Effect of Microwave Heating on the Acrylamide Formation in Foods
Abstract
:1. Introduction
2. Structure and Properties of Acrylamide
3. Characteristics and Application of Microwave Treatment versus Traditional Heating
3.1. Blanching
3.2. Drying
3.3. Thawing and Tempering
3.4. Pasteurization and Sterilization
3.5. Cooking and Baking
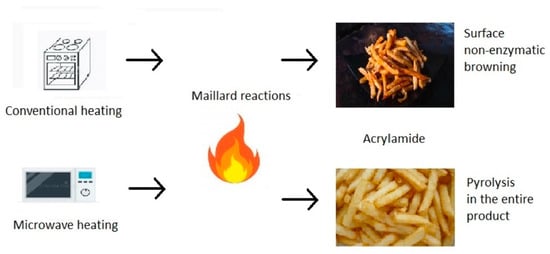
4. Acrylamide in Microwave Heating
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guo, C.; Xie, Y.-J.; Zhu, M.-T.; Xiong, Q.; Chen, Y.; Yu, Q.; Xie, J.-H. Influence of different cooking methods on the nutritional and potentially harmful components of peanuts. Food Chem. 2020, 316, 126269. [Google Scholar] [CrossRef] [PubMed]
- Bent, G.-A.; Maragh, P.; Dasgupta, T.P. Acrylamide in Caribbean foods—Residual levels and their relation to reducing sugar and asparagine content. Food Chem. 2012, 133, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Capuano, E.; Fogliano, V. Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT Food Sci. Technol. 2011, 44, 793–810. [Google Scholar] [CrossRef]
- Claeys, W.L.; De Vleeschouwer, K.; Hendrickx, M. Quantifying the formation of carcinogens during food processing: Acrylamide. Trends Food Sci. Technol. 2005, 16, 181–193. [Google Scholar] [CrossRef]
- Jia, Y.; Khalifa, I.; Hu, L.; Zhu, W.; Li, J.; Li, K.; Li, C. Influence of three different drying techniques on persimmon chips’ characteristics: A comparison study among hot-air, combined hot-air-microwave, and vacuum-freeze drying techniques. Food Bioprod. Process. 2019, 118, 67–76. [Google Scholar] [CrossRef]
- Maan, A.A.; Anjum, M.A.; Khan, M.K.I.; Nazir, A.; Saeed, F.; Afzaal, M.; Aadil, R.M. Acrylamide Formation and Different Mitigation Strategies during Food Processing—A Review. Food Rev. Int. 2020, 1–18. [Google Scholar] [CrossRef]
- Cartus, A.T.; Schrenk, D. Current methods in risk assessment of genotoxic chemicals. Food Chem. Toxicol. 2017, 106, 574–582. [Google Scholar] [CrossRef]
- Devi, S.; Zhang, M.; Ju, R.; Mujumdar, A.S. Co-influence of ultrasound and microwave in vacuum frying on the frying kinetics and nutrient retention properties of mushroom chips. Dry. Technol. 2019, 1–12. [Google Scholar] [CrossRef]
- Jozinović, A.; Šarkanj, B.; Ačkar, D.; Balentić, J.P.; Šubarić, D.; Cvetković, T.; Ranilović, J.; Guberac, S.; Babić, J. Simultaneous Determination of Acrylamide and Hydroxymethylfurfural in Extruded Products by LC-MS/MS Method. Molecules 2019, 24, 1971. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-S.; Han, J.-W.; Jung, M.Y.; Lee, K.-W.; Chung, M.-S. Effects of Thawing and Frying Methods on the Formation of Acrylamide and Polycyclic Aromatic Hydrocarbons in Chicken Meat. Foods 2020, 9, 573. [Google Scholar] [CrossRef]
- Lodolini, E.M.; Cabrera-Bañegil, M.; Fernández, A.; Delgado-Adámez, J.; Ramírez, R.; Martín-Vertedor, M. Monitoring of acrylamide and phenolic compounds in table olive after high hydrostatic pressure and cooking treatments. Food Chem. 2019, 286, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Aalami, M.; Shoeibi, S.; Kashaninejad, M.; Ghorbani, M.; Delavar, M. Effects of different roasting methods on formation of acrylamide in pistachio. Food Sci. Nutr. 2020, 8, 2875–2881. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.-S. Computer Simulation with a Temperature-Step Frying Approach to Mitigate Acrylamide Formation in French Fries. Foods 2020, 9, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koszucka, A.; Nowak, A. Thermal processing food-related toxicants: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3579–3596. [Google Scholar] [CrossRef]
- Michalak, J.; Gujska, E.; Czarnowska-Kujawska, M.; Nowak, F. Effect of different home-cooking methods on acrylamide formation in pre-prepared croquettes. J. Food Compos. Anal. 2017, 56, 134–139. [Google Scholar] [CrossRef]
