- 1College of Biological Science and Technology, Shenyang Agricultural University, Shenyang, China
- 2College of Life Engineering, Shenyang Institute of Technology, Fushun, China
- 3Primorye State Agricultural Academy, Ussuriisk, Russia
- 4Federal Scientific Center of the East Asia Terrestrial Biodiversity, Far Eastern Branch of Russian Academy of Sciences, Vladivostok, Russia
Artificial cultivation of Morchella sextelata and other morels is expanding in China, but continuous cropping reduces Morchella for unknown reasons. Here, we investigated soil that had been used or not used for M. sextelata cultivation for 0, 1, and 2 years. We found that the continuous cropping of M. sextelata substantially reduced the pH and the nutrient content of the hyphosphere soil and increased sclerotia formation by M. sextelata. Changes in the structure of bacterial and fungal communities were associated with levels of available nitrogen (N) and phosphorus in the soil. With continuous cropping, the richness and diversity of fungal and bacterial communities increased, but the abundance of Bacillus and Lactobacillus decreased and the abundance of pathogenic fungi increased. FAPROTAX analysis indicated that N cycle functions were enriched more with than without continuous cultivation, and that enrichment of N cycle and sulfate respiration functions was higher in the second than in the first year of cultivation. FunGuild analysis indicated that the functions related to pathotrophs and wood saprotrophs were enriched by M. sextelata cultivation. Overall, the results suggest that continuous cropping may reduce M. sextelata production by acidifying the soil and increasing the abundance of pathogenic fungi. Additional research is needed to determine whether increases in the abundance of pathogenic fungi and changes in soil chemistry result in the declines in production that occur with continuous M. sextelata cultivation.
Introduction
In addition to being flavorful, the fruiting bodies of morel mushrooms (Morchella spp., Ascomycetes) contain a variety of nutrients and antioxidant, antibacterial, and immune-regulating compounds. As a result, Morchella spp. are highly valued (Li et al., 2017; Tietel and Masaphy, 2018; Meng et al., 2019; Badshah et al., 2021). The market demand for morels has been rising but cannot be met by wild resources (Du et al., 2016; Yang et al., 2019), such that morel cultivation is necessary. In recent years, the cultivation of Morchella spp. in China has gradually increased but so have associated problems including outbreaks of diseases and pests that reduce yields (Guo et al., 2016; Liu et al., 2021b). Guo et al. (2016) reported a serious rot disease of Morchella caused by Fusarium incarnatum-F. equiseti species complex from China. The natural incidence rate of the disease was more than 30%. Fusarium nematophilum was also identified as one of the main pathogens of stipe rot disease, which caused heavy losses in Morchella sextelata cultivation (Liu et al., 2021b).
The term “continuous cropping” refers to the practice of cultivating the same crop in the same place for more than 1 year. In addition to increasing the incidence and severity of diseases and pests, continuous cropping can alter soil physicochemical properties and soil microbial communities in ways that are detrimental to the growth of plant crop (Ren et al., 2015; Dong et al., 2017; Shen et al., 2018). Continuous cropping obstacle have been widely studied in plants, primarily based on high-throughput amplicon sequencing and microbiome analyses. The continuous cropping of sugar beet, for example, reduces the abundance of beneficial soil microorganisms, increases the abundance of pathogenic soil microorganisms, and leads to the accumulation of compounds responsible for allelopathic autotoxicity (Huang et al., 2019, 2021). For sugarcane, continuous cropping reduces the abundance of soil bacteria related to the nitrogen (N) and sulfur cycle in hyphosphere soil and increases the abundance of plant-pathogenic bacteria (Pang et al., 2021). In the case of tea, continuous cropping causes changes in the soil fungal community that were correlated with changes in soil pH and exchangeable aluminum content and the enrichment of functions related to pathotroph fungi (Li et al., 2020). Functional pathway analyses have indicated that the problems resulting from the continuous cropping of plants involve changes in soil physicochemical properties, in microbial community structure, and autotoxicity (Coskun et al., 2017; Cheng et al., 2020; Li et al., 2021a).
Regarding the cultivation of edible or medicinal mushrooms, the continuous cropping of Agaricus bisporus was found to increase the abundance of Penicillium and Mucor species in soil (Huang, 2006). Problems resulting from the continuous cropping of Ganoderma lingzhi may result from Penicillium spp. (Yuan et al., 2019). To our knowledge, problems resulting from the continuous cropping of Morchella spp. have not been previously investigated.
Consequently, it was hypothesized that the continuous cropping obstacle of Morchella is caused by the inhibition of rhizosphere exudates and the accumulation of pathogenic microorganisms. To test the hypothesis, in the current study, high throughput amplicon sequencing was used to assess fungal (ITS rDNA) and prokaryotic (16S rDNA) communities from the morel cultivation environment. We would determine whether problems in the continuous cropping of the morel Morchella sextelata are associated with changes in the composition and functional pathways of the soil microbial community, soil physicochemical properties, and soil allelochemicals.
Materials and methods
Description of the site, soil, and collection of samples
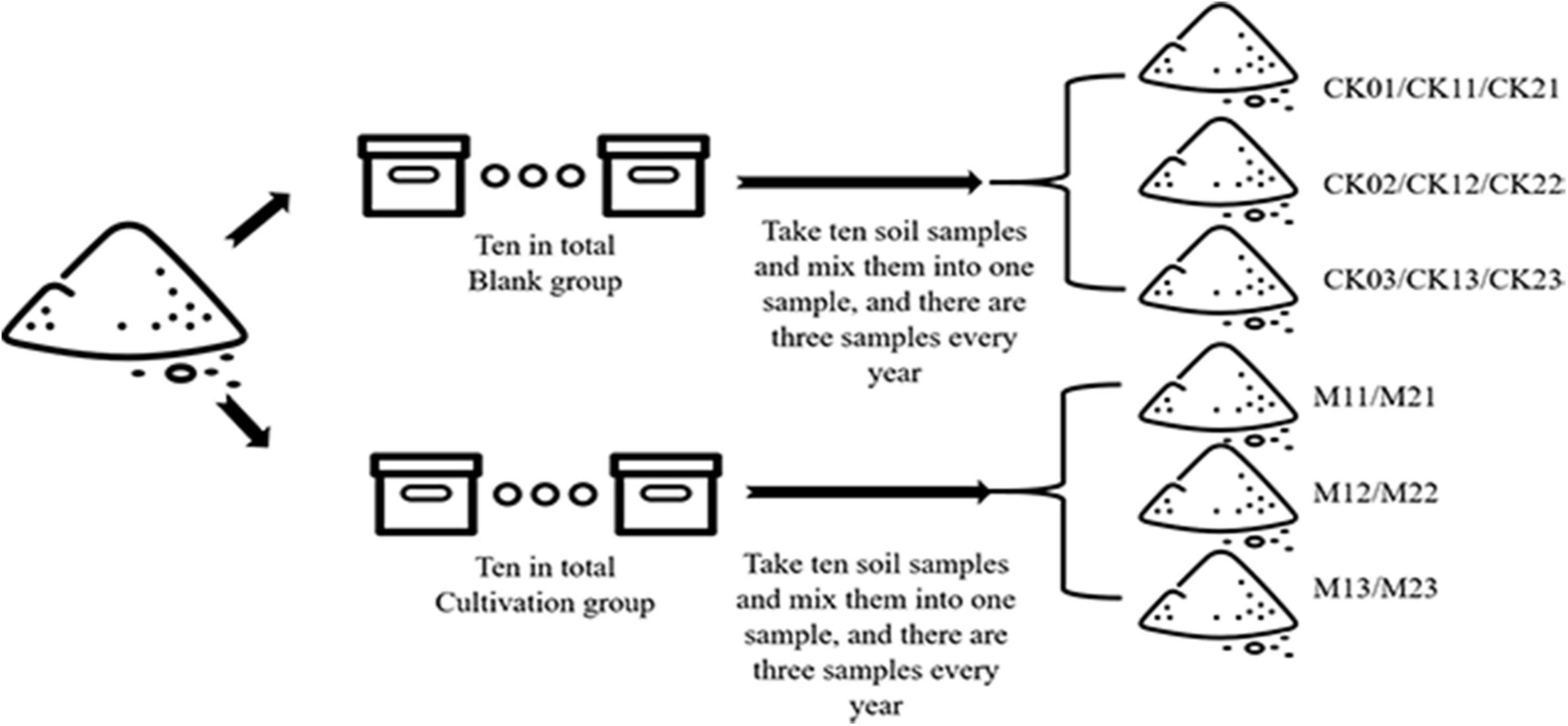
The experiment was conducted in 20 wooden boxes (83 cm x 58 cm x 51 cm) that were kept outside in Shenyang City, Liaoning province, China (123°45′N, 41°80′E). The experiment was conducted as described in Figure 1. Soil was collected Tian-zhu Mountain in Shenyang City and was amended with 10% humus. First, the soil was exposed, sieved (2 mm), and then evenly divided into 20 parts, and one part (≈ 245 cm3) was placed in each of 20 boxes (83 cm × 58 cm × 51 cm). All the boxes were placed in a greenhouse in experimental base of Shenyang Agriculture University. Among the 20 boxes, 10 boxes were cultivated with M. sextelata, and 10 boxes without M. sextelata were used as controls. The cultivation method followed the previous studies (Liu, 2017; Benucci et al., 2019). The criteria for the determination of Morchella infection is that the fruiting bodies of Morchella have lesions, damage, rot, developmental deformities, death, etc., which are regarded as Morchella infection (Liu, 2017).
M. sextelata was planted in November of 2019 and 2020 and was harvested about 3 months later, in February of 2020 and 2021. The process of soil sampling is outlined in Table 1. Soil samples (10 g) were collected from each box and were combined, mixed, and divided into three samples for CK boxes (without M. sextelata) and three samples for M boxes (with M. sextelata). For CK boxes, this was done at the start of the experiment (samples CK01, CK02, and CK03) and again in February 2020 and February 2021 (see Table 1). For M boxes, this was done in February 2020 and February 2021 (see Table 1). Each of the nine CK samples and each of the six M samples was divided into three parts: one part was dried for analysis of soil physicochemical properties; a second part was dried to determine the autotoxicity of soil allelochemicals; and a third part was stored at −80°C and was used for extraction of soil DNA.
