/j.1469-185X.1964.tb00948.x
Toews D. P. L., Brelsford A. (2012). The biogeography of mitochondrial and nuclear discordance in animals. Mol. Ecol. 21, 3907–3930. doi: 10.1111/j.1365-294X.2012.05664.x
Tulchinsky A. Y., Norenburg J. L., Turbeville J. M. (2012). Phylogeography of the marine interstitial nemertean Ototyphlonemertes parmula (Nemertea, Hoplonemertea) reveals cryptic diversity and high dispersal potential. Mar. Biol. 159, 661–674. doi: 10.1007/s00227-011-1844-y
Veron J. E. N., DeVantier L. M., Turak E., Green A. L., Kininmonth S., Stafford-Smith M., et al. (2011). “The Coral Triangle,” in Coral reefs: An ecosystem in transition. Eds. Dubinsky Z., Stambler N. (London: Springer), 47–55. doi: 10.1007/978-94-007-0114-4_5
Vieites D. R., Wollenberg K. C., Andreone F., Köhler J., Glaw F., Vences M. (2009). Vast underestimation of Madagascar’s biodiversity evidenced by an integrative amphibian inventory. Proc. Natl. Acad. Sci. U.S.A. 106, 8267–8272. doi: 10.1073/pnas.0810821106
Vilgalys R., Sun B. L. (1994). Ancient and recent patterns of geographic speciation in the oyster mushroom Pleurotus revealed by phylogenetic analysis of ribosomal DNA sequences. Proc. Natl. Acad. Sci. U.S.A. 91, 4599–4603. doi: 10.1073/pnas.91.10.4599
Zhang J., Kapli P., Pavlidis P., Stamatakis A. (2013). A general species delimitation method with applications to phylogenetic placements. Bioinformatics 29, 2869–2876. doi: 10.1093/bioinformatics/btt499
Keywords: species diversity, Nemertea, Ototyphlonemertes, integrative taxonomy, South China Sea, zoogeographical distribution
Citation: Liu H-L, Kajihara H and Sun S-C (2022) Diversity of interstitial nemerteans of the genus Ototyphlonemertes (Nemertea: Monostilifera: Ototyphlonemertidae) in the South China Sea, with a comment on the distribution pattern of the genus. Front. Mar. Sci. 9:1009536. doi: 10.3389/fmars.2022.1009536
Received: 02 August 2022; Accepted: 11 November 2022;
Published: 08 December 2022.
Edited by:
Peter Von Dassow, Pontificia Universidad Católica de Chile, ChileReviewed by:
Chunsheng Wang, Ministry of Natural Resources, ChinaAlexei Chernyshev, A.V. Zhirmunsky National Scientific Center of Marine Biology, Far Eastern Branch, Russian Academy of Sciences (NSCMB FEB RAS), Russia
Copyright © 2022 Liu, Kajihara and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shi-Chun Sun, sunsc@ouc.edu.cn
 Hai-Long Liu
Hai-Long Liu Hiroshi Kajihara
Hiroshi Kajihara Shi-Chun Sun
Shi-Chun Sun