- Martinez, E.; Rodríguez, J.A.; Mondragon, A.C.; Lorenzo, J.M.; Santos, E.M. Influence of Potato Crisps Processing Parameters on Acrylamide Formation and Bioaccesibility. Molecules 2019, 24, 3827. [Google Scholar] [CrossRef] [Green Version]
- Nooshkam, M.; Varidi, M.; Bashash, M. The Maillard reaction products as food-born antioxidant and antibrowning agents in model and real food systems. Food Chem. 2019, 275, 644–660. [Google Scholar] [CrossRef]
- Shi, J.; Shao, Z.; Li, H.; Zhang, Y.; Wang, S. Co-Extraction and Co-Purification Coupled with HPLC-DAD for Simultaneous Detection of Acrylamide and 5-hydroxymethyl-2-furfural in Thermally Processed Foods. Molecules 2019, 24, 3734. [Google Scholar] [CrossRef] [Green Version]
- Tepe, Y.; Çebi, A.; Aydin, H. Acrylamide content and color formation of hazelnuts roasted at different processing temperatures and times. Eur. Food Res. Technol. 2020, 246, 1543–1549. [Google Scholar] [CrossRef]
- Ekezie, F.-G.C.; Sun, D.-W.; Han, Z.; Cheng, J.-H. Microwave-assisted food processing technologies for enhancing product quality and process efficiency: A review of recent developments. Trends Food Sci. Technol. 2017, 67, 58–69. [Google Scholar] [CrossRef]
- Tekkeli, S.E.K.; Onal, C.; Önal, A. A Review of Current Methods for the Determination of Acrylamide in Food Products. Food Anal. Methods 2011, 5, 29–39. [Google Scholar] [CrossRef]
- Zamani, E.; Shokrzadeh, M.; Fallah, M.; Shaki, F. A review of acrylamide toxicity and its mechanism. Pharm. Biomed. Res. 2017, 3, 1–7. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC Acrylamide. In IARC Monographs on the Evaluation of Carcinogen Risk to Humans IARC: Some Industrial Chemicals; International Agency for Research on Cancer: Lyon, France, 1994; Volume 60, pp. 389–433. [Google Scholar]
- ECB. European Union Risk Assessment Report Acrylamide. In EUR 19835 EN 2002; Office for Official Publications of the European Communities: Luxembourg, 2002; Volume 24, pp. 1–207. [Google Scholar]
- European Chemicals Agency ECHA 2010 (The European Chemicals Agency). Available online: https://echa.europa.eu/documents/10162/13585/pr_10_05_acrylamide_20100330_en.pdf (accessed on 10 February 2019).
- SNFA Swedish National Food Administration. Acrylamide Is Formed during the Preparation of Food and Occurs in Many Foodstuffs. Available online: http://www.slv.se/engdefault.asp (accessed on 10 March 2020).
- WHO. Summary Report of the Sixty-Fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additive (JECFA); The ILSI Press International Life Sciences Institute: Washington, DC, USA, 2005; pp. 1–47. [Google Scholar]
- Dybing, E.; Farmer, P.; Andersen, M.E.; Fennell, T.; Lalljie, S.; Müller, D.; Olin, S.; Petersen, B.; Schlatter, J.; Scholz, G.; et al. Human exposure and internal dose assessments of acrylamide in food. Food Chem. Toxicol. 2005, 43, 365–410. [Google Scholar] [CrossRef] [PubMed]
- Fohgelberg, P.; Rosén, J.; Hellenäs, K.-E.; Abramsson-Zetterberg, L. The acrylamide intake via some common baby food for children in Sweden during their first year of life—an improved method for analysis of acrylamide. Food Chem. Toxicol. 2005, 43, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Keramat, J.; LeBail, A.; Prost, C.; Jafari, M. Acrylamide in Baking Products: A Review Article. Food Bioprocess Technol. 2010, 4, 530–543. [Google Scholar] [CrossRef]
- Krishnakumar, T.; Visvanathan, R. Acrylamide in Food Products: A Review. J. Food Process. Technol. 2014, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Michalak, J.; Gujska, E.; Czarnowska, M.; Klepacka, J.; Nowak, F. Effect of Storage on Acrylamide and 5-hydroxymethylfurfural Contents in Selected Processed Plant Products with Long Shelf-life. Plant Foods Hum. Nutr. 2016, 71, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of Acrylamide, a Carcinogen Formed in Heated Foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef]
- Tateo, F.; Bononi, M. Preliminary study on acrylamide in baby foods on the Italian market. Ital. J. Food Sci. 2003, 15, 593–599. [Google Scholar]