Analysis of soil physicochemical properties and determination of the autotoxicity of soil allelochemicals
All the soil physicochemical properties in this study were testedy in Analytical and Testing Center, Shenyang Agricultural University. The standards for the physicochemical properties were listed as follows. The pH value: NY/T 1121.2-2006; total nitrogen (TN): NY/T 53-1987; total potassium (TK): NY/T 87-1988; total phosphorus (TP): NY/T 88-1988; available nitrogen (AN): NY/T 1121.7-2014; available potassium (AK): NY/T 889-2004; available phosphorus (AP): NY/T 1121.7-2014; organic matter (OM): NY/T 1121.6-2006; humic substances (HS): NY/T 1867-2010. The steps for testing the autotoxicity of soil allelochemicals were as follow: A 0.5-g subsample of each replicate of the five samples (CK0, CK1, CK2, M1, and M2) was sterilized at 121°C for 20 min. After the five sterilized subsamples were placed in five equally spaced locations along the edge of a 9-cm-diameter Petri plate (one subsample per sample per location) containing potato dextrose agar (PDA), the center of each place was inoculated with M. sextelata. The plates (10 per sample type) were kept at 25 °C, and the radial growth of M. sextelata toward each subsample was measured after 4, 6, 8 days, respectively.
DNA extraction, PCR amplification, and Illumina MiSeq sequencing
The procedures described in this section were performed by Novogene Bioinformatics Technology Co., Ltd. Total genome DNA from samples was extracted using the CTAB method. 16S rRNA genes of distinct regions (16S V3-V4) were amplified with the 16S-specific primers V3-V4:341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACNNGGGTATCTAAT-3′). ITS genes of distinct regions were amplified using the specific primers ITS2: ITS3-2024F (5′-GCATCGATGAAGAACGCAGC-3′) and ITS4-2409R (5′-TCCTCC GCTTATTGATATGC-3′). All PCR reactions were carried out with 15 μL of Phusion® High-Fidelity PCR Master Mix (New England Biolabs), 0.2 μM of forward and reverse primers, and about 10 ng of template DNA. Thermal cycling consisted of initial denaturation at 98°C for 1 min; followed by 30 cycles of denaturation at 98°C for 10 s, annealing at 50°C for 30 s, and elongation at 72°C for 30 s; and a final extension at 72°C for 5 min. PCR products were mixed in equidensity ratios. The mixture of PCR products was then purified with the Qiagen Gel Extraction Kit (Qiagen, Germany). Sequencing libraries were generated using the TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina, United States) following the manufacturer’s recommendations, and index codes were added. Library quality was assessed with the Qubit @ 2.0 Fluorometer (Thermo Fisher Scientific) and Agilent Bioanalyzer 2100 system. Finally, the library was sequenced on an Illumina NovaSeq platform, and 250-bp paired-end reads were generated.
Operational taxonomic unit cluster and species annotation
The Uparse algorithm (Haas et al., 2011) was used to cluster all effective tags of all samples. By default, the sequences were clustered into operational taxonomic units (OTUs) with 97% identity. At the same time, the representative sequences of OTUs were selected. The sequence with the highest frequency in OTUs was selected as the representative sequence of OTUs. Species annotation of bacterial OTUs sequences was performed using the mothur method and the SSUrRNA database (Wang et al., 2007) of SILVA138 (Edgar, 2013) to obtain taxonomic information (the threshold was set at 0.8-1.0). Species annotation of fungal OTUs sequences was performed using the blast method1 (Altschul et al., 1990) in Qiime software (Version 1.9.1) and the Unite (v8.2) database2 (Kõljalg et al., 2013). The community composition of each sample was then determined at each taxonomic level: kingdom, phylum, class, order, family, genus, and species. MUSIC software (Quast et al., 2013) was used for fast multi sequence alignment to determine the phylogenetic relationships among all OTU representative sequences. Finally, the data of each sample were homogenized, and the data with the least amount of data in the sample was used as the standard for homogenization. The subsequent Alpha diversity and Beta diversity analyses were based on the homogenized data.
Alpha diversity
Alpha diversity was applied to analyze the complexity of species diversity for a sample through 6 indices, including Observed-species, Chao1, Shannon, Simpson. All these indices in our samples were calculated with QIIME (Version 1.7.0) and were displayed with R software (Version 2.15.3).
Beta diversity
Beta diversity analysis was used to evaluate community differences between groups. Beta diversity on both weighted and unweighted unifrac were calculated by QIIME software (Version 1.9.1). Non-metric multi-dimensional scaling (NMDS) and principal component analysis (PCA) diagrams were drawn with R software (Version 2.15.3). The vegan package of R software was used for NMDS analysis, and the ade4 package and ggplot2 package of R software were used for PCA analysis. Unweighted pair-group method with Arithmetic Means (UPGMA) clustering was performed as a type of hierarchical clustering method to interpret the distance matrix using average linkage and was conducted with QIIME software (Version 1.9.1).
Correlation analysis of environmental factors
For canonical correlation analysis (CCA) and redundancy analysis (RDA), the CCA and RDA functions in the vegan package were used for ranking analysis. The r2 and P-values for the relationships between each environmental factor and species distribution were calculated with the envfit function, and environmental factors with significant P-values were then screened for CCA and RDA analysis. After the BioEnv function in vegan package was used to identify the environmental factors or combinations with the greatest Spearman correlation with the species matrix, the identified factors were subjected to targeted CCA and RDA analysis. Variance inflation factor (Vif) used the vif.cca function in vegan package for screening the environmental factors with redundancy constraints, and then non-redundant environmental factors were used for CCA and RDA analysis. According to the results of a degraded response analysis (DCA), RDA analysis was selected to reflect the relationship between soil microorganisms and environmental factors. The corr.test function of the psych package in R was used to calculate the Spearman correlation coefficients between species and environmental factors, and the pheatmap function in pheatmap package was then used for visualization.
Functional prediction
Phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt) was used to identify the gene function spectrum of their common ancestor based on the gene information on the tree of OTUs in the Greenenes database; And analyze the gene function spectrum of other unmeasured species in the Greenenes database, constructs the gene function prediction spectrum of the whole lineage of Archaea and bacterial domain; Finally, maps the composition of the sequenced soil microorganisms into the database, we predicted the metabolic function of soil microorganisms. FAPROTAX was the environmental function database of prokaryotes, which classified the ecological role of bacteria and archaea in the environment according to the published literature evidence, and summarized it into FAPROTAX database. Based on the annotation results of amplified sub species, the database can be queried to obtain the environmental function information of species supported by existing literature.
FunGuid was the environmental function database of fungi. Based on the existing literature, the ecological functions of fungi were classified and the FunGuid database was constructed. Based on the species information obtained by amplicon analysis, the ecological functions of existing species in the literature can be forecasted.
Statistical analysis
Soil physicochemical properties were analyzed using one-way analysis of variance (ANOVA), followed by Duncan’s multiple range tests. The Paired Samples Test showed significant differences in yield and infection rates between M1 and M2. For all parameters, data were calculated using SPSS Statistics software version 17.0 (SPSS Inc., United States). Differences at P < 0.05 were regarded as statistically significant.
Results
Morchella sextelata fruiting body production
After 2 years of continuous cultivation, we counted the production of 2-year fruiting body and diseased fruiting body (Supplementary Table 1.1). The production of M2 was significantly lower than that of M1 (113.0 g for M2 vs. 221.7 g for M1). However, the infection rate of fruiting bodies of M2 was significantly higher than that of fruiting bodies of M1 (35.9% for M2 vs. 9.0% for M1). The significant differences in yield and infection rates showed in Supplementary Tables 1.2, 1.3.
Soil physicochemical properties and the autotoxicity of allelochemicals
Soil pH and the contents of AK, AP, and TK decreased significantly or tended to decrease over time (Table 2). AN, TN, and OM did not significantly differ between CK0 and M1, but were significantly lower in M2 than in CK0. Four days after Petri plates with the five kinds of soil samples were inoculated with M. sextelata (Supplementary Figure 1), the mycelium of the fungus had grown over the entire surface of all plates. At the stage of sclerotia formation, however, sclerotia formation was particularly vigorous toward the M2 soil sample.
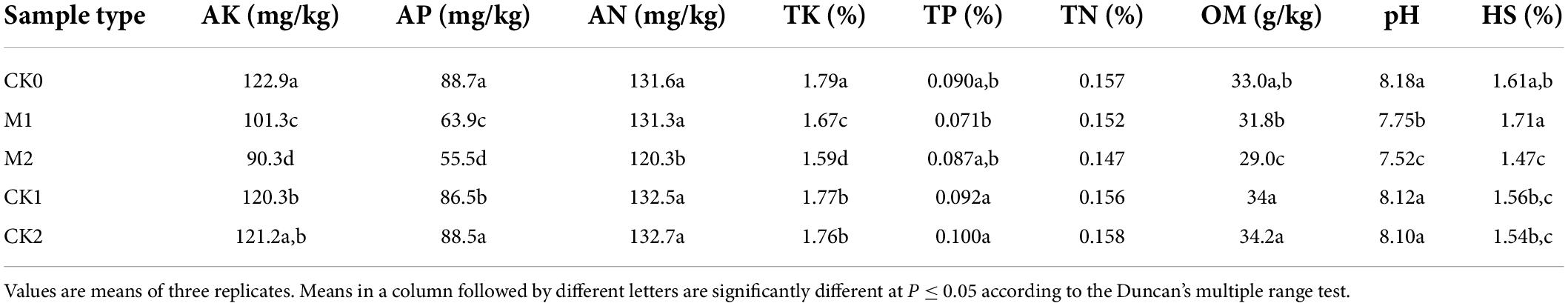
Table 2. Effects of continuous cropping of Morchella sextelata on the physicochemical properties of hyphosphere soil.
