Zingiber Officinale Roscoe: The Antiarthritic Potential of a Popular Spice—Preclinical and Clinical Evidence
Abstract
:1. Introduction
1.1. Inflammatory Arthritis Prevalence and Treatment
1.2. Osteoarthritis
1.3. Rheumatoid Arthritis
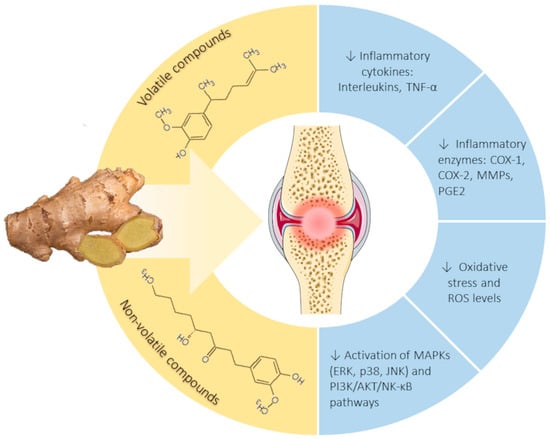
1.4. Ginger Phytochemistry and Rationale for Further Review and Investigation
2. In Vitro Preclinical Studies
| Type of Extract/Active Compound | Dose | Type of Cells | Results | Ref. |
|---|---|---|---|---|
| EV.EXT®77 (Z. officinale, A. galanga) | 1 mg/mL | FLSs from healthy patients or patients with RA and OA | ↓IL-8 similarly to betamethasone | [71] |
| EV.EXT®77 (Z. officinale, A. galanga) | 100 µg/mL | FLSs from patients with OA | ↓activation of TNF-α and COX-2 expression, ↓synthesis of PGE2 and TNF-α, and ↓induction of NF-κB and IκB-α | [72] |
| Z. officinale and A. galanga combined extracts | 100 µg/mL | FLSs from patients with OA | ↓chemokines, ↓MCP-1, and ↓IP-10; the immunosuppressive effect of combined extracts were higher than either extract alone | [73] |
| Zingerone | 20–40 µM | SW1353 cell culture and explanted cells from pig cartilage | ↓MMP-13 ↓expression of TNF-α, IL-6, and IL-8 mRNA levels | [74] |
| Zingerone | 5–20 µM | FLSs from rats with collagen-induced arthritis | Inhibition of cell proliferation and migration; ↓ROS formation, and ↓ expression of IL-1β and IL-6 transcripts | [75] |
| 6-shogaol | 5, 10, 20, 40 µM | FLSs and MH7A cell lines from patients with RA | Apoptosis of FLSs and MH7A cells and inhibition of their migration and proliferation; ↓synthesis of MMP-2, MMP-9, IL-1β, IL-6, IL-8, and TNF-α | [76] |
| Cedrol | 1, 5, 10 µM | FLSs from mice with collagen-induced arthritis | Downregulation of ERK/MAPK and p65 NF-κB activation, ↓MMP-13, ↓MCP-1, and ↓expression of TNF-α, IL-1β, COX-1, and COX-2 | [77] |
| Z. officinale extract in DMSO | 5, 25 µg/mL | C28I2 human chondrocyte cells | ↓ROS, ↓lipid peroxidation, Bax/Bcl-2 ratio, and capsase-3 activity; boosted mRNA expression of genes related to SOD1, GPX1, GPX2, GPX4, and CAT | [79] |
| Z. officinale aqueous extract (Aquaresin ginger) | 10–100 µg/mL | Healthy and osteoarthritic chondrocytes isolated from sow cartilage explants | ↓PGE2 and NO (doses > 10 g/mL) in OA chondrocytes and, at low concentrations, in normal chondrocytes | [80] |
| 6-shogaol | 5 µM | Human chondrocyte cells | ↓MCP-1 and IL-6 ↓ MMP-2 and MMP-9 activity | [81] |
3. In Vivo Preclinical Studies
4. Clinical Effects of Ginger in the Treatment of Osteoarthritis and Rheumatoid Arthritis
| Author Year [Ref.] | Study Design | No. of Participants (Age) | Type of Intervention | Comparison | Result | Condition | Duration | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Aryaeian et al. 2019 [91] | Randomized double-blind clinical trial | 70 (19–69 years old) | Z. officinale (750 mg) two times a day | Placebo (wheat flour) |
| RA | 12 weeks | None reported |
| Aryaeian et al. 2019 [90] | Randomized double-blind clinical trial | 66 (19–69 years old) | Z. officinale (750 mg) two times a day | Placebo (roasted wheat flour) |
| RA | 12 weeks | None reported |
| Mozaffari-Khosravi et al. 2016 [92] | Randomized double-blind clinical trial | 120 (50–70 years old) | Z. officinale (500 mg) two times a day | Placebo (starch) |
| Knee OA | 3 months | None reported |
