Fucosylated Chondroitin Sulfates with Rare Disaccharide Branches from the Sea Cucumbers Psolus peronii and Holothuria nobilis: Structures and Influence on Hematopoiesis
Abstract
:1. Introduction
2. Results and Discussion
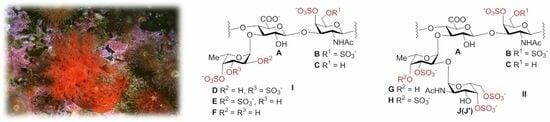
2.1. Preparation and Structural Assessment of FCS
2.2. Studies of the Ability of FCS to Influence Hematopoiesis
3. Materials and Methods
3.1. General Methods
3.2. Isolation of Sulfated Polysaccharides
3.3. Polyacrylamide Gel Electrophoresis (PAGE) and Gel-Permeation Chromatography
3.4. Cell Model
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiew, P.L.; Don, M.M. Jewel of the sea bed: Sea cucumbers as nutritional and drug candidates. Int. J. Food Sci. Nutr. 2012, 63, 616–636. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. Holothurian fucosylated chondroitin sulfate. Mar. Drugs 2014, 12, 232–254. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhou, Q.; Liu, B.; Chen, F.; Wang, M. Holothurian fucosylated chondroitin sulfates and their potential benefits for human health: Structures and biological activities. Carbohydr. Polym. 2022, 275, 118691. [Google Scholar] [CrossRef] [PubMed]
- Mourão, P.A.S. Perspective on the use of sulfated polysaccharides from marine organisms as a source of new antithrombotic drugs. Mar. Drugs. 2015, 13, 2770–2784. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, M.; Xu, B.; Li, Z.; Qi, Y.; Song, Z.; Zhao, Q.; Du, B.; Yang, Y. The current status and future perspective in combination of the processing technologies of sulfated polysaccharides from sea cucumbers: A comprehensive review. J. Funct. Foods 2021, 87, 104744. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Q.; Lv, K.; Ma, H.; Gao, C.; Liu, Y.; Zhang, S.; Zhao, L. Low-molecular-weight fucosylated glycosaminoglycan and its oligosaccharides from sea cucumber as novel anticoagulants: A review. Carbohydr. Polym. 2021, 251, 117034. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Wu, L.; Yang, H.; Wey, C.; Ding, C.; Linhardt, R.J.; Zheng, X.; Ye, X.; Chen, S. Ultrasound-assisted fast preparation of low molecular weight fucosylated chondroitin sulfate with antitumor activity. Carbohydr Polym. 2019, 209, 82–91. [Google Scholar] [CrossRef]
- Vasconcelos, A.A.; Pomin, V.H. Marine carbohydrate-based compounds with medical properties. Mar. Drugs 2018, 16, 233. [Google Scholar] [CrossRef]
- Pomin, V.H.; Vignovich, W.P.; Gonzales, A.V.; Vasconcelos, A.A.; Mulloy, B. Galactosaminoglycans: Medical applications and drawbacks. Molecules 2019, 24, 2803. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Sulfated polysaccharides in sea cucumbers and their biological properties: A review. Int. J. Biol. Chem. 2023, 253, 127329. [Google Scholar] [CrossRef]
- Yan, L.; Li, L.; Li, J.; Yu, Y.; Liu, X.; Ye, X.; Linhardt, R.J.; Chen, S. Bottom-up analysis using chromatography-Fourier transform mass spectrometry to characterize fucosylated chondroitin sulfates from sea cucumbers. Glycobiology 2019, 29, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Shashkov, A.S.; Kusaykin, M.I.; Stonik, V.A.; Nifantiev, N.E.; Usov, A.I. Structural characterization and anticoagulant properties of sulfated polysaccharides of the sea cucumber Cucumaria japonica. Glycobiology 2016, 26, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Mourao, P.A.S.; Vilanova, E.; Soares, P.A.G. Unveiling the structure of sulfated fucose-rich polysaccharides via nuclear magnetic resonance spectroscopy. Curr. Opinion Struct. Biol. 2018, 50, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.; Gao, N.; Yin, R.; Lin, L.; Xiao, C.; Zhou, L.; Li, Z.; Purcell, S.W.; Wu, M.; Zhao, J. Precise structures of fucosylated glycosaminoglycan and its oligosaccharides as novel intrinsic factor Xase inhibitors. Eur. J. Med. Chem. 2018, 148, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Shashkov, A.S.; Kusaykin, M.I.; Stonik, V.A.; Nifantiev, N.E.; Usov, A.I. The struc-402 ture of a fucosylated chondroitin sulfate from the sea cucumber Cucumaria frondosa. Carbohydr. Polym. 