Illumina sequencing data analysis
By splicing reads, we measured on average of 106,033 tags for each bacterial sample; 90,433 raw tags were obtained through quality control, with 65,307 effective tags and a 61.65% effective rate of quality control. An average of 102,621 tags were measured for each fungal sample; 98,369 raw tags were obtained through quality control, with 64,082 effective tags and a 62.51% effective rate of quality control. The sequences were clustered into OTUs with 97% identity. A total of 5,210 bacterial OTUs and 1,813 fungal OTUs were obtained. In the annotation results, 1,847 (35.45%) bacterial OTUs and 1,291 (71.21%) fungal OTUs were annotated to the genus level. We then calculated the α-diversity for bacteria and fungi as affected by the treatments (the CKs and Ms) and as related to environmental properties.
Alpha diversity of the microbial community in the hyphosphere of Morchella sextelata
The rarefaction curves (Supplementary Figure 2) of all groups of bacteria and fungi approached a saturation plateau, indicating that the amount of data sequenced was sufficient to reflect the information for most microorganisms in the samples. The microbial richness and diversity between the groups were analyzed by Chao1 index and Shannon index (Supplementary Figure 2 and Supplementary Table 2.1). The significances were also analyzed by Wilcoxon’s test (Supplementary Tables 2.2, 2.3). After planting Morchella, the richness and diversity of both fungal and bacterial communities in the soil were significantly increased compared with the control groups (M1 vs. CK1 and M2 vs. CK2), indicating that the number of Morchella-related microorganisms increased over time.
Bacterial community composition in hyphosphere soil with continuous cropping of Morchella sextelata
A total of 2,810 prokaryotic OTUs were shared by the five sample types (Supplementary Figure 3). Unique prokaryotic microorganisms were more abundant in the M samples than in the CK samples, and the unique prokaryotic microorganisms were more abundant in M1 than in CK1 and were more abundant in M2 than in CK2. For CK0, M1, and M2, M1 and M2 shared the highest number of OTUs (351), indicating that the composition of bacterial communities in M1 and M2 was highly similar. M1 and M2 shared 3,653 OTUs, and M1 had more unique OTUs (533) than M2 (505). For CK0, CK1, and CK2, there were 3,125 OTUs, and the number of unique OTUs was in the order CK0 (295) > CK1 (257) > CK2 (228), indicating that the number of unique OTUs in the CKs decreased year by year.
To gain a deeper understanding of changes in bacterial community abundance and composition during continuous cropping of M. sextelata, bacterial communities were analyzed at the phylum level (Figure 2A) and the genus level (Figure 2B). We also assessed differences in the relative abundance of some bacteria in the five types of samples (Supplementary Table 3). At the phylum level, the dominant phylum of in each sample type was Proteobacteria. The abundance of Acidobacteriota was slightly lower in the Ms than in the CKs. The abundance of Firmicutes was 68.5% higher in M1 than in CK0, but decreased by 34.5% in M2; the trend in changes from CK0 to CK1 and CK2 was opposite to that from M1 to M2. There was no significant difference in the abundance of Proteobacteria among the five sample types, but at the order level, the abundance of Enterobacterales was much higher in the Ms than in the CKs, and was higher in M2 than in M1. In the Bacteroidota, the abundance of Bacteroidales was higher in the Ms than in the CKs, and the abundance of Cytohagales was higher in the CKs than in the Ms.
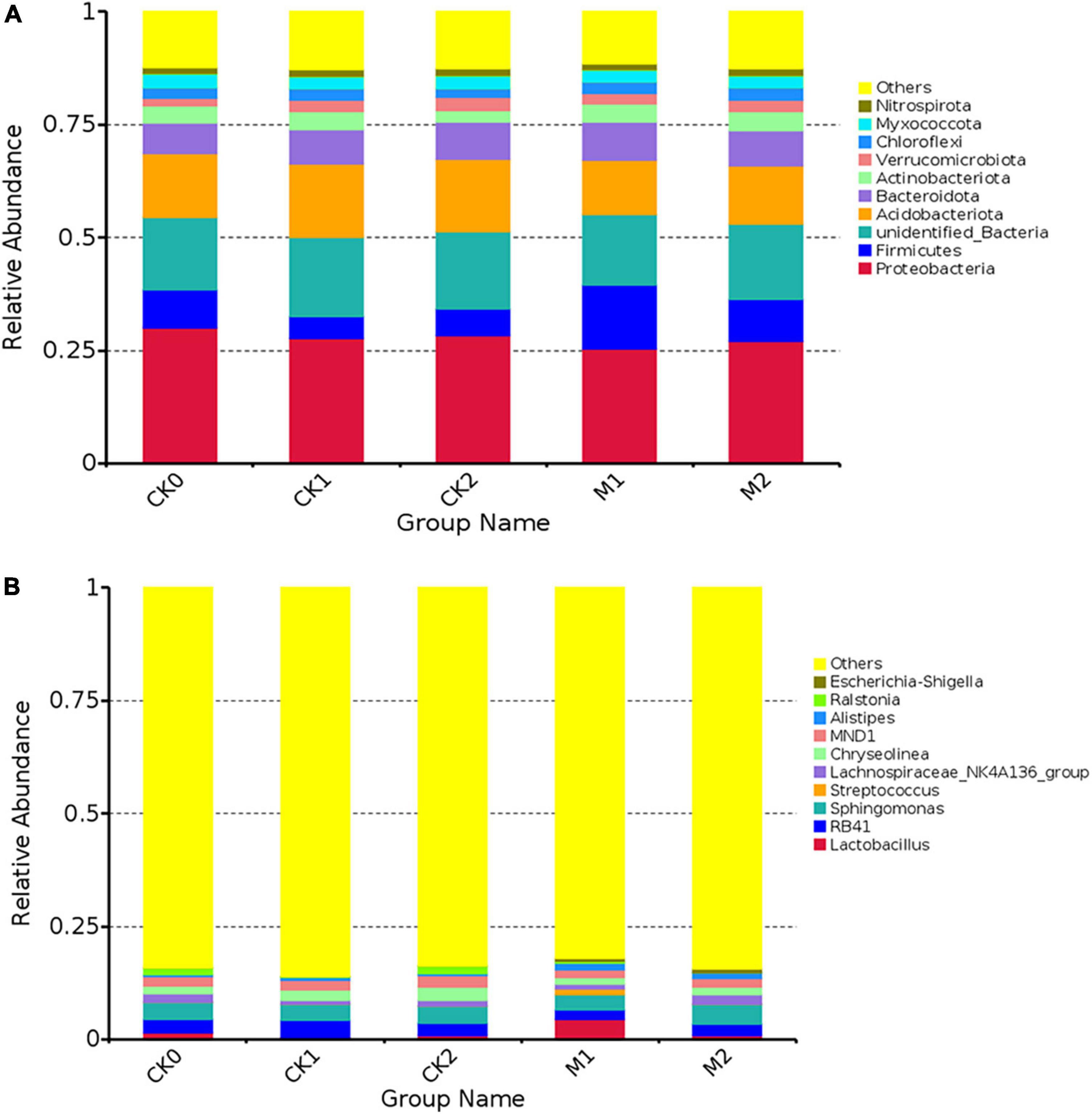
Figure 2. The composition of bacterial communities at the phylum level (A) and genus level (B) in the five types of samples. The 10 species with the highest abundance at each level were selected for each sample type. “Others” represents of the sum of the relative abundance of all other phyla (genus) other than these 10 phyla or genera.
At the genus level, the dominant genus in M1 was Lactobacillus, while the dominant genus in the other four sample types was Sphingomona. The abundances of Lactobacillus, Escherichia, Shigella, and Alitipies were much higher in M1 than in CK0, but their abundances were similar among CK1, CK2, and CK3. Interestingly, the abundances of some bacterial genera had the opposite trend in M1 vs. M2; For example, Lactobacillus and Streptococcus increased in M1 and then decreased in M2, and RB41 and Streptococcus decreased in M1 and then increased in M2, such that the abundances of these bacteria in M2 were similar to their abundance in CK0.
Fungal community composition in the hyphosphere soil with continuous cropping of Morchella sextelata
There were 510 eukaryotic OTUs shared among the five sample types (Supplementary Figure 4). The unique eukaryotic microorganisms were more abundant in the Ms than in the CKs, and were more abundant in M1 than in CK1 and in M2 than in CK2. For CK0, M1, and M2, M1 and M2 shared the most OTUs (263), indicating that the similarity in the composition of fungal communities was high for M1 and M2. M1 and M2 shared 936 OTUs, and the number of OTUs was higher in M2 (373) than in M1 (256). A total of 3,125 OTUs were shared by the three CKs, and the order of the number of OTUs was CK2 (161) > CK0 (149) > CK1 (126).
The fungal community was analyzed at the phylum level (Figure 3A) and genus level (Figure 3B). The dominant phylum for all five sample types was Ascomycota. We also assessed the differences in the relative abundance of some fungi in the sample types (Supplementary Table 3). The abundance of Basidiomycota was 140% higher in M1 than in CK0, and its abundance was nearly 11 times higher in CK1 than in CK0. The abundance of Mucoromycota was 85% higher in M2 than in M1, and the abundance of Glomeromycota was 168% higher in M1 than in CK0 and was 23% higher in M2 than in M1. In contrast to the changes in the abundance of these two phyla in the Ms, the changes in the CKs were small.