| Naderi et al. 2016 [93] | Randomized double-blind clinical trial | 120 (50–70 years old) | Z. officinale (500 mg) two times a day | Placebo (starch) |
| Knee OA | 3 months | None reported |
| Kordi Yoosefinejad et al. 2021 [96] | Randomized single-blind clinical trial | 40 (≥40 years old) | 5% Z. officinale gel formulation (70% Z. officinale solution + physiological aqua gel) five times a week during physical therapy | Ultrasonography with physiological aqua gel |
| Knee OA | 2 weeks | None reported |
| Zakeri et al. 2011 [95] | Randomized double-blind clinical trial | 204 (37–75 years old) | Z. officinale (250 mg) two times a day | Placebo (starch) |
| Knee OA | 6 weeks | None reported |
| Niempoog et al. 2012 [89] | Randomized double-blind clinical trial | 60 | Z. officinale (500 mg) two times a day | Placebo |
| Knee OA | 8 weeks | Heartburn (one patient) |
| Author Year [Ref.] | Study Design | No. of Participants (Age) | Type of Intervention | Comparison | Result | Condition | Duration | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Altman et al. 2001 [101] | Randomized double-blind clinical trial | 261 (≥18 years old) | Ginger extract EV.EXT77 from Z. officinale and Alpinia galanga (255 mg) twice a day | Placebo (coconut oil) | ↓ knee pain during standing compared to the placebo group | Knee OA | 6 weeks | Mild gastrointestinal effects |
| Bliddal et al. 2000 [102] | Randomized double-blind clinical trial | 56 (24–87 years old) | Z. officinale extract, Eurovita Extract 33 (170 mg), three times a day | Placebo or Ibuprofen (400 mg) | Statistical difference between Ibupofen, Z. officinale, and the placebo, but no difference between the placebo and Z. officinale | Hip or knee OA | 3 weeks | None reported |
| Heidari-Beni et al. 2020 [103] | Randomized double-blind clinical trial | 60 (35–75 years old) | Capsule with turmeric extract, ginger, and black pepper, containing curcumin (300 mg), gingerols (7.5 mg), piperine (3.75 mg), taken twice a day | Naproxen (250 mg) | ↓ PGE2 | Knee OA | 4 weeks | None reported |
| Zahmatkash et al. 2011 [105] | Randomized double-blind clinical trial | 92 (52.2 ± 12.4 years old) | Ointment containing cinnamon, ginger, mastic, and sesame oil (2 g) three times a day | Salicylate ointment | ↓ pain, morning stiffness, and limited motion after ointment use (comparable to the effect of a salicylate ointment) | OA | 6 weeks | None reported |
| Niempoog et al. 2012 [104] | Randomized double-blind clinical trial | 99 | Combination of 4% Z. officinale and Z. cassumunar extract in Plygersic gel (1 g) four times a day | 1% Diclofenac gel | ↓ pain and ↑ QoL after application of ginger gel (an effect similar to the 1% diclofenac gel) | Knee OA | 6 weeks | Contact dermatitis (one patient) |
| Amorndoljai et al. 2017 [106] | Randomized double-blind clinical trial | 118 (50–75 years old) | Nanostructure lipid carrier loaded with ginger extract in a ratio of 5% by weight (total amount 28.8 ± 8.7 mg) | 1% Diclofenac gel | ↓ knee pain, ↓ stiffness, and better physical function (ss) Effect of ginger similar to diclophenac | Knee OA | 12 weeks | Skin reaction (one patient) |
5. Ginger-Loaded Delivery Systems for Arthritis Inflammatory Treatment
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| APC | antigen-presenting cell |
| BDDCS | biopharmaceutical drug disposition categorization system |
| CAT | catalase |
| CFA | Chronic Freund’s adjuvant |
