Radiation is energy that moves from one place to another in a form that can be described as waves or particles. We are exposed to radiation in our everyday life. Some of the most familiar sources of radiation include the sun, microwave ovens in our kitchens and the radios we listen to in our cars. Most of this radiation carries no risk to our health. But some does. In general, radiation has lower risk at lower doses but can be associated with higher risks at higher doses. Depending on the type of radiation, different measures must be taken to protect our bodies and the environment from its effects, while allowing us to benefit from its many applications.
What is Radiation?
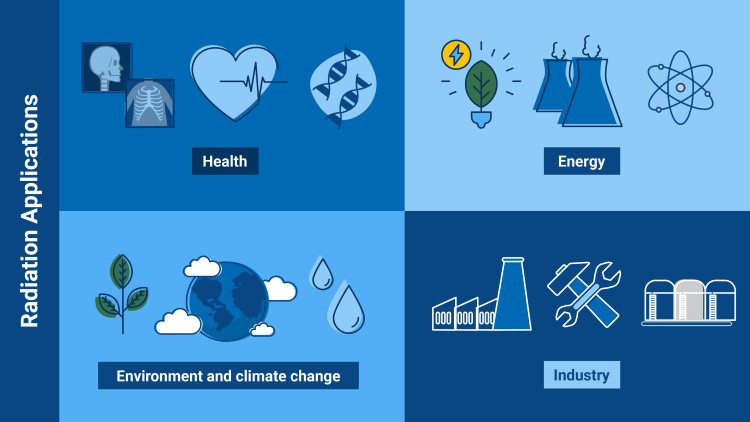
What is radiation good for? – Some examples
- Health: thanks to radiation, we can benefit from medical procedures, such as many cancer treatments, and diagnostic imaging methods.
- Energy: radiation allows us to produce electricity via, for example, solar energy and nuclear energy.
- Environment and climate change: radiation can be used to treat wastewater or to create new plant varieties that are resistant to climate change.
- Industry and science: with nuclear techniques based on radiation, scientists can examine objects from the past or produce materials with superior characteristics in, for instance, the car industry.
If radiation is beneficial, why should we protect ourselves from it?
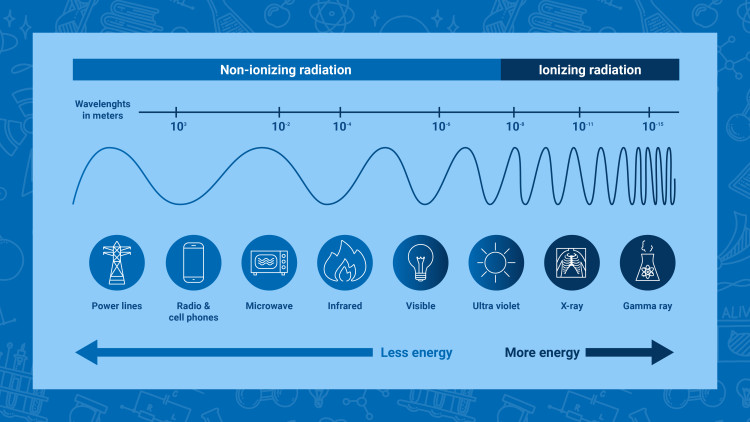
Radiation has many beneficial applications but, as in every activity, when there are risks associated with its use specific actions need to be put in place to protect the people and the environment. Different types of radiation require different protective measures: a low energy form, called “non-ionizing radiation”, may require fewer protective measures than the higher energy “ionizing radiation”. The IAEA establishes standards for protection of the people and the environment in relation to the peaceful use of ionizing radiation – in line with its mandate.
Types of radiation
Non-ionizing radiation

Some examples of non-ionizing radiation are the visible light, the radio waves, and the microwaves (Infographic: Adriana Vargas/IAEA)
Non-ionizing radiation is lower energy radiation that is not energetic enough to detach electrons from atoms or molecules, whether in matter or living organisms. However, its energy can make those molecules vibrate and so produce heat. This is, for instance, how microwave ovens work.
For most people, non-ionizing radiation does not pose a risk to their health. However, workers that are in regular contact with some sources of non-ionizing radiation may need special measures to protect themselves from, for example, the heat produced.
Some other examples of non-ionizing radiation include the radio waves and visible light. The visible light is a type of non-ionizing radiation that the human eye can perceive. And the radio waves are a type of non-ionizing radiation that is invisible to our eyes and other senses, but that can be decoded by traditional radios.
Ionizing radiation

Some examples of ionizing radiation include some types of cancer treatments using gamma rays, the X-rays, and the radiation emitted from radioactive materials used in nuclear power plants (Infographic: Adriana Vargas/IAEA)
Ionizing radiation is a type of radiation of such energy that it can detach electrons from atoms or molecules, which causes changes at the atomic level when interacting with matter including living organisms. Such changes usually involve the production of ions (electrically charged atoms or molecules) – hence the term “ionizing” radiation.
In high doses, ionizing radiation can damage cells or organs in our bodies or even cause death. In the correct uses and doses and with the necessary protective measures, this kind of radiation has many beneficial uses, such as in energy production, in industry, in research and in medical diagnostics and treatment of various diseases, such as cancer. While regulation of use of sources of radiation and radiation protection are national responsibility, the IAEA provides support to lawmakers and regulators through a comprehensive system of international safety standards aiming to protect workers and patients as well as members of the public and the environment from the potential harmful effects of ionizing radiation.
The science behind radioactive decay and the resulting radiation
Ionizing radiation can originate from, for example, unstable (radioactive) atoms as they are transitioning into a more stable state while releasing energy.
Most atoms on Earth are stable, mainly thanks to an equilibrated and stable composition of particles (neutrons and protons) in their centre (or nucleus). However, in some types of unstable atoms, the composition of the number of protons and neutrons in their nucleus does not allow them to hold those particles together. Such unstable atoms are called “radioactive atoms”. When radioactive atoms decay, they release energy in the form of ionizing radiation (for example alpha particles, beta particles, gamma rays or neutrons), which, when safely harnessed and used, can produce various benefits.
What are the most common types of radioactive decay? How can we protect ourselves against the harmful effects of the resulting radiation?
Depending on the type of particles or waves that the nucleus releases to become stable, there are various kinds of radioactive decay leading to ionizing radiation. The most common types are alpha particles, beta particles, gamma rays and neutrons.
Alpha radiation
In alpha radiation, the decaying nuclei release heavy, positively charged particles in order to become more stable. These particles cannot penetrate our skin to cause harm and can often be stopped by using even a single sheet of paper.
However, if alpha-emitting materials are taken into the body by breathing, eating, or drinking, they can expose internal tissues directly and may, therefore, damage health.
Americium-241 is an example of an atom that decays via alpha particles, and it is used in smoke detectors across the world.
Beta radiation
In beta radiation, the nuclei release smaller particles (electrons) that are more penetrating than alpha particles and can pass through e.g., 1-2 centimetres of water, depending on their energy. In general, a sheet of aluminium a few millimetres thick can stop beta radiation.
Some of the unstable atoms that emit beta radiation include hydrogen-3 (tritium) and carbon-14. Tritium is used, among others, in emergency lights to for instance mark exits in the dark. This is because the beta radiation from tritium cause phosphor material to glow when the radiation interacts, without electricity. Carbon-14 is used to, for example, date objects from the past.
Gamma rays
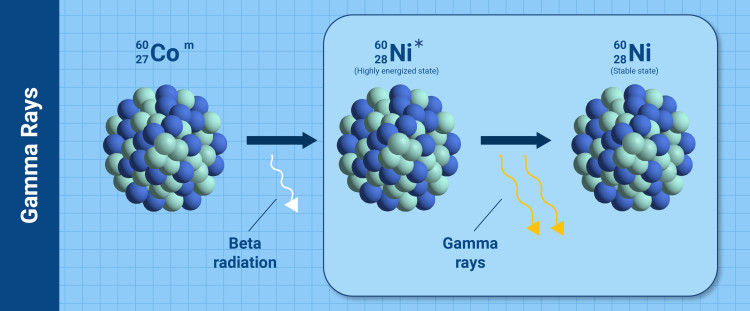
Cobalt-60 is used in cancer treatments because of its ability to produce gamma rays, which can be used to combat tumours. A simplified mechanism is given in the figure above. At first, beta radiation is emitted from the cobalt-60 nucleus, resulting in a transformation to nickel-60 in a highly energized state (*Ni-60). This state quickly loses energy by emitting two gamma rays resulting in stable nickel-60. (Infographic: A. Vargas/IAEA).
Gamma rays, which have various applications, such as cancer treatment, are electromagnetic radiation, similar to X-rays. Some gamma rays pass right through the human body without causing harm, while others are absorbed by the body and may cause damage. The intensity of gamma rays can be reduced to levels that pose less risk by thick walls of concrete or lead. This is why the walls of radiotherapy treatment rooms in hospitals for cancer patients are so thick.
Neutrons

Nuclear fission inside a nuclear reactor is an example of a radioactive chain reaction sustained by neutrons (Graphic: A. Vargas/IAEA).
Neutrons are relatively massive particles that are one of the primary constituents of the nucleus. They are uncharged and therefore do not produce ionization directly. But their interaction with the atoms of matter can give rise to alpha-, beta-, gamma- or X-rays, which then result in ionization. Neutrons are penetrating and can be stopped only by thick masses of concrete, water or paraffin.
Neutrons can be produced in a number of ways, for example in nuclear reactors or in nuclear reactions initiated by high-energy particles in accelerator beams. Neutrons can represent a significant source of indirectly ionizing radiation.
What is the role of the IAEA?
- The IAEA assists its Member States in the use of nuclear technologies, including the use of radiation, for health, agriculture, environmental protection, water management, energy, and industry. To do so, the IAEA assists in the research and development of the practical use of radiation and radioactive sources, and coordinates research activities and implements projects in countries around the world.
- Through its safeguards and verification activities, the IAEA oversees that materials capable of producing radiation are not diverted from peaceful uses.
- Finally, the IAEA develops safety standards and security guidance and report on best practices for the protection of people, society and the environment from the harmful effects of ionizing radiation.
This article was first published on iaea.org on 3 March 2022.