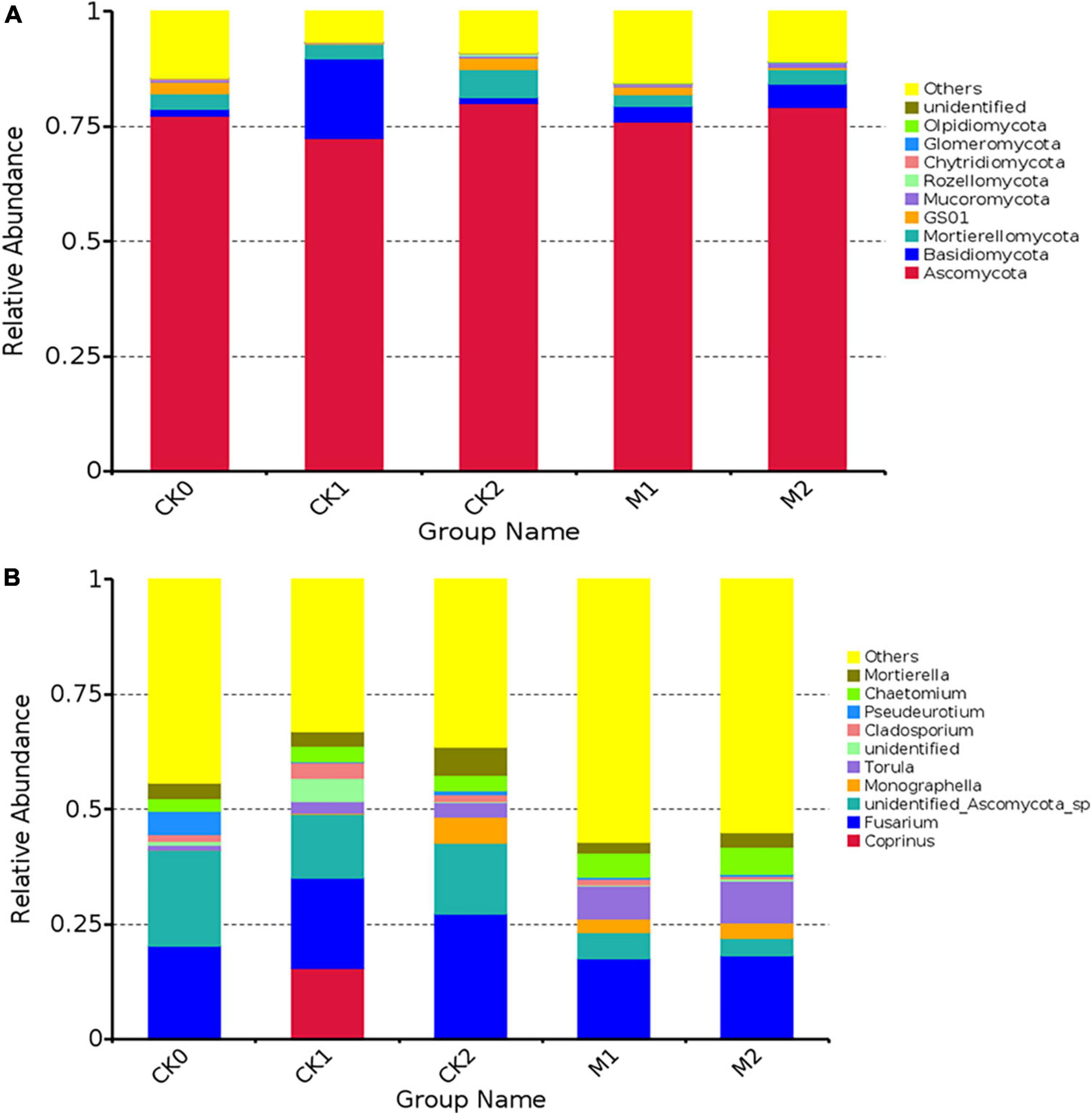
Figure 3. The composition of the fungal community at phylum level (A) and genus level (B) in the five types of samples. The 10 species with the highest abundance at the phylum or genus level were selected for each sample type. “Others” represents the sum of the relative abundances of all other phyla (genera) other than these 10 phyla or genera.
At the genus level, the dominant genus in CK0 was an unidentified Ascomycota sp., and the dominant genus in the other sample types was Fusarium. The abundance of Fusarium was similar in CK1 and CK2, and was slightly lower in the Ms than in the CKs. The abundance of Penicillium was 157% higher in M1 than in CK0, and was 105% higher in M2 than in M1, but the variation in the abundance of Penicillium in the CKs was small. The abundance of Trichoderma was 422% higher in M1 than in CK0, but the variation of the abundance of Trichoderma in the CKs was small. In addition, the abundance of some common pathogenic fungi, such as Fusarium oxysporum, Botrytis cinerea, Clonostachys rosea, and Aspergillus niger, increased during continuous cropping of M. sextelata.
Effects of continuous cropping on the microbial community structure in the Morchella sextelata hyphosphere
NMDS analysis and UMGPA clustering tree analysis were performed on the microbial communities in the five sample types. For both the bacterial community (Figure 4A) and the fungal community (Figure 4B), M1 and M2 were well separated from the CKs in the NMDS analysis, indicating that microbial community structure differed in soil in which M. sextelata had or had not been continuously cropped. The small distance between M1 and M2 indicated that the microbial community structures of M1 and M2 were similar. Similar results were obtained in the gate level analysis of the UMGPA clustering tree (Supplementary Figure 5). In these analyses, the microbial community structure was similar for the CKs, i.e., CK0, CK1, and CK2.
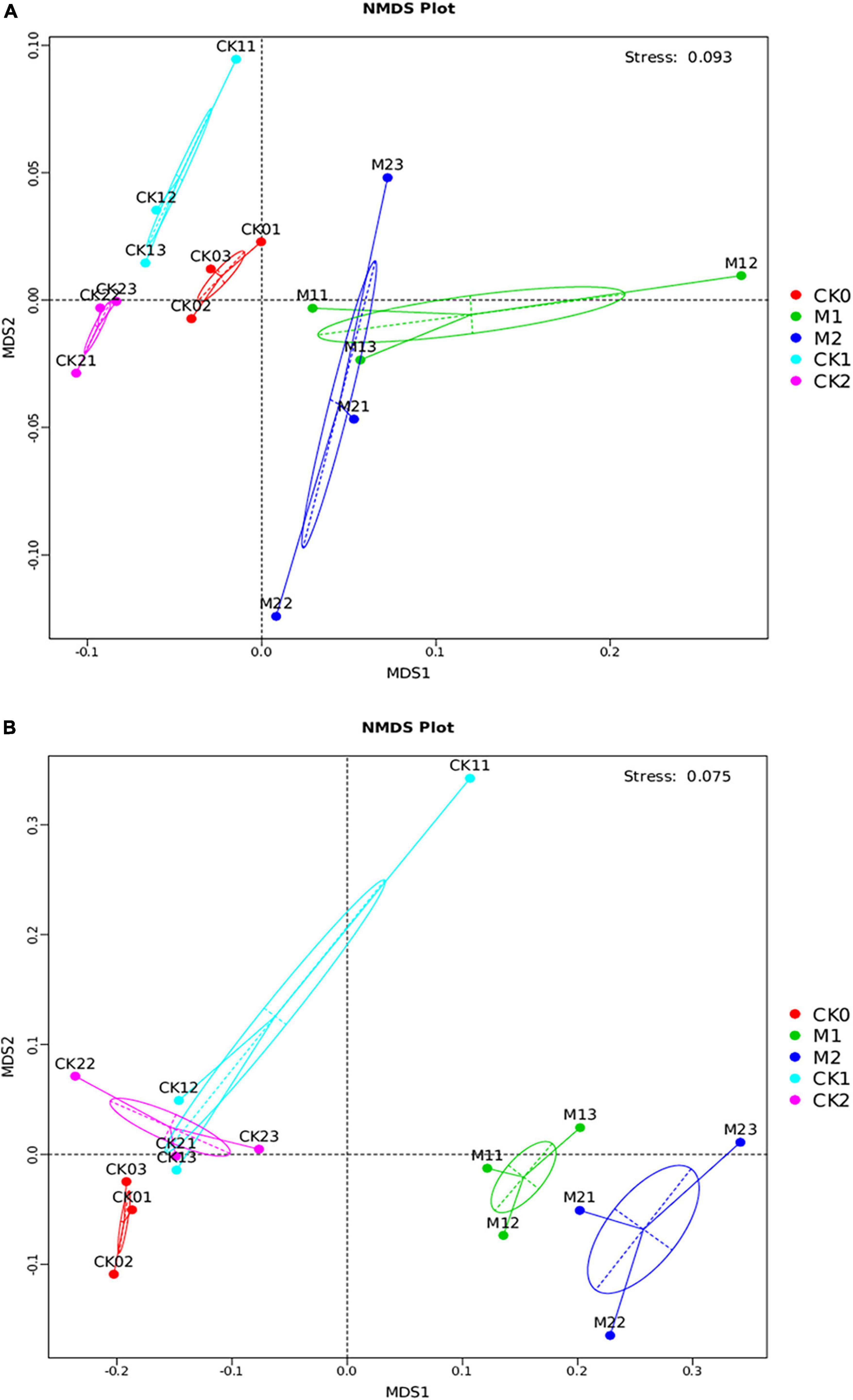
Figure 4. Differences in microbial community structure among the five sample types. Bray curtis NMDS analysis of bacterial community (A) and fungal community (B) at the OTU level. The top right corner of (A,B) shows the stress value of the NMDS analysis.
Relationships between environmental factors and on bacterial and fungal community composition
RDA analysis was performed on the bacterial and fungal communities (Figure 5). RDA components (RDA1 and RDA2) explained 59 and 41% of total variation in the bacterial community, and 65 and 35% of total variation in the fungal community. In addition, r2 and P-values were calculated to investigate the possible relationships between soil environmental factors and microbial community composition. Among soil environmental factors, the bacterial community composition (Figure 5A) was significantly related to AN (r2 = 0.984, P = 0.025) and AP (r2 = 0.973, P = 0.033), and the fungal community composition (Figure 5B) was also significantly related to AN (r2 = 0.995, P = 0.05) and AP (r2 = 0.985, P = 0.033) in the five types of samples.