| CIA | collagen-induced arthritis |
| COX-1 | cyclooxygenase-1 |
| COX-2 | cyclooxygenase-2 |
| CRP | c-reactive protein |
| DMARD | disease-modifying antirheumatic drug |
| DMSO | dimethyl sulfoxide |
| ECM | extracellular matrix |
| ERK | extracellular signal-regulated kinase |
| EULAR | European League against Rheumatism |
| FLS | fibroblast-like synoviocyte cells |
| FoxP3 | forkhead box P3 |
| GPX | glutathione peroxidase |
| IκB-alfa | nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha |
| IFN-gamma | interferon-gamma |
| IP-10 | interferon-gamma-inducible protein 10 kD |
| JAK/STAT | Janus kinase/signal transducers and activators of transcription |
| JNK | c-Jun N-terminal kinase |
| MAPK | mitogen-activated protein kinases |
| MCP-1 | monocyte chemoattractant protein-1 |
| MMP | matrix metalloproteinase |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLC | nanostructure lipid carrier |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| OA | osteoarthritis |
| p38-MAPK | p38 mitogen-activated protein kinases |
| PGE2 | prostaglandin E2 |
| PIK3/AKT | phosphatidylinositol 3’-kinase-Akt signaling pathway |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| RA | rheumatoid arthritis |
| RORγt | RAR-related orphan receptor gamma |
| ROS | reactive oxygen species |
| SCW | streptococcal cell wall |
| SOD1 | superoxide dismutase type 1 |
| TGF | transforming growth factor |
| TNF-α | tumor necrosis factor |
| TRPV1 | transient receptor potential vanilloid 1 |
| VCAM-1 | vascular cell adhesion molecule 1 |
| VEGF | vascular endothelial growth factor |
References
- Kin-Hoo Koo, K.; Chinoy, H.; Creaney, L.; Hayton, M. Inflammatory Arthropathy in the Elite Sports Athlete. Curr. Sports Med. Rep. 2021, 20, 577–583. [Google Scholar] [CrossRef]
- Schett, G.; Rahman, P.; Ritchlin, C.; McInnes, I.B.; Elewaut, D.; Scher, J.U. Psoriatic Arthritis from a Mechanistic Perspective. Nat. Rev. Rheumatol. 2022, 18, 311–325. [Google Scholar] [CrossRef]
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of Osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef]
- Black, R.J.; Cross, M.; Haile, L.M.; Culbreth, G.T.; Steinmetz, J.D.; Hagins, H.; Kopec, J.A.; Brooks, P.M.; Woolf, A.D.; Ong, K.L.; et al. Global, Regional, and National Burden of Rheumatoid Arthritis, 1990–2020, and Projections to 2050: A Systematic Analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e594–e610. [Google Scholar] [CrossRef]
- Charlier, E.; Relic, B.; Deroyer, C.; Malaise, O.; Neuville, S.; Collée, J.; Malaise, M.; De Seny, D. Insights on Molecular Mechanisms of Chondrocytes Death in Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2146. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Pope, J.E. Novel Treatment Strategies in Rheumatoid Arthritis. Lancet 2017, 389, 2338–2348. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, H.; Liu, L. Side Effects of Methotrexate Therapy for Rheumatoid Arthritis: A Systematic Review. Eur. J. Med. Chem. 2018, 158, 502–516. [Google Scholar] [CrossRef]
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef]
- Guilak, F. Biomechanical Factors in Osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2011, 25, 815–823. [Google Scholar] [CrossRef]