- Yuan, Y.; Chen, F.; Zhao, G.-H.; Liu, J.; Zhang, H.-X.; Hu, X.-S. A Comparative Study of Acrylamide Formation Induced by Microwave and Conventional Heating Methods. J. Food Sci. 2007, 72, C212–C216. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.; Zhang, Y. Occurrence and analytical methods of acrylamide in heat-treated foods. J. Chromatogr. A 2005, 1075, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Stadler, R.H.; Studer, A. Chapter 1—Acrylamide Formation Mechanisms. In Acrylamide in Food, Analysis, Content and Potential Health Effects 2016; Academic Press Academic Press: Waltham, MA, USA, 2016; pp. 1–17. [Google Scholar]
- Miśkiewicz, K.; Rosicka-Kaczmarek, J.; Nebesny, E. Effects of Chickpea Protein on Carbohydrate Reactivity in Acrylamide Formation in Low Humidity Model Systems. Foods 2020, 9, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedegaard, R.V.; Frandsen, H.; Skibsted, L.H. Kinetics of formation of acrylamide and Schiff base intermediates from asparagine and glucose. Food Chem. 2008, 108, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Li, X.; Hu, X.; Ma, Z.; Liu, L.; Ma, X. Effect of buckwheat extracts on acrylamide formation and the quality of bread. J. Sci. Food Agric. 2019, 99, 6482–6489. [Google Scholar] [CrossRef] [PubMed]
- Torres-Gonzalez, J.D.; Dueik, V.; Carré, D.; Bouchon, P. Effect of the Addition of Soluble Dietary Fiber and Green Tea Polyphenols on Acrylamide Formation and In Vitro Starch Digestibility in Baked Starchy Matrices. Molecules 2019, 24, 3674. [Google Scholar] [CrossRef] [Green Version]
- Salazar, R.; Arámbula-Villa, G.; Luna-Bárcenas, G.; Figueroa-Cárdenas, J.; Azuara, E.; Vazquez-Landaverde, P. Effect of added calcium hydroxide during corn nixtamalization on acrylamide content in tortilla chips. LWT Food Sci. Technol. 2014, 56, 87–92. [Google Scholar] [CrossRef]
- Muttucumaru, N.; Elmore, J.S.; Curtis, T.; Mottram, N.S.; Parry, M.A.J.; Halford, N.G. Reducing Acrylamide Precursors in Raw Materials Derived from Wheat and Potato. J. Agric. Food Chem. 2008, 56, 6167–6172. [Google Scholar] [CrossRef]
- Nerín, C.; Aznar, M.; Carrizo, D. Food contamination during food process. Trends Food Sci. Technol. 2016, 48, 63–68. [Google Scholar] [CrossRef]
- Stadler, R.H.; Blank, I.; Varga, N.; Robert, F.; Hau, J.; Guy, P.A.; Robert, M.-C.; Riediker, S. Acrylamide from Maillard reaction products. Nature 2002, 419, 449–450. [Google Scholar] [CrossRef]
- Michalak, J.; Czarnowska-Kujawska, M.; Gujska, E. Acrylamide and Thermal-Processing Indexes in Market-Purchased Food. Int. J. Environ. Res. Public Health 2019, 16, 4724. [Google Scholar] [CrossRef] [Green Version]
- Michalak, J.; Gujska, E.; Kuncewicz, A. RP-HPLC-DAD studies on acrylamide in cereal-based baby foods. J. Food Compos. Anal. 2013, 32, 68–73. [Google Scholar] [CrossRef]
- EFSA. Results on Acrylamide Levels in Food from Monitoring Years 2007–2009 and Exposure Assessment. EFSA J. 2011, 9, 2133. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Scientific Opinion on Acrylamide in Food. EFSA Panel on Contaminants in the Food Chain (CONTAM). EFSA J. 2015, 13, 1–321. [Google Scholar] [CrossRef] [Green Version]
- JECFA 2005. Joint FAO/WHO Expert Committee on Food Additives. Available online: http://www.who.int/ipcs/food/jecfa/summaries/summary_report_64_final.pdf (accessed on 11 January 2020).
- EU European Commission Recommendation of 3 May 2007 on the Monitoring of Acrylamide Levels in Food (2007/331/EC), L 123/33, 12.5.2007. 2007. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32007H0331 (accessed on 11 January 2020).
- EU European Commission Recommendation of 10 January 2011 on Investigations into the Levels of Acrylamide in Food (2010/307/EU). Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/cs_contaminants_catalogue_acrylamide_recommendation_10012011_food_en.pdf (accessed on 10 January 2020).