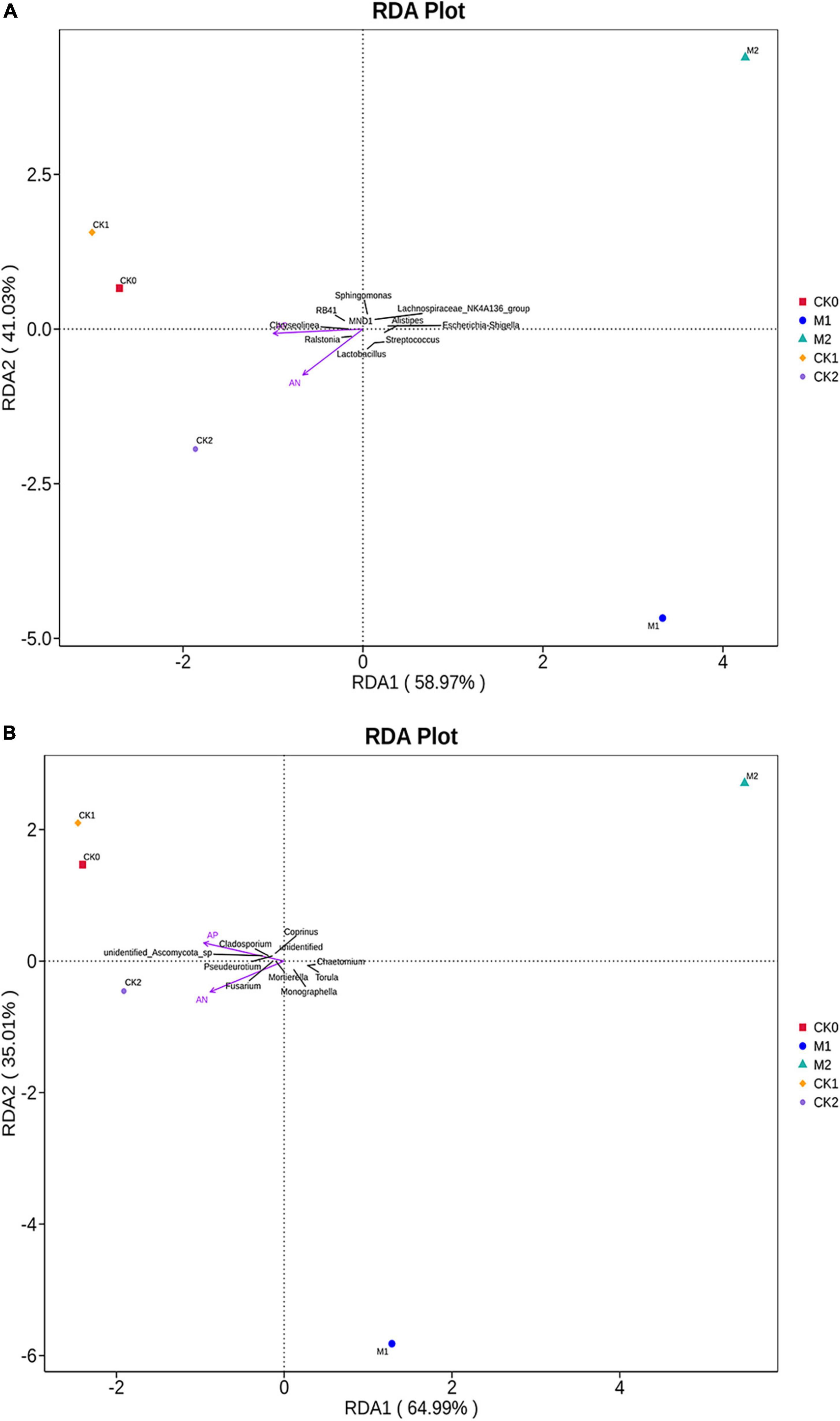
Figure 5. Redundancy analysis plots of the correlation between environmental factors (after Vif screening) and the (A) bacterial community and (B) fungal community in the five types of samples.
Spearman rank correlation analysis was used to determine the relationships between environmental factors and the α-diversity indices of the key microorganisms (Supplementary Table 4). In the bacterial community, Bacillus was negatively correlated with AN (P < 0.05), TP (P < 0.01), and OM (P < 0.05), and Lactobacillus was negatively correlated with TP (P < 0.01). The α-diversity index of the bacterial community was negatively correlated with AN, TP, TN, and OM. The abundances of most pathogenic fungi were negatively correlated with environmental factors; more specifically, the abundances of Penicillium, Stachybotrys, and Trichoderma and the fungal community α-diversity index were negatively correlated with most environmental factors.
Effects of continuous cropping of Morchella sextelata on the predicted functions of soil microorganisms in the hyphosphere
The results of PICRUSt showed the KEGG metabolic pathway Level2 contains seven classes and 41 metabolic pathways (Supplementary Figure 6). The pathway amino acid metabolism was the most enriched, followed by the pathways’ membrane transport, carbohydrate metabolism, replication and repair, energy metabolism, and undesirable characteristics. FAPROTAX was then used to predict the ecological functions of bacteria. The functions chemoheterotrophy and fermentation were more enriched in M1 than in the other four sample types (Figure 6A). The heatmap (Figure 7A) indicates that genes related to N cycle functions were significantly more abundant in the Ms than in the CKs, and were more abundant in M2 than in M1. Genes related to sulfate consumption and intracellular parasites also increased year by year during the continuous cropping of M. sextelata. Genes associated with chemoheterotrophy, fermentation, phototrophy, oxygenic photoautotrophy, and cyanobacteria were more abundant in the Ms than in the CKs, but were less enriched in M2 than in M1. Genes associated with cellulolysis, chitunolysis, xylanolysis, chloroplasts, and ureolysis were more abundant in the CKs than in the Ms. Principal components analysis (Supplementary Figure 7) showed that some bacterial community functions differed between the CKs and the Ms.
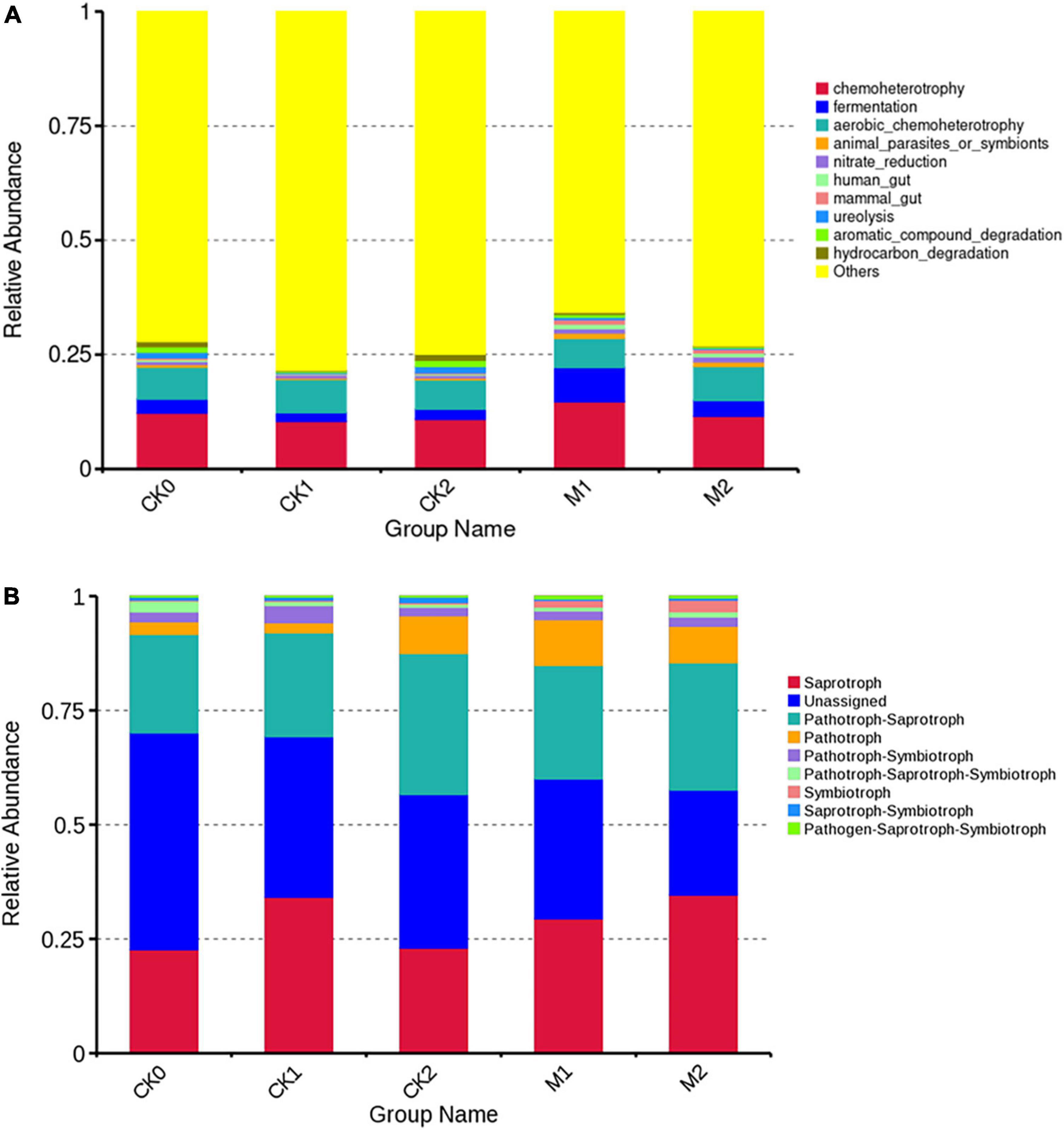
Figure 6. Ecological function relative abundance of bacterial community (A) and fungal community (B).