- Roos, E.M. Joint Injury Causes Knee Osteoarthritis in Young Adults. Curr. Opin. Rheumatol. 2005, 17, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Serge, P.; Anne-Priscille, T. Pain in Osteoarthritis from a Symptom to a Disease. Best Pract. Res. Clin. Rheumatol. 2023, 37, 101825. [Google Scholar] [CrossRef]
- Sohn, D.; Sokolove, J.; Sharpe, O.; Erhart, J.C.; Chandra, P.E.; Lahey, L.J.; Lindstrom, T.M.; Hwang, I.; Boyer, K.A.; Andriacchi, T.P.; et al. Plasma Proteins Present in Osteoarthritic Synovial Fluid Can Stimulate Cytokine Production via Toll-like Receptor 4. Arthritis Res. Ther. 2012, 14, R7. [Google Scholar] [CrossRef] [PubMed]
- Bondeson, J.; Wainwright, S.D.; Lauder, S.; Amos, N.; Hughes, C.E. The Role of Synovial Macrophages and Macrophage-Produced Cytokines in Driving Aggrecanases, Matrix Metalloproteinases, and Other Destructive and Inflammatory Responses in Osteoarthritis. Arthritis Res. Ther. 2006, 8, R187. [Google Scholar] [CrossRef] [PubMed]
- Sakao, K.; Takahashi, K.A.; Arai, Y.; Saito, M.; Honjo, K.; Hiraoka, N.; Asada, H.; Shin-Ya, M.; Imanishi, J.; Mazda, O.; et al. Osteoblasts Derived from Osteophytes Produce Interleukin-6, Interleukin-8, and Matrix Metalloproteinase-13 in Osteoarthritis. J. Bone Min. Metab. 2009, 27, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Marcu, K.B. Cartilage Homeostasis in Health and Rheumatic Diseases. Arthritis Res. Ther. 2009, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Tetlow, L.C.; Adlam, D.J.; Woolley, D.E. Matrix Metalloproteinase and Proinflammatory Cytokine Production by Chondrocytes of Human Osteoarthritic Cartilage: Associations with Degenerative Changes. Arthritis Rheum. 2001, 44, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Sandell, L.J.; Aigner, T. Articular Cartilage and Changes in Arthritis: Cell Biology of Osteoarthritis. Arthritis Res. Ther. 2001, 3, 107. [Google Scholar] [CrossRef] [PubMed]
- Pascarelli, N.; Collodel, G.; Moretti, E.; Cheleschi, S.; Fioravanti, A. Changes in Ultrastructure and Cytoskeletal Aspects of Human Normal and Osteoarthritic Chondrocytes Exposed to Interleukin-1β and Cyclical Hydrostatic Pressure. Int. J. Mol. Sci. 2015, 16, 26019–26034. [Google Scholar] [CrossRef]
- Mehana, E.-S.E.; Khafaga, A.F.; El-Blehi, S.S. The Role of Matrix Metalloproteinases in Osteoarthritis Pathogenesis: An Updated Review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef]
- Shlopov, B.V.; Lie, W.-R.; Mainardi, C.L.; Cole, A.A.; Chubinskaya, S.; Hasty, K.A. Osteoarthritic Lesions. Involvement of Three Different Collagenases. Arthritis Rheum. 1997, 40, 2065–2074. [Google Scholar] [CrossRef]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef]
- Galasso, O.; Familiari, F.; De Gori, M.; Gasparini, G. Recent Findings on the Role of Gelatinases (Matrix Metalloproteinase-2 and -9) in Osteoarthritis. Adv. Orthop. 2012, 2012, 834208. [Google Scholar] [CrossRef]
- Blom, A.; Van Der Kraan, P.; Van Den Berg, W. Cytokine Targeting in Osteoarthritis. Curr. Drug Targets 2007, 8, 283–292. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Fan, H.W.; Liu, G.Y.; Zhao, C.F.; Li, X.F.; Yang, X.Y. Differential Expression of COX-2 in Osteoarthritis and Rheumatoid Arthritis. Genet. Mol. Res. 2015, 14, 12872–12879. [Google Scholar] [CrossRef]
- Karan, A.; Karan, M.A.; Vural, P.; Erten, N.; Taşçioglu, C.; Aksoy, C.; Canbaz, M.; Öncel, A. Synovial Fluid Nitric Oxide Levels in Patients with Knee Osteoarthritis. Clin. Rheumatol. 2003, 22, 397–399. [Google Scholar] [CrossRef]