- EU European Commission Recommendation of 8 November 2013 on Investigations into the Levels of Acrylamide in Food (2013/647/EU). Available online: https://www.fsai.ie/uploadedFiles/Recomm_2013_647.pdf (accessed on 10 January 2020).
- EU European Commission Regulation of 20 November 2017 Establishing Mitigation Measures and Benchmark Levels for the Reduction of the Presence of Acrylamide in Food (2017/2158). Available online: https://eur-lex.europa.eu/eli/reg/2017/2158/oj (accessed on 12 January 2020).
- EU European Commission Recommendation of 7 November 2019 on the Monitoring of the Presence of Acrylamide in Certain Foods (2019/1888/EU). Commission Recommendation. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32019H1888 (accessed on 2 July 2020).
- EFSA European Food Safety Authority. Update on acrylamide levels in food from monitoring years 2007 to 2010. EFSA J. 2012, 10, 2938. [Google Scholar] [CrossRef]
- Mojska, H. Secular Trends in Food Acrylamide. In Acrylamide in Food: Analysis, Content and Potential Health Effects; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 39–59. [Google Scholar]
- FDE. Food Drink Europe Acrylamide Toolbox. 2011. Available online: http://www.fooddrinkeurope.eu/uploads/publications_documents/Toolboxfinal260911.pdf (accessed on 22 April 2019).
- FDE. Food Drink Europe Acrylamide Toolbox. 2013. Available online: http://www.fooddrinkeurope.eu/uploads/publications_documents/AcrylamideToolbox_2013.pdf (accessed on 22 April 2019).
- Sumnu, G.; Sahin, S. Recent Developments in Microwave Heating. In Emerging Technologies for Food Processing; Academic Press: Cambridge, MA, USA, 2005; pp. 419–444. [Google Scholar]
- Datta, A.K. Handbook of Microwave Technology for Food Application; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Guo, Q.; Sun, D.-W.; Cheng, J.-H.; Han, Z. Microwave processing techniques and their recent applications in the food industry. Trends Food Sci. Technol. 2017, 67, 236–247. [Google Scholar] [CrossRef]
- Kalla, A.M.; Devaraju, R. Microwave energy and its application in food industry: A reveiw. Asian J. Dairy Food Res. 2017, 36, 37–44. [Google Scholar] [CrossRef]
- Chavan, R.; Chavan, S. Microwave Baking in Food Industry: A Review. Int. J. Dairy Sci. 2010, 5, 113–127. [Google Scholar] [CrossRef] [Green Version]
- Datta, A.K.; Davidson, P.M. Microwave and Radio Frequency Processing. J. Food Sci. 2000, 65, 32–41. [Google Scholar] [CrossRef]
- Fernandez, Y.; Arenillas, A.; Menendez, J.A. Microwave heating applied to pyrolysis. In Advances in Induction and Microwave Heating of Mineral and Organic Materials; Grundas, S., Ed.; InTech: Rijeka, Croatia, 2011; pp. 723–752. Available online: https://core.ac.uk/reader/36042930 (accessed on 2 July 2020).
- Shaheen, M.S.; El-Massry, K.F.; El-Ghorab, A.H.; Anjum, F.M. Microwave Applications in Thermal Food Processing. In The Development and Application of Microwave Heating; IntechOpen: London, UK, 2012; pp. 3–16. [Google Scholar]
- Xiao, H.-W.; Pan, Z.; Deng, L.-Z.; El-Mashad, H.; Yang, X.-H.; Mujumdar, A.S.; Gao, Z.-J.; Zhang, Q. Recent developments and trends in thermal blanching—A comprehensive review. Inf. Process. Agric. 2017, 4, 101–127. [Google Scholar] [CrossRef]
- Khan, I.; Tango, C.N.; Miskeen, S.; Lee, B.H.; Oh, D.-H. Hurdle technology: A novel approach for enhanced food quality and safety—A review. Food Control. 2017, 73, 1426–1444. [Google Scholar] [CrossRef]
- Archer, M.; Edmonds, M.; George, M. Seafood thawing. Seafish Res. Dev. 2008, SR598, 10–11. [Google Scholar]
- Ahmed, J.; Ramaswamy, H.S. Microwave pasteurization and sterilization of foods. In Handbook of Food Preservation, 2nd ed.; Rahman, M.S., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 691–711. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Choi, W.; Lee, S.H.; Jun, S. Exploring the heating patterns of multiphase foods in a continuous flow, simultaneous microwave and ohmic combination heater. J. Food Eng. 2013, 116, 65–71. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, D.-W.; Han, Z. Applications of electromagnetic fields for nonthermal inactivation of microorganisms in foods: An overview. Trends Food Sci. Technol. 2017, 64, 13–22. [Google Scholar] [CrossRef]
- Reverte-Ors, J.D.; Pedreno-Molina, J.; Fernandez, P.S.; Lozano-Guerrero, A.J.; Periago, P.M.; Díaz-Morcillo, A. A Novel Technique for Sterilization Using a Power Self-Regulated Single-Mode Microwave Cavity. Sensors 2017, 17, 1309. [Google Scholar] [CrossRef] [PubMed]
- Vadivambal, R.; Jayas, D.S. Non-uniform Temperature Distribution During Microwave Heating of Food Materials—A Review. Food Bioprocess Technol. 2010, 3, 161–171. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Kinetics of Microbial Inactivation for Alternative Food Processing Technologies—Microwave and Radio Frequency Processing. Available online: http://www.fda.gov/Food/ScienceResearch/ResearchAreas/SafePracticesforFoodProcesses/ucm100250.htm (accessed on 18 April 2019).
- Salazar-Gonzalez, C.; San Martin-Gonzalez, M.F.; Lopez-Malo, A.; Sosa-Morales, M.E. Recent studies related to microwave processing of fluid foods. Food Bioprocess Technol. 2012, 5, 31–46. [Google Scholar] [CrossRef]
- Al-Ansi, W.; Mahdi, A.A.; Al-Maqtari, Q.A.; Fan, M.; Wang, L.; Li, Y.; Qian, H.; Zhang, H. Evaluating the role of microwave-baking and fennel (Foeniculum vulgare L.)/nigella (Nigella sativa L.) on acrylamide growth and antioxidants potential in biscuits. J. Food Meas. Charact. 2019, 13, 2426–2437. [Google Scholar] [CrossRef]
- Sumnu, G.; Datta, A.K.; Sahin, S.; Keskin, S.O.; Rakesh, V. Transport and related properties of breads baked using various heating modes. J. Food Eng. 2007, 78, 1382–1387. [Google Scholar] [CrossRef]
- Gökmen, V.; Palazoglu, T.K.; Şenyuva, H.Z. Relation between the acrylamide formation and time-temperature history of surface and core regions of French fries. J. Food Eng. 2006, 77, 972–976. [Google Scholar] [CrossRef]
- Gökmen, V.; Palazoglu, T.K. Acrylamide Formation in Foods during Thermal Processing with a Focus on Frying. Food Bioprocess Technol. 2007, 1, 35–42. [Google Scholar] [CrossRef]
- Hamid, N.A.; Omar, S.; Sanny, M. Effect of thawing conditions and corresponding frying temperature profiles on the formation of acrylamide in French fries. J. Saudi Soc. Agric. Sci. 2019, 18, 396–400. [Google Scholar] [CrossRef]
- Matthäus, B.; Haase, N.U.; Vosmann, K. Factors affecting the concentration of acrylamide during deep-fat frying of potatoes. Eur. J. Lipid Sci. Technol. 2004, 106, 793–801. [Google Scholar] [CrossRef]
- Michalak, J.; Gujska, E.; Klepacka, J. The effect of domestic preparation of some potato products on acrylamide content. Plant Food Hum. Nutr. 2011, 66, 307–312. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Fang, H.; Zhang, Y. Study on formation of acrylamide in asparagine–sugar microwave heating systems using UPLC-MS/MS analytical method. Food Chem. 2008, 108, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Takatsuki, S.; Nemoto, S.; Sasaki, K.; Maitani, T. Production of acrylamide in agricultural products by cooking. J. Food Hyg. Soc. Jpn. 2004, 45, 44–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, H.; Miao, Y.; Zhao, C.; Yuan, Y. Acrylamide and methylglyoxal formation in potato chips by microwaving and frying heating. Int. J. Food Sci. Technol. 2011, 46, 1921–1926. [Google Scholar] [CrossRef]
- Zyzak, D.V.; Sanders, R.A.; Stojanovic, M.; Tallmadge, D.H.; Eberhart, B.L.; Ewald, D.K.; Gruber, D.C.; Morsch, T.R.; Strothers, M.A.; Rizzi, G.P.; et al. Acrylamide Formation Mechanism in Heated Foods. J. Agric. Food Chem. 2003, 51, 4782–4787. [Google Scholar] [CrossRef]
- Juodeikiene, G.; Zadeike, D.; Vidziunaite, I.; Bartkiene, E.; Bartkevics, V.; Pugajeva, I. Effect of heating method on the microbial levels and acrylamide in corn grits and subsequent use as functional ingredient for bread making. Food Bioprod. Process. 2018, 112, 22–30. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Luo, H.-M.; Hsu, P.-H.; Sung, W.C. Effects of calcium supplements on the quality and acrylamide content of puffed shrimp chips. J. Food Drug Anal. 2016, 24, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Anese, M.; Manzocco, L.; Calligaris, S.; Nicoli, M.C. Industrially Applicable Strategies for Mitigating Acrylamide, Furan, and 5-Hydroxymethylfurfural in Food. J. Agric. Food Chem. 2013, 61, 10209–10214. [Google Scholar] [CrossRef]
- Barutcu, I.; Sahin, S.; Sumnu, G. Acrylamide formation in different batter formulations during microwave frying. LWT Food Sci. Technol. 2009, 42, 17–22. [Google Scholar] [CrossRef]
- Leatherhead International Limited; Burch, R. Examination of the Effect of Domestic Cooking on Acrylamide Levels in Food; Food Standards Agency: London, UK, 2007; Available online: http://www.foodbase.org.uk/results.php?f_report_id=46 (accessed on 10 December 2019).