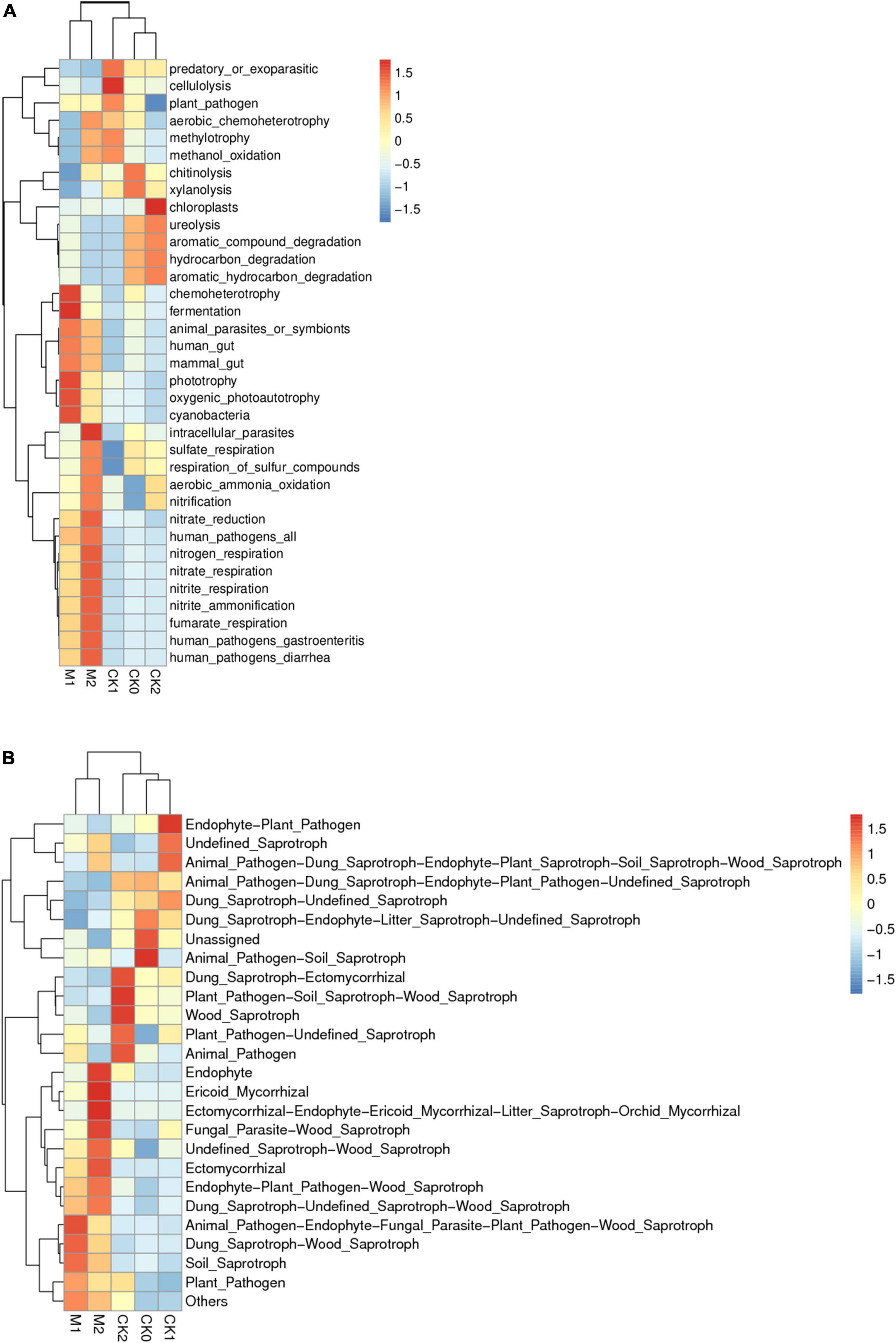
Figure 7. Ecological function heatmap of bacterial community (A); ecological function heatmap of fungal community (B).
The results of FunGuild (Figure 6B) showed that the relative abundances of saprotrophs and pathograph-saprotrophs were high in all groups except unassigned. Pathotrophs were more abundant in the Ms than in the CKs, and symbiotrophs were more abundant in M2 than in M1. According to the heatmap (Figure 7B), wood saprotrophs were more abundant in the Ms than in the CKs, and ectomycorrhizal fungi, endophytic fungi, and parasitic fungi were more abundant in M2 than in M1 or the CKs. Soil saprotrophs and plant pathogens were more abundant in M1 than in M2. PCA was performed based on the statistical results of the abundance of functional annotations in the database (Supplementary Figure 7). The close distances in the dimension reduction diagram indicate that the functions of fungi were similar in M1 and M2. Similarly, the close distances in the dimension reduction diagram indicate that the fungi in CK0, CK1, and CK2 have similar functions. The long distances between the Ms and the CKs indicate that the fungi have different functions in the Ms than in the CKs.
Discussion
Analysis of soil allelochemicals and physicochemical properties
In a Petri dish assay, we found that the growth and development of M. sextelata mycelium did not differ among the five sample types. However, more M. sextelata sclerotia formed near the M2 sample type than near the other four sample types. A possible reason is that exudates in the M. sextelata hyphosphere promoted the growth and development of M. sextelata sclerotia. The release of such allelochemicals could lead to an excessive loss of available nutrients, resulting in reduced production when M. sextelata is continuously cropped. Future studies will identify the allelochemicals and explore their properties and driving mechanisms through metabolome and transcriptome analysis.
In the current study, the continuous cropping of M. sextelata resulted in a decrease in available nutrients and in soil pH. A previous study found that changes in soil physicochemical properties in the M. sextelata hyphosphere are closely related to changes in the microbial community and especially the bacterial community (Zhang et al., 2019). We found that AN was significantly lower in M2 than in M1 or CK0. Soil N content can affect the growth of Morchella spp. and especially the growth of Morchella spp. sclerotia (Volk and Leonard, 1989; Mu, 2019). We infer that the allelochemicals in M2 soil enhanced the growth of M. sextelata sclerotia, resulting in the consumption of a large amount of AN, such that the content of AN was significantly reduced in M2.
Analysis of the microbial community composition in the hyphosphere of continuously cropped Morchella sextelata
For the bacterial community, the abundance of Bacillus was 151% higher in M1 than in CK0, but was 70% lower than in M2. Bacillius spp. substantially affected the growth and development of Morchella rufobrunnea fruiting bodies in a recent study (Longley et al., 2019). This is because Bacillus can not only inhibit the pathogenic fungus Trichoderma harzianum in Pleurotus (Velázquez-Cedeño et al., 2008), but can also produce a lipopeptide that inhibits pathogenic microorganisms such as Fusarium oxysporum (Sarwar et al., 2018). The results of the current study suggest that although Bacillus spp. may have increased the growth of M. sextelata during the first year of cropping, the abundance of Bacillus spp. declined in the second year, which may have increased the risk that M. sextelata was attacked by other microorganisms. The abundance of Bacillius may have decreased in M2 because of competition with Clostridium or the influence of environmental factors. The abundance of Lactobacillus was 192% higher in M1 than in CK0, but was 79% lower in M2 than in M1. Polysaccharides produced by edible fungi stimulate Lactobacillus growth and provide fermentation substrates for Lactobacillus (Nowak et al., 2018; Tohno et al., 2019). In the current study, M. sextelata grew normally in the first year and may have produced a large amount of polysaccharides, which may have increased the abundance of Lactobacillus. At the same time, Lactobacillus can produce lactic acid, which could reduce the pH of the soil. The decrease of soil pH would provide favorable conditions for the growth of pathogenic fungi such as Trichoderma, Mucor, and Penicillium, thereby increasing the probability that M. sextelata was infected by pathogens. In the second year of cropping, however, M. sextelata growth was decreased, which may have led to a sharp decrease in the content of polysaccharides in the soil and therefore in the abundance of Lactobacillus.
In the current study, we found that changes in the abundance of pathogenic fungi with the continuous cultivation of M. sextelata had three patterns. In one pattern, the cultivation of M. sextelata in the first year resulted in an increase in the abundance of pathogenic fungi, i.e., the abundance of pathogenic fungi was higher in M1 than in CK0. It is therefore possible that this increase in pathogenic fungi led increased infection of M. sextelata by these fungi in the second year of cropping. The pathogenic fungi that increased in abundance included Penicillium, Trichoderma, Aspergillus sp., Fusarium oxysporum, Botrytis cinerea, and Clonostachys rosea. Penicillium is a common pathogenic fungus in the production of edible fungi. In this study, the abundance of Penicillium increased with the years of continuous cropping of M. sextelata. Penicillium was previously found to interfere with the cultivation of Pleutus ostreatus, Lentinus edodes, and other mushrooms (Xiang, 2007; Liu, 2017). By inhibiting the mycelial growth of edible fungi, Penicillium can prevent the fungi from forming fruiting bodies or might cause the fruiting bodies to rot and become brown. Trichoderma is also a main pathogen of Morchella (Liu, 2017; Chen et al., 2018) found that the abundance of Trichoderma was higher in a soil in which Morchella stipe rot disease occurred than in a soil without the disease. In our study, Trichoderma abundance was much higher in the Ms than in the CKs. Aspergillus sp. can cause white mildew of Morchella (Yu et al., 2020). In our study, Aspergillus abundance was 105% higher in M1 than in CK0, and was 62% higher in M2 than in M1. In addition to causing disease of plant roots, Fusarium spp. can also cause disease of Morchella (Scherm et al., 2013; SMa et al., 2013; Guo et al., 2016). For example, Fusarium nematophilum was identified as the pathogen of stipe rot disease of M. sextelata (Liu et al., 2021b). Although this species was not identified in the current study, other species might have a similar effect. We found that the abundance of F. oxysporum increased year by year with M. sextelata cultivation, i.e., its abundance was 92% higher in M1 than in CK0 and 19% higher in M2 than in M1. The F. oxysporum species complex had been reported to infect more than 120 plant species (Pietro et al., 2003; Michielse and Rep, 2009; Li et al., 2021b). Botrytis cinerea has a wide host range and can cause disease in more than 200 plant species (Williamson et al., 2007; Cheung et al., 2020). The abundance of B. cinerea was 66% higher in M1 than in CK0, and was 40% higher in M2 than in M1; in contrast, there was little change in B. cinerea abundance in the CKs. Clonostachys rosea is considered a pathogen of Cordyceps militaris and naked barley (Li et al., 2018; Liu et al., 2021a); in the current study, its abundance was 36% greater in M1 than in CK0 and was 42% greater in M2 than in CK0. The increase in the abundance of these pathogenic fungi began in the first year, which increased the chances of infection in the next year.