2017, 165, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.A.G.; Ribeiro, K.A.; Valente, A.P.; Capille, N.V.; Jliveira, S.-N.M.C.G.; Tovar, A.M.F.; Pereira, M.S.; Vilanova, E.; Mourão, P.A.S. A unique fucosylated chondroitin sulfate type II with strikingly homogeneous and neatly distributed α-fucose branches. Glycobiology 2018, 28, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Qui, P.; Wu, F.; Yi, L.; Chen, L.; Jin, Y.; Ding, X.; Ouyang, Y.; Yao, Y.; Jiang, Y.; Zhang, Z. Structure characterization of a heavily fucosylated chondroitin sulfate from sea cucumber (H. leucospilota) with bottom-up strategies. Carbohydr Polym. 2020, 240, 116337. [Google Scholar] [CrossRef]
- Yin, R.; Zhou, L.; Gao, N.; Lin, L.; Sun, H.; Chen, D.; Cai, Y.; Zuo, Z.; Hu, K.; Huang, S.; et al. Unveiling the disaccharide-branched glycosaminoglycan and anticoagulant potential of its derivatives. Biomacromolecules 2021, 22, 1244–1255. [Google Scholar] [CrossRef]
- Mao, H.; Cai, Y.; Li, S.; Sun, H.; Lin, L.; Pan, Y.; Yang, W.; He, Z.; Chen, R.; Zhou, L.; et al. A new fucosylated glycosaminoglycan containing disaccharide branches from Acaudina molpadioides: Unusual structure and anti-intrinsic tenase activity. Carbohydr. Polym. 2020, 245, 116503. [Google Scholar] [CrossRef]
- Li, S.; Zhong, W.; Pan, Y.; Lin, L.; Cai, Y.; Mao, H.; Zhang, T.; Li, S.; Chen, R.; Zhou, L.; et al. Structural characterization and anticoagulant analysis of the novel branched fucosylated glycosaminoglycan from sea cucumber Holothuria nobilis. Carbohydr. Polym. 2021, 269, 118290. [Google Scholar] [CrossRef]
- Yin, R.; Pan, Y.; Cai, Y.; Yang, F.; Gao, N.; Ruzemaimaiti, D.; Zhao, J. Re-understanding of structure and anticoagulation: Fucosylated chondroitin sulfate from sea cucumber Ludwigothurea grisea. Carbohydr. Polym. 2022, 294, 119826. [Google Scholar] [CrossRef] [PubMed]
- Lan, D.; Zhang, J.; Shang, X.; Yu, L.; Xu, C.; Wang, P.; Cui, L.; Cheng, N.; Sun, H.; Ran, J.; et al. Branch distribution pattern and anticoagulant activity of a fucosylated chondroitin sulfate from Phyllophorella kohkutiensis. Carbohydr. Polym. 2023, 321, 121304. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.P.; Mourão, P.A.S. Occurrence of a unique fucose-branched chondroitin sulfate in the body wall of a sea cucumber. J. Biol. Chem. 1988, 263, 18176–18183. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, G.; Wu, N.; Guo, X.; Liao, N.; Ye, X.; Liu, D.; Xue, C.; Chai, W. Sulfation pattern of the fucose branch is important for the anticoagulant and antithrombotic activities of fucosylated chondroitin sulfates. Biochim. Biophys. Acta 2013, 1830, 3054–3066. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. Medical gains of chondroitin sulfate upon fucosylation. Curr. Med. Chem. 2015, 22, 4166–4176. [Google Scholar] [CrossRef] [PubMed]
- Selenka, E. Beiträge zur Anatomie und Systematik der Holothurien. Z. Für Wiss. Zool. 1867, 17, 291–374. [Google Scholar]
- Bell, F.J. Studies in the Holothuroidea. I. On the Genus Psolus and the forms allied thereto. In Proceedings of the Zoological Society of London; Blackwell Publishing Ltd.: Oxford, UK, 1882; pp. 641–650. [Google Scholar]
- Luo, L.; Wu, M.; Xu, L.; Lian, W.; Xiang, J.; Lu, F.; Gao, N.; Xiao, C.; Wang, S.; Zhao, J. Comparison of physicochemical characteristics and anticoagulant activities of polysaccharides from three sea cucumbers. Mar. Drugs 2013, 11, 399–417. [Google Scholar] [CrossRef]
- Dong, X.; Pan, R.; Deng, X.; Chen, Y.; Zhao, G.; Wang, C. Separation, purification, anticoagulant activity and preliminary structural characterization of two sulfated polysaccharides from sea cucumber Acaudina molpadioidea and Holothuria nobilis. Process. Biochem. 2014, 49, 1352–1361. [Google Scholar] [CrossRef]
- Dong, X.; Pan, R.; Zou, S.; He, M.; Wang, C. Oxidative degradation of the sulfated polysaccharide isolated from sea cucumber Holothuria nobilis. Process. Biochem. 2015, 50, 94–301. [Google Scholar] [CrossRef]
- Zou, S.; Pan, R.; Dong, X.; He, M.; Wang, C. Physicochemical properties and antioxidant activities of two fucosylated chondroitin sulfate from sea cucumber Acaudina molpadioidea and Holothuria nobilis. Process. Biochem. 2016, 51, 650–658. [Google Scholar] [CrossRef]
- Miller, A.K.; Kerr, A.M.; Paulay, G.; Reich, M.; Wilson, N.G.; Carvajal, J.I.; Rouse, G.W. Molecular phylogeny of extant Holothuroidea (Echinodermata). Mol. Phylogenetics Evol. 2017, 111, 110–131. [Google Scholar] [CrossRef]
- Vieira, R.P.; Mulloy, B.; Mourão, P.A.S. Structure of a fucose-branched chondroitin sulfate from sea cucumber. Evidence for the presence of 3-O-sulfo-β-D-glucuronosyl residues. J. Biol. Chem. 1991, 266, 13530–13536. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Borodina, E.Y.; Stonik, V.A.; Nifantiev, N.E.; Usov, A.I. A highly regular fucosylated chondroitin sulfate from the sea cucumber Massinium magnum: Structure and effects on coagulation. Carbohydr. Polym. 2017, 167, 20–26. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Tsvetkova, E.A.; Nikogosova, S.P.; Hang, C.T.T.; Thinh, P.D.; Trung, D.T.; Van, T.T.T.; Shashkov, A.S.; et al. Fucose-rich sulfated polysaccharides from two Vietnamese sea cucumbers Bohadschia argus and Holothuria (Theelothuria) spinifera: Structures and anticoagulant activity. Mar. Drugs 2022, 20, 380. [Google Scholar] [CrossRef]
- Anisimova, N.Y.; Ustyuzhanina, N.E.; Bilan, M.I.; Donenko, F.V.; Usov, A.I.; Kiselevskiy, M.V.; Nifantiev, N.E. Fucoidan and fucosylated chondroitin sulfate stimulate hematopoiesis in cyclophosphamide-Induced mice. Mar. Drugs 2017, 15, 301. [Google Scholar] [CrossRef]
- Anisimova NYu Ustyuzhanina, N.E.; Bilan, M.I.; Donenko, F.V.; Ushakova, N.A.; Usov, A.I.; Kiselevskiy, M.V.; Nifantiev, N.E. Influence of modified fucoidan and related sulfated oligosaccharides on hematopoiesis in cyclophosphamide-induced mice. Mar. Drugs 2018, 16, 333. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Anisimova, N.Y.; Bilan, M.I.; Donenko, F.V.; Morozevich, G.E.; Yashunskiy, D.V.; Usov, A.I.; Siminyan, N.G.; Kirgisov, K.I.; Varfolomeeva, S.R.; et al. Chondroitin sulfate and fucosylated chondroitin sulfate as stimulators of hematopoiesis. Pharmaceuticals 2021, 14, 074. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Anisimova, N.Y.; Dmitrenok, A.S.; Tsvetkova, E.A.; Kiselevskiy, M.V.; Nifantiev, N.E.; Usov, A.I. Depolymerization of a fucosylated chondroitin sulfate from the sea cucumber Cucumaria japonica: Structure and biological activity of the product. Carbohydr. Polym. 2022, 281, 119072. [Google Scholar] [CrossRef]