- Sansano, M.; Reyes, R.D.L.; Andrés, A.; Heredia, A. Effect of Microwave Frying on Acrylamide Generation, Mass Transfer, Color, and Texture in French Fries. Food Bioprocess Technol. 2018, 11, 1934–1939. [Google Scholar] [CrossRef]
- Erdoğdu, S.B.; Palazoglu, T.K.; Gökmen, V.; Şenyuva, H.Z.; Ekiz, I. Reduction of acrylamide formation in French fries by microwave pre-cooking of potato strips. J. Sci. Food Agric. 2006, 87, 133–137. [Google Scholar] [CrossRef]
- Akkarachaneeyakorn, S.; Laguerre, J.; Tattiyakul, J.; Neugnot, B.; Boivin, P.; Morales, F.J.; Birlouez-Aragon, I. Optimization of Combined Microwave—Hot Air Roasting of Malt Based on Energy Consumption and Neo-Formed Contaminants Content. J. Food Sci. 2010, 75, E201–E207. [Google Scholar] [CrossRef] [PubMed]
- Elfaitouri, T.A.; Ghazali, H.M.; Sumnu, G.; Ariffin, A.A.; Tan, C.P. Effect of Microwave Frying on Acrylamide Formation in Potato Chips. World J. Food Sci. Technol. 2018, 2, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Ahrné, L.; Andersson, C.-G.; Floberg, P.; Rosén, J.; Lingnert, H. Effect of crust temperature and water content on acrylamide formation during baking of white bread: Steam and falling temperature baking. LWT Food Sci. Technol. 2007, 40, 1708–1715. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, M.; Wang, Q.; Cheng, J. Structure-guided unravelling: Phenolic hydroxyls contribute to reduction of acrylamide using multiplex quantitative structure–activity relationship modelling. Food Chem. 2016, 199, 492–501. [Google Scholar] [CrossRef]
- Soncu, E.D.; Kolsarici, N. Microwave thawing and green tea extract efficiency for the formation of acrylamide throughout the production process of chicken burgers and chicken nuggets. J. Sci. Food Agric. 2017, 97, 1790–1797. [Google Scholar] [CrossRef]
- Rydberg, P.; Eriksson, S.; Tareke, E.; Karlsson, P.; Ehrenberg, L.; Törnqvist, M. Factors That Influence the Acrylamide Content of Heated Foods. In Chemistry and Safety of Acrylamide in Food; Springer: Boston, MA, USA, 2005; pp. 317–328. [Google Scholar] [CrossRef]
- Friedman, M.; Mottram, D. Chemistry and Safety of Acrylamide in Food; Springer Press: New York, NY, USA, 2005. [Google Scholar]
- Janković, S.M.; Milosev, M.; Novakovic, M. The effects of microwave radiation on microbial cultures. Hosp. Pharmacol. Int. Multidiscip. J. 2014, 1, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Maldonado, A.F.; Lee, A.; Farber, J. Methods for the Control of Foodborne Pathogens in Low-Moisture Foods. Annu. Rev. Food Sci. Technol. 2018, 9, 177–208. [Google Scholar] [CrossRef]
- Lingnert, H.; Grivas, S.; Jägerstad, M.; Skog, K.; Törnqvist, M.; Aman, P. Acrylamide in food: Mechanisms of formation and influencing factors during heating of foods. Scand. J. Nutr. 2002, 46, 159–172. [Google Scholar] [CrossRef]
- Matthäus, B.; Haase, N.U. Acrylamide—Still a matter of concern for fried potato food? Eur. J. Lipid. Sci. Technol. 2014, 116, 675–687. [Google Scholar] [CrossRef]
- Romani, S.; Bacchiocca, M.; Rocculi, P.; Rosa, M.D. Influence of frying conditions on acrylamide content and other quality characteristics of French fries. J. Food Compos. Anal. 2009, 22, 582–588. [Google Scholar] [CrossRef]
- Gökmen, V.; Açar, Özge, Ç.; Arribas-Lorenzo, G.; Morales, F.J. Investigating the correlation between acrylamide content and browning ratio of model cookies. J. Food Eng. 2008, 87, 380–385. [Google Scholar] [CrossRef]