A second pattern of increased abundance of pathogenic fungi was also detected in the current study. In this case, the abundance of pathogenic fungi was similar in M1 and CK0 but was greater in M2 than in M1 or CK0; in other words, these fungi did not increase until the second year of M. sextelata cultivation. Pathogenic fungi that conformed to this pattern included Mucor, Stachybotrys, Aspergillus niger, and others. Mucor is a common pathogen of edible genus such as Agrocybe aegerita and Morchella (Choi et al., 2010; Liu, 2017). Mucor is a common fungus that infects via spores. In our study, the abundance of Mucor was 1,158% higher in M2 than in M1 but did not differ greatly between M1 and the CKs. Stachybotrys usually has a high abundance in the soil where Morchella stipe rot disease occurs (Chen et al., 2018). In our study, the abundance of Stachybotrys was 189% higher in M2 than in M1, while there was little difference in its abundance between CK0 and M1 or between CK1 and CK2. Aspergillus niger is also a common pathogen of edible fungi including Morchella and Cordyceps militaris (Liu, 2017; Liu et al., 2021a). In our study, the abundance of A. niger was 200% higher in M2 than in M1.
In the third pattern, the abundance of pathogenic fungi increased over time whether M. sextelata was present or not. The fungus Cephalotrichum exhibited this pattern and is a potential pathogen of Agaricus bisporus (Fergus, 1978; Dugan et al., 2012). Cephalotrichum may parasitize Morchella mycelium or may inhibit the development of its fruiting bodies (Tan et al., 2021). Cephalotrichum abundance did not significantly differ between CK0 and M1, but it was 78% higher in M2 than in M1. However, its abundance in CK2 also increased by 69% compared with CK1, indicating that Cephalotrichum was also apparently increasing in response to environmental factors that were common to the CKs and the Ms. Therefore, the spores of pathogenic fungi such as Cephalotrichum may spread in the environment, it will increase the risk of infection by Morchella.
The abundance of cellulose-degrading microorganisms was also interesting. The abundance of cellulose-degrading bacteria such as Cellvibrio and Cytohaga was higher in the CKs than in the Ms. At the same time, the abundance of cellulose-degrading fungi such as Chaetomium and Trichoderma was higher in the Ms than in the CKs. Cellulose-degrading bacteria and fungi in soil may have a competitive relationship in different soil (Eichorst and Kuske, 2012). Perhaps the cultivation of M. sextelata altered the soil environment, increasing the competition between cellulose-degrading fungi and bacteria. Bacteria were more dominant than fungi in the CKs, while fungi were more dominant than bacteria in the Ms.
Effects of environmental factors on microbial community structure
RDA analysis in the current study indicated that AN and AP contents in soil were strongly related to the structure of the bacterial community and fungal communities when M. sextelata was continuously cropped. According to Spearman rank correlation analysis, most of the measured environmental factors were significantly related to the diversity and richness of microbial communities. The relationships between environmental factors and pathogenic fungi were mostly negative correlated; the abundances of Penicillium, Stachybotrys, and Trichoderma, for example, were negatively correlated with multiple environmental factors. We suspect that the continuous cropping of M. sextelata decreases the content of AK, AP, AN in soil and reduces soil pH, resulting in an increase in the abundance of fungi that can infect M. sextelata.
Functional predictive analysis of microbial community
Our KEGG analysis indicated that with continuous cropping of M. sextelata in all five sample types soil bacteria were enriched in the functions of amino acid metabolism, membrane transport, and carbohydrate metabolism. For soil bacteria, N cycle functions (e.g., nitrification and denitrification) were enriched significantly more in the Ms than in the CKs, and the enrichment of N cycle functions and sulfate respiration was higher in M2 than in M1. This may be because, as the richness and diversity of the bacterial community increases, so does the total amount of N that the bacterial community requires. Intracellular parasites increased year by year during the continuous cropping of M. sextelata, and M2 had the highest intracellular abundance, which would also increase the probability of M. sextelata infection. The prediction results of soil fungal functions prediction showed that enrichment of pathotrophs was higher in the Ms than in the CKs, and that enrichment of symbiotrophs was higher in M2 than M1. The enrichment of wood saprotrophs was greater in the Ms than in the CKs, and the enrichment of ectomycorrhizal, endophyte, and fungal parasites was greater in M2 than in M1 and the CKs. These increases are likely to increase the chances that M. sextelata will be negatively affected by competitors or pathogens with continuous cropping. PCA analysis showed that the functional structure of microbial communities and especially of fungal communities differed between the CKs and the Ms. This suggested that changes in the fungal community may help explain why M. sextelata production decreases with continuous cropping.
Conclusion
Our results suggest that the decline in M. sextelata production with continuous cropping might result from several factors. Although the yield of Morchella fruiting bodies is generally high in the first year of cultivation, during that year the content of soil available nutrients decreases as does soil pH. The decrease in soil pH is associated with a gradual accumulation of pathogenic fungi such as Penicillium, Trichoderma, and Aspergillus in the second year of cultivation. Also in the second year of cultivation, the reduced fruiting rate of Morchella could lead to a decline in the abundance of Bacillus and other beneficial microorganisms in the soil. The increased abundance of pathogenic fungi and reduced abundance of beneficial microorganisms may explain why M. sextelata production decreases with continuous cultivation. Additional research is needed to determine whether these correlations are causal, i.e., whether increases in pathogenic fungi and decreases in beneficial microorganisms truly cause the decreases in M. sextelata production with continuous cultivation. The effects of allelochemicals on sclerotia production by M. sextelata also warrants further study.
Data availability statement
The datasets presented in this study can be found in online repositories. The name of the repository and accession number can be found below: National Center for Biotechnology Information (NCBI) BioProject, https://www.ncbi.nlm.nih.gov/bioproject/, PRJNA822526.
Author contributions
YX-D and GB: conceptualization. LY, GB, and BX: data curation. YX-D: formal analysis and funding acquisition. LY and GB: methodology and writing—original draft. GB and QJ: resources. LY: picture processing. YX-D and SA: writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (grant no. 31770014) and Science and Technology Plan Project of Liaoning Province (2020-MZLH-33).
Acknowledgments
We thank the staff of College of Biological Science and Technology, Shenyang Agricultural University, for field management. We also thank Prof. Bruce Jaffee for correcting the English.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.903983/full#supplementary-material
Footnotes
References
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., and Lipman, D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215, 403–410. doi: 10.1016/S0022-2836(05)80360-2
Badshah, S. L., Riaz, A., Muhammad, A., Tel Çayan, G., Çayan, F., Emin Duru, M., et al. (2021). Isolation, Characterization, and Medicinal Potential of Polysaccharides of Morchella esculenta. Molecules 26:1459. doi: 10.3390/molecules26051459
Benucci, G., Longley, R., Zhang, P., Zhao, Q., Bonito, G., and Yu, F. (2019). Microbial communities associated with the black morel Morchella sextelata cultivated in greenhouses. PeerJ 7:e7744. doi: 10.7717/peerj.7744
Chen, C., Li, Q., Wang, J., Fu, R., Jin, X., Xiong, C., et al. (2018). Effects of morel (Morchella sp.) stipe rot disease occurrence on soil fungal community structure. J. Microbiol. 38, 39–45.
Cheng, F., Ali, M., Liu, C., Deng, R., and Cheng, Z. (2020). Garlic Allelochemical Diallyl Disulfide Alleviates Autotoxicity in the Root Exudates Caused by Long-Term Continuous Cropping of Tomato. J. Agric. Food Chem. 68, 11684–11693. doi: 10.1021/acs.jafc.0c03894
Cheung, N., Tian, L., Liu, X., and Li, X. (2020). The Destructive Fungal Pathogen Botrytis cinerea-Insights from Genes Studied with Mutant Analysis. Pathogens 9:923. doi: 10.3390/pathogens9110923
Choi, I. Y., Choi, J. N., Sharma, P. K., and Lee, W. H. (2010). Isolation and Identification of Mushroom Pathogens from Agrocybe aegerita. Mycobiology 38, 310–315. doi: 10.4489/MYCO.2010.38.4.310
Coskun, D., Britto, D. T., Shi, W., and Kronzucker, H. J. (2017). How Plant Root Exudates Shape the Nitrogen Cycle. Trends Plant Sci. 22, 661–673. doi: 10.1016/j.tplants.2017.05.004
Dong, L., Xu, J., Zhang, L., Yang, J., Liao, B., Li, X., et al. (2017). High-throughput sequencing technology reveals that continuous cropping of American ginseng results in changes in the microbial community in arable soil. Chin. Med. 12:18. doi: 10.1186/s13020-017-0139-8
Du, X. H., Zhao, Q., Xu, J., and Yang, Z. L. (2016). High inbreeding, limited recombination and divergent evolutionary patterns between two sympatric morel species in China. Sci. Rep. 6:22434. doi: 10.1038/srep22434
Dugan, F. M., Lupien, S. L., and Chen, W. (2012). Clonostachys rhizophaga and other fungi from chickpea debris in the Palouse region of the Pacific Northwest, USA. N. Am. Fungi 7, 1–11. doi: 10.2509/naf2012.007.006
Edgar, R. C. (2013). UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998. doi: 10.1038/nmeth.2604
Eichorst, S. A., and Kuske, C. R. (2012). Identification of cellulose-responsive bacterial and fungal communities in geographically and edaphically different soils by using stable isotope probing. Appl. Environ. Microbiol. 78, 2316–2327. doi: 10.1128/AEM.07313-11
Fergus, C. L. (1978). The fungus flora of compost during mycelium colonization by the cultivated mushroom Agaricus brunnescens. Mycologia 70, 636–644. doi: 10.2307/3759400
Guo, M. P., Chen, K., Wang, G. Z., and Bian, Y. B. (2016). First report of stipe rot disease on Morchella importuna caused by Fusarium incarnatum–F. equiseti species complex in China. Plant Dis. 100, 2530–2530. doi: 10.1094/pdis-05-16-0633-pdn
Haas, B. J., Gevers, D., Earl, A. M., Feldgarden, M., Ward, D. V., Giannoukos, G., et al. (2011). Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 21, 494–504. doi: 10.1101/gr.112730.110
Huang, M. (2006). Study on Soil Microorganism of Agaricus Bisporus Replant Field, Ph D thesis, Sichuan: Sichuan Agricultural University
Huang, W., Sun, D., Fu, J., Zhao, H., Wang, R., and An, Y. (2019). Effects of Continuous Sugar Beet Cropping on Rhizospheric Microbial Communities. Genes 11:13. doi: 10.3390/genes11010013
Huang, W., Sun, D., Wang, R., and An, Y. (2021). Integration of Transcriptomics and Metabolomics Reveals the Responses of Sugar Beet to Continuous Cropping Obstacle. Front. Plant Sci. 12:711333. doi: 10.3389/fpls.2021.711333
Kõljalg, U., Nilsson, R. H., Abarenkov, K., Tedersoo, L., Taylor, A. F., Bahram, M., et al. (2013). Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 22, 5271–5277. doi: 10.1111/mec.12481
Li, C., Chen, G., Zhang, J., Zhu, P., Bai, X., Hou, Y., et al. (2021a). The comprehensive changes in soil properties are continuous cropping obstacles associated with American ginseng (Panax quinquefolius) cultivation. Sci Rep. 11:5068. doi: 10.1038/s41598-021-84436-x
Li, J., Fokkens, L., and Rep, M. (2021b). A single gene in Fusarium oxysporum limits host range. Mol. Plant Pathology 22, 108–116. doi: 10.1111/mpp.13011
Li, X., Li, J., Li, M., Yang, F., Qi, Y., and Guo, C. (2018). “First Report of Root Rot on Naked barley (Hordeum vulgare L. var. nudum Hook. f.) Caused by Clonostachys rosea in Qinghai-tibet plateau, China,” in International Congress of Plant Pathology (ICPP) 2018: Plant Health in A Global Economy. APSNE, (Boston: FAO).