- Li, C.; Niu, Q.; Li, S.; Zhang, X.; Liu, C.; Cai, C.; Li, G.; Yu, G. Fucoidan from sea cucumber Holothuria polii: Structural elucidation and stimulation of hematopoietic activity. Int. J. Biol. Macromol. 2020, 154, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Li, G.; Li, C.; Li, Q.; Li, J.; Liu, C.; Pan, L.; Li, S.; Cai, C.; Hao, J.; et al. Two different fucosylated chondroitin sulfates: Structural elucidation, stimulating hematopoiesis and immune-enhancing effects. Carbohydr. Polym. 2020, 230, 115698. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, E.A.; Lortat-Jacob, H.; Priestley, G.V.; Nakamoto, B.; Papayannopoulou, T. Sulfated polysaccharides increase plasma levels of SDF-1 in monkeys and mice: Involvement in mobilization of stem/progenitor cells. Blood 2002, 99, 44–51. [Google Scholar] [CrossRef]
- Ma, W.P.; Yin, S.N.; Chen, J.P.; Geng, X.C.; Liu, M.F.; Li, H.H.; Liu, M.; Liu, H.B. Stimulating the hematopoietic effect of simulated digestive product of fucoidan from Sargassum fusiforme on cyclophosphamide-induced hematopoietic damage in mice and its protective mechanisms based on serum lipidomics. Mar. Drugs 2022, 20, 201. [Google Scholar] [CrossRef]
- Ziegler, P.; Boettcher, S.; Takizawa, H.; Manz, M.G.; Brümmendorf, T.H. LPS-stimulated human bone marrow stroma cells support myeloid cell development and progenitor cell maintenance. Ann. Hematol. 2016, 95, 173–178. [Google Scholar] [CrossRef]
- Syrjälä, M.; Ruutu, T.; Jansson, S.E. A flow cytometric assay of CD34-positive cell populations in the bone marrow. Br. J. Haematol. 1994, 88, 679–684. [Google Scholar] [CrossRef]
- Pierelli, L.; Scambia, G.; Bonanno, G.; Rutella, S.; Puggioni, P.; Battaglia, A.; Mozzetti, S.; Marone, M.; Menichella, G.; Rumi, C.; et al. CD34+/CD105+ cells are enriched in primitive circulating progenitors residing in the G0 phase of the cell cycle and contain all bone marrow and cord blood CD34+/CD38low/- precursors. Br. J. Haematol. 2000, 108, 610–620. [Google Scholar] [CrossRef]
- Braun, J.; Kurtz, A.; Barutcu, N.; Bodo, J.; Thiel, A.; Dong, J. Concerted regulation of CD34 and CD105 accompanies mesenchymal stromal cell derivation from human adventitial stromal cell. Stem Cells Dev. 2013, 22, 815–827. [Google Scholar] [CrossRef]
- Mackey, M.C.; Glisovic, S.; Leclerc, J.; Pastore, Y.; Krajinovic, M.; Craig, M. The timing of cyclic cytotoxic chemotherapy can worsen neutropenia and neutrophilia. Br. J. Clin. Pharmacol. 2020, 87, 687–693. [Google Scholar] [CrossRef]
- Craig, M.; Humphries, A.; Mackey, M. A mathematical model of granulopoiesis incorporating the negative feedback dynamics and kinetics of G-CSF/neutrophil binding and internalisation. Bull Math Biol. 2016, 78, 2304–2357. [Google Scholar] [CrossRef]
- Cabrera-Rivera, G.L.; Madera-Sandoval, R.L.; León-Pedroza, J.I.; Ferat-Osorio, E.; Salazar-Rios, E.; Hernández-Aceves, J.A.; Guadarrama-Aranda, U.; López-Macías, C.; Wong-Baeza, I.; Arriaga-Pizano, L.A. Increased TNF-α production in response to IL-6 in patients with systemic inflammation without infection. Clin. Exp. Immunol. 2022, 209, 225–235. [Google Scholar] [CrossRef]
- Cartmell, T.; Poole, S.; Turnbull, A.V.; Rothwell, N.J.; Luheshi, G. Circulating interleukin-6 mediates the febrile response to localised inflammation in rats. J. Physiol. 2000, 526, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, H.; Yin, Y.L.; Guo, W.Z.; Ma, Y.Q.; Wang, Y.B.; Shu, C.; Dong, L.Q. Role of interleukin-6 to differentiate sepsis from non-infectious systemic inflammatory response syndrome. Cytokine 2016, 88, 126–135. [Google Scholar] [CrossRef]