- Gökmen, V.; Mogol, B.A. Computer vision-based image analysis for rapid detection of acrylamide in heated foods. Qual. Assur. Saf. Crop. Foods 2010, 2, 203–207. [Google Scholar] [CrossRef]
- Mustafa, A.; Andersson, R.; Rosén, J.; Kamal-Eldin, A.; Aman, P. Factors Influencing Acrylamide Content and Color in Rye Crisp Bread. J. Agric. Food Chem. 2005, 53, 5985–5989. [Google Scholar] [CrossRef]
- Pedreschi, F.; Kaack, K.; Granby, K. Acrylamide content and color development in fried potato strips. Food Res. Int. 2006, 39, 40–46. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V. Evaluation of the Maillard reaction in potato crisps by acrylamide, antioxidant capacity and color. J. Food Compos. Anal. 2009, 22, 589–595. [Google Scholar] [CrossRef]
- Surdyk, N.; Rosén, J.; Andersson, R.; Aman, P. Effects of Asparagine, Fructose, and Baking Conditions on Acrylamide Content in Yeast-Leavened Wheat Bread. J. Agric. Food Chem. 2004, 52, 2047–2051. [Google Scholar] [CrossRef]
- Capuano, E.; Ferrigno, A.; Acampa, I.; Serpen, A.; Açar, Özge, Ç.; Gökmen, V.; Fogliano, V. Effect of flour type on Maillard reaction and acrylamide formation during toasting of bread crisp model systems and mitigation strategies. Food Res. Int. 2009, 42, 1295–1302. [Google Scholar] [CrossRef]
- Mestdagh, F.; De Wilde, T.; Castelein, P.; Nemeth, O.; Van Peteghem, C.; De Meulenaer, B. Impact of the reducing sugars on the relationship between acrylamide and Maillard browning in French fries. Eur. Food Res. Technol. 2008, 227, 69–76. [Google Scholar] [CrossRef]

| Food Product | µg/kg | References |
|---|---|---|
| Baby foods | ||
| Cereal-based (ready-to-eat) | 13 | [47] |
| Instant cereal based | 345 | [47] |
| Candy-bars | 54 | [47] |
| Biscuits | 87 | [32] |
| Jarred baby food | 32–47 | [48] |
| Ready-to-eat meal cereal-based | 13 | [49] |
| Porridge | 29 | [49] |
| Infant formulae | 14 | [49] |
| Fruit purée | 22 | [49] |
| Juice | 12 | [49] |
| Bread | ||
| Crisp bread | 443 | [32] |
| Wheat soft bread | 38 | [49] |
| Other soft bread | 57 | [49] |
| Cereal products | ||
| Wheat- and rye-based products | 170 | [49] |
| Bran products and whole grain cereals | 211 | [49] |
| Crackers | 231 | [49] |
| Biscuits and wafers | 201 | [49] |
| Gingerbread | 407 | [49] |
| Pasta | 13 | [49] |
| Beer | 14 | [49] |
| Cacao | ||
| Cacao (100% cocoa powder) | 347 | [32] |
| Cacao (cocoa-containing beverages powder: sugars and 20% cocoa powder) | 248 | |
| Coffee and coffee substitutes (dry) | ||
| Roasted coffee (dry) | 249 | [49] |
| Instant coffee (dry) | 710 | [49] |
| Substitute coffee (dry), based on cereals | 510 | [49] |
| Substitute coffee (dry), based on chicory | 2942 | [49] |
| Potato products | ||
| French fries | 326–328 | [48] |
| Potato crisps | 689–693 | [48] |
| Deep fried home-cooked potato products | 234–241 | [48] |
| Oven baked home-cooked potato products | 317 | [48] |
| Other products | ||
| Roasted nuts and seeds | 93 | [49] |
| Black olives in brine | 454 | [49] |
| Prunes and dates | 89 | [49] |
| Paprika powder | 379 | [49] |
| Fish and sea food | 25 | [50] |
| Milk and milk products | 6 | [50] |
| Pizza | 33 | [50] |
| Green tea roasted | 306 | [50] |
| Sugars and honey | 24 | [50] |
| Vegetables | 17 | [50] |
| Vegetable crisps | 1846 | [49] |
| Fruits dried and processed | 131 | [50] |
| Dried food | 121 | [50] |
| Food Product | Preparation Method | Acrylamide (µg/kg) | References | |
|---|---|---|---|---|
| French fries | Before final preparation | 416 | [84] | |
| Pan frying 180 °C/3 min | 561 | |||
| Deep frying 180 °C/3 min | 597 | |||
| Roasting 220 °C/10 min | 727 | |||
| Microwaving 220 °C (700 W)/10 min | 790 | |||
| Deep frying 180 °C/from 1 to 8 min | 21–231 | [94] | ||
| Microwave frying 315 W/from 1 to 10 min | 46–182 | |||
| Microwave frying 430 W/from 1 to 8 min | 44–337 | |||
| Microwave frying 600 W/from 1 to 6 min | 23–172 | |||
| Unthawed and deep frying at 180 °C/3.5 min | 85 | [82] | ||
| Thawing at room temp. and deep frying at 180 °C/3.5 min | 84 | |||
| Thawing in a chiller (5 °C overnight) and deep frying at 180 °C/3.5 min | 77 | |||
| Thawing in a microwave oven (30% power/5 min) and deep frying at 180 °C/3.5 min | 106 | |||
| Potato pancakes | Before final preparation | 286 | [84] | |
| Pan frying 180 °C/3 min | 437 | |||
| Deep frying 180 °C/3 min | 422 | |||
| Roasting 220 °C/10 min | 564 | |||
| Microwaving 220 °C (700 W)/10 min | 694 | |||
| Potato chips | Frying 180 °C/4 min | 645 | [35] | |
| Microwaving 750 W/2.5 min | 897 | |||
| Frying 160 °C/7 min | 3110 | [87] | ||
| Frying 180 °C/6 min | 3604 | |||
| Microwaving 750 W/3 min | 5184 | |||
| Microwave frying at 160 °C (200 W)/30–150 s | 542–895 | [97] | ||
| Microwave frying at 170 °C (400 W)/30–150 s | 669–1739 | |||
| Microwave frying at 180 °C (800 W)/30–150 s | 1139–11,423 | |||
| Grated potatoes | Frying | 447 | [33] | |
| Microwaving | 551 | |||
| Potato | Baking 220 °C/5 min | approx.70 | [86] | |
| Microwave precooking 150 W/60 s and baking (220 °C/5 min) | approx. 180 | |||
| Asparagus | Baking 220 °C/5 min | approx. 90 | ||
| Microwave precooking 150 W/60 s and baking 220 °C/5 min | approx. 160 | |||
| Green gram sprouts | Baking 220 °C/5 min | approx. 340 | ||
| Microwave precooking 150 W/60 s and baking 220 °C/5 min | approx. 580 | |||
| Pistachios | Raw | 57 | [12] | |
| Sun-dried | 93 | |||
| Hot air roasting from 100 °C/5min to 150 °C/5min | salted | 130–463 | ||
| unsalted | 204–594 | |||
| IR (infrared) roasting from 75 V/10 min to 95 V/30 min | salted | 242–697 | ||
| unsalted | 318–851 | |||
| Microwaving roasting form 180 W/12 min to 360 W/16 min | salted | 105–307 | ||
| unsalted | 119–344 | |||
| Pre-cooked flour-based croquettes | Before final preparation | 190 | [15] | |
| Pan frying 180 °C/5 min | 285 | |||
| Deep frying 180 °C/5min | 298 | |||
| Roasting 200 °C/10 min | 360 | |||
| Microwaving 200 °C (700 W)/10 min | 420 | |||
| Fried chicken with batter formulation of chickpea flour | Deep frying (180 °C/5 min) | 110 | [92] | |
| Microwave frying (180 °C/350 W/2 min) | 79 | |||
| Fried chicken with batter formulation of rice flour | Deep frying (180 °C/5 min) | 111 | ||
| Microwave frying (180 °C/350 W/2 min) | 73 | |||
| Fried chicken with batter formulation of soy flour | Deep frying (180 °C/5 min) | 100 | ||
| Microwave frying (180 °C/350 W/2 min) | 76 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalak, J.; Czarnowska-Kujawska, M.; Klepacka, J.; Gujska, E. Effect of Microwave Heating on the Acrylamide Formation in Foods. Molecules 2020, 25, 4140. https://doi.org/10.3390/molecules25184140
Michalak J, Czarnowska-Kujawska M, Klepacka J, Gujska E. Effect of Microwave Heating on the Acrylamide Formation in Foods. Molecules. 2020; 25(18):4140. https://doi.org/10.3390/molecules25184140
Chicago/Turabian StyleMichalak, Joanna, Marta Czarnowska-Kujawska, Joanna Klepacka, and Elżbieta Gujska. 2020. "Effect of Microwave Heating on the Acrylamide Formation in Foods" Molecules 25, no. 18: 4140. https://doi.org/10.3390/molecules25184140