Li, Y., Li, Z., Arafat, Y., and Lin, W. (2020). Studies on fungal communities and functional guilds shift in tea continuous cropping soils by high-throughput sequencing. Ann. Microbiol. 70, 1–12. doi: 10.1186/s13213-020-01555-y
Li, Y., Yuan, Y., Lei, L., Li, F., Zhang, Y., Chen, J., et al. (2017). Carboxymethylation of polysaccharide from Morchella angusticepes Peck enhances its cholesterol-lowering activity in rats. Carbohydr. Polym. 172, 85–92. doi: 10.1016/j.carbpol.2017.05.033
Liu, Q., Wang, F., Xu, F., Xu, Y., and Dong, C. (2021a). Pathogenic fungi of artificially cultivated Cordyceps militaris. Mycosystema 40, 2962–2980.
Liu, T., Zhou, J., Wang, D., He, X., Tang, J., Chen, Y., et al. (2021b). A new stipe rot disease of the cultivated Morchella sextelata. Mycosystema 40, 2229–2243. doi: 10.13346/j.mycosystema.210055
Liu, W. (2017). Biology and Cultivation Techniques of Morchella. Jilin: Jilin Science and Technology Press.
Longley, R., Benucci, G., Mills, G., and Bonito, G. (2019). Fungal and bacterial community dynamics in substrates during the cultivation of morels (Morchella rufobrunnea) indoors. FEMS Microbiol. Lett. 366:fnz215. doi: 10.1093/femsle/fnz215
Meng, X., Che, C., Zhang, J., Gong, Z., Si, M., Yang, G., et al. (2019). Structural characterization and immunomodulating activities of polysaccharides from a newly collected wild Morchella sextelata. Int. J. Biol. Macromol. 129, 608–614. doi: 10.1016/j.ijbiomac.2019.01.226
Michielse, C. B., and Rep, M. (2009). Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathology 10, 311–324. doi: 10.1111/j.1364-3703.2009.00538.x
Mu, X. (2019). Physicochemical characteristics and microbial community of Morchella esculenta Cultivated Soil in Sichuan Province, Ph.D thesis, Sichuan: Sichuan Agricultural University.
Nowak, R., Nowacka-Jechalke, N., Juda, M., and Malm, A. (2018). The preliminary study of prebiotic potential of Polish wild mushroom polysaccharides: The stimulation effect on Lactobacillus strains growth. Eur. J. Nutr. 57, 1511–1521. doi: 10.1007/s00394-017-1436-9
Pang, Z., Dong, F., Liu, Q., Lin, W., Hu, C., and Yuan, Z. (2021). Soil Metagenomics Reveals Effects of Continuous Sugarcane Cropping on the Structure and Functional Pathway of Rhizospheric Microbial Community. Front. Microbiol. 12:627569. doi: 10.3389/fmicb.2021.627569
Pietro, A. D., Madrid, M. P., Caracuel, Z., Delgado Jarana, J., and Roncero, M. I. G. (2003). Fusarium oxysporum: Exploring the molecular arsenal of a vascular wilt fungus. Mol. Plant Pathology 4, 315–325. doi: 10.1046/j.1364-3703.2003.00180.x
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., et al. (2013). The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596. doi: 10.1093/nar/gks1219
Ren, X., He, X., Zhang, Z., Yan, Z., Jin, H., Li, X., et al. (2015). Isolation, Identification, and Autotoxicity Effect of Allelochemicals from Rhizosphere Soils of Flue-Cured Tobacco. J. Agric. Food Chem. 63, 8975–8980. doi: 10.1021/acs.jafc.5b03086
Sarwar, A., Brader, G., Corretto, E., Aleti, G., Ullah, M. A., Sessitsch, A., et al. (2018). Qualitative analysis of biosurfactants from Bacillus species exhibiting antifungal activity. PLoS One 13:e0198107. doi: 10.1371/journal.pone.0198107
Scherm, B., Balmas, V., Spanu, F., Pani, G., Delogu, G., Pasquali, M., et al. (2013). Fusarium culmorum: Causal agent of foot and root rot and head blight on wheat. Mol. Plant Pathology 14, 323–341. doi: 10.1111/mpp.12011
Shen, Z., Penton, C. R., Lv, N., Xue, C., Yuan, X., Ruan, Y., et al. (2018). Banana Fusarium Wilt Disease Incidence Is Influenced by Shifts of Soil Microbial Communities Under Different Monoculture Spans. Microbial. Ecol. 75, 739–750. doi: 10.1007/s00248-017-1052-5
SMa, L. J., Geiser, D. M., Proctor, R. H., Rooney, A. P., O’Donnell, K., Trail, F., et al. (2013). Fusarium pathogenomics. Annu. Rev. Microbiol. 67, 399–416. doi: 10.1146/annurev-micro-092412-155650
Tan, H., Liu, T., Yu, Y., Tang, J., Jiang, L., Martin, F. M., et al. (2021). Morel Production Related to Soil Microbial Diversity and Evenness. Microbiol. Spectr. 9:e0022921. doi: 10.1128/Spectrum.00229-21
Tietel, Z., and Masaphy, S. (2018). True morels (Morchella)-nutritional and phytochemical composition, health benefits and flavor: A review. Crit. Rev. Food Sci. Nutr. 58, 1888–1901. doi: 10.1080/10408398.2017.1285269
Tohno, M., Tanizawa, Y., Kojima, Y., Sakamoto, M., Nakamura, Y., Ohkuma, M., et al. (2019). Lactobacillus salitolerans sp. nov., a novel lactic acid bacterium isolated from spent mushroom substrates. Int. J. Syst. Evol. Microbiol. 69, 964–969. doi: 10.1099/ijsem.0.003224
Velázquez-Cedeño, M., Farnet, A. M., Mata, G., and Savoie, J. M. (2008). Role of Bacillus spp. in antagonism between Pleurotus ostreatus and Trichoderma harzianum in heat-treated wheat-straw substrates. Bioresour. Technol. 99, 6966–6973. doi: 10.1016/j.biortech.2008.01.022
Volk, T. J., and Leonard, T. J. (1989). Physiological and Environmental Studies of Sclerotium Formation and Maturation in Isolates of Morchella crassipes. Appl. Environ. Microbiol. 55, 3095–3100. doi: 10.1128/aem.55.12.3095-3100.1989
Wang, Q., Garrity, G. M., Tiedje, J. M., and Cole, J. R. (2007). Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73, 5261–5267. doi: 10.1128/AEM.00062-07
Williamson, B., Tudzynski, B., Tudzynski, P., and van Kan, J. A. (2007). Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathology 8, 561–580. doi: 10.1111/j.1364-3703.2007.00417.x
Xiang, J. (2007). The Study on Isolation and Identification and Application of Antagonistic Strain Against Penicillium in the Polluted Edible Fungus, Ph.D thesis, Sichuan: Sichuan Agricultural University
Yang, C., Zhou, X., Meng, Q., Wang, M., Zhang, Y., and Fu, S. (2019). Secondary Metabolites and Antiradical Activity of Liquid Fermentation of Morchella sp. Isolated from Southwest China. Molecules 24:1706. doi: 10.3390/molecules24091706
Yu, M., Yin, Q., and He, P. (2020). Isolation and Identification of Pathogen of Morel White Rot. Northern Hortic. 7, 142–145.
Yuan, Y., Huang, H., Ye Li Fu, J., and Wu, X. (2019). Analysis of fungal community in continuous cropping soil of Ganoderma lingzhi. Mycosystema 38, 2112–2121.
Keywords: Morchella, continuous cropping, microbial community, pathogenic fungi, function prediction
Citation: Wei-Ye L, Hong-Bo G, Ke-Xin B, Alekseevna SL, Xiao-Jian Q and Xiao-Dan Y (2022) Determining why continuous cropping reduces the production of the morel Morchella sextelata. Front. Microbiol. 13:903983. doi: 10.3389/fmicb.2022.903983
Received: 25 March 2022; Accepted: 16 August 2022;
Published: 12 September 2022.
Edited by:
Anna Gałązka, Institute of Soil Science and Plant Cultivation, PolandReviewed by:
Pratiksha Singh, Guangxi University for Nationalities, ChinaShuo Jiao, Northwest A&F University, China
Copyright © 2022 Wei-Ye, Hong-Bo, Ke-Xin, Alekseevna, Xiao-Jian and Xiao-Dan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Xiao-Dan, yuxd126@126.com
†These authors have contributed equally to this work
 Liu Wei-Ye
Liu Wei-Ye Guo Hong-Bo2,3†
Guo Hong-Bo2,3† Sibirina Lidiya Alekseevna
Sibirina Lidiya Alekseevna Yu Xiao-Dan
Yu Xiao-Dan