- Mikaeili, H.; Taghizadieh, A.; Nazemiyeh, M.; Rezaeifar, P.; Zununi Vahed, S.; Safiri, S.; Ardalan, M.; Ansarin, K. The early start of hemoperfusion decreases the mortality rate among severe COVID-19 patients: A preliminary study. Hemodial. Int. 2022, 26, 176–182. [Google Scholar] [CrossRef]
- Le Blanc, K.; Rasmusson, I.; Sundberg, B.; Götherström, C.; Hassan, M.; Uzunel, M.; Ringdén, O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004, 393, 1439–1441. [Google Scholar] [CrossRef]
- Zhu, G.R.; Zhou, X.Y.; Lu, H.; Zhou, J.W.; Li, A.P.; Xu, W.; Li, J.Y.; Wang, C.Y. Human bone marrow mesenchymal stem cells express multiple hematopoietic growth factors. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2003, 11, 115–119. [Google Scholar]
- Li, Y.; Chen, S.; Yuan, J.; Yang, Y.; Li, J.; Ma, J.; Wu, X.; Freund, M.; Pollok, K.; Hanenberg, H.; et al. Mesenchymal stem/progenitor cells promote the reconstitution of exogenous hematopoietic stem cells in Fancg-/- mice in vivo. Blood 2009, 113, 2342–2351. [Google Scholar] [CrossRef]
- D’Souza, A.; Fretham, C.; Lee, S.J.; Arora, M.; Brunner, J.; Chhabra, S.; Devine, S.; Eapen, M.; Hamadani, M.; Hari, P.; et al. Current use of and trends in hematopoietic cell transplantation in the United States. Biol. Blood Marrow Transplant. 2020, 26, 177–182. [Google Scholar] [CrossRef]
- Korngold, R.; Marini, J.C.; de Baca, M.E.; Murphy, G.F.; Giles-Komar, J. Role of tumor necrosis factor-alpha in graft-versus-host disease and graft-versus-leukemia responses. Biol. Blood Marrow Transplant. 2003, 9, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.; Lorentino, F.; Nitti, R.; Lupo Stanghellini, M.T.; Giglio, F.; Clerici, D.; Xue, E.; Lazzari, L.; Piemontese, S.; Mastaglio, S.; et al. Interleukin-6 as biomarker for acute GvHD and survival after allogeneic transplant with post-transplant cyclophosphamide. Front. Immunol. 2019, 1, 2319. [Google Scholar] [CrossRef] [PubMed]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef]
- Scott, R.W. Colorimetric determination of hexuronic acids in plant materials. Anal. Chem. 1979, 51, 936–941. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ustyuzhanina, N.E.; Bilan, M.I.; Anisimova, N.Y.; Nikogosova, S.P.; Dmitrenok, A.S.; Tsvetkova, E.A.; Panina, E.G.; Sanamyan, N.P.; Avilov, S.A.; Stonik, V.A.; et al. Fucosylated Chondroitin Sulfates with Rare Disaccharide Branches from the Sea Cucumbers Psolus peronii and Holothuria nobilis: Structures and Influence on Hematopoiesis. Pharmaceuticals 2023, 16, 1673. https://doi.org/10.3390/ph16121673
Ustyuzhanina NE, Bilan MI, Anisimova NY, Nikogosova SP, Dmitrenok AS, Tsvetkova EA, Panina EG, Sanamyan NP, Avilov SA, Stonik VA, et al. Fucosylated Chondroitin Sulfates with Rare Disaccharide Branches from the Sea Cucumbers Psolus peronii and Holothuria nobilis: Structures and Influence on Hematopoiesis. Pharmaceuticals. 2023; 16(12):1673. https://doi.org/10.3390/ph16121673
Chicago/Turabian StyleUstyuzhanina, Nadezhda E., Maria I. Bilan, Natalia Yu. Anisimova, Sofya P. Nikogosova, Andrey S. Dmitrenok, Evgenia A. Tsvetkova, Elena G. Panina, Nadezhda P. Sanamyan, Sergey A. Avilov, Valentin A. Stonik, and et al. 2023. "Fucosylated Chondroitin Sulfates with Rare Disaccharide Branches from the Sea Cucumbers Psolus peronii and Holothuria nobilis: Structures and Influence on Hematopoiesis" Pharmaceuticals 16, no. 12: 1673. https://doi.org/10.3390/ph16121673