- Rousset, F.; Hazane-Puch, F.; Pinosa, C.; Nguyen, M.V.C.; Grange, L.; Soldini, A.; Rubens-Duval, B.; Dupuy, C.; Morel, F.; Lardy, B. IL-1beta Mediates MMP Secretion and IL-1beta Neosynthesis via Upregulation of P22phox and NOX4 Activity in Human Articular Chondrocytes. Osteoarthr. Cartil. 2015, 23, 1972–1980. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic Signaling Pathways and Therapeutic Targets. Sig. Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Li, Z.; Dai, A.; Yang, M.; Chen, S.; Deng, Z.; Li, L. p38MAPK Signaling Pathway in Osteoarthritis: Pathological and Therapeutic Aspects. J. Inflamm. Res. 2022, 15, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Blom, A.B.; Brockbank, S.M.; Van Lent, P.L.; Van Beuningen, H.M.; Geurts, J.; Takahashi, N.; Van Der Kraan, P.M.; Van De Loo, F.A.; Schreurs, B.W.; Clements, K.; et al. Involvement of the Wnt Signaling Pathway in Experimental and Human Osteoarthritis: Prominent Role of Wnt-induced Signaling Protein 1. Arthritis Rheum. 2009, 60, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.-N.; Cai, W.-J.; Shi, J.; Yi, Z.-J. MAPK Inhibitors Protect against Early-stage Osteoarthritis by Activating Autophagy. Mol. Med. Rep. 2021, 24, 829. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid Arthritis: Pathological Mechanisms and Modern Pharmacologic Therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef]
- Wehr, P.; Purvis, H.; Law, S.-C.; Thomas, R. Dendritic Cells, T Cells and Their Interaction in Rheumatoid Arthritis. Clin. Exp. Immunol. 2019, 196, 12–27. [Google Scholar] [CrossRef]
- Gregersen, P.K.; Silver, J.; Winchester, R.J. The Shared Epitope Hypothesis. an Approach to Understanding the Molecular Genetics of Susceptibility to Rheumatoid Arthritis. Arthritis Rheum. 1987, 30, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.H.S.; Panayi, G.S. Cytokine Pathways and Joint Inflammation in Rheumatoid Arthritis. N. Engl. J. Med. 2001, 344, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Zafar, A.; Moin, S.; Khan, A.Q.; Zubair, S. Understanding the Role of Cytokines in the Pathogenesis of Rheumatoid Arthritis. Clin. Chim. Acta. 2016, 455, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Warrington, K.J.; Takemura, S.; Goronzy, J.J.; Weyand, C.M. CD4+,CD28? T Cells in Rheumatoid Arthritis Patients Combine Features of the Innate and Adaptive Immune Systems. Arthritis Rheum. 2001, 44, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Quiñonez-Flores, C.M.; González-Chávez, S.A.; Del Río Nájera, D.; Pacheco-Tena, C. Oxidative Stress Relevance in the Pathogenesis of the Rheumatoid Arthritis: A Systematic Review. BioMed Res. Int. 2016, 2016, 6097417. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.L.; Lyon, M.; Chapman, E.A.; Moots, R.J.; Edwards, S.W. Rheumatoid Arthritis Synovial Fluid Neutrophils Drive Inflammation Through Production of Chemokines, Reactive Oxygen Species, and Neutrophil Extracellular Traps. Front. Immunol. 2021, 11, 584116. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Hu, W.; Wang, R.; Yang, Q.; Zhu, M.; Li, M.; Cai, J.; Rose, P.; Mao, J.; Zhu, Y.Z. Signaling Pathways in Rheumatoid Arthritis: Implications for Targeted Therapy. Sig. Transduct. Target. Ther. 2023, 8, 68. [Google Scholar] [CrossRef]
- Dissanayake, K.G.C.; Liyanage, W.A.; Waliwita, C.; Liyanage, R.P. A Review on Medicinal Uses of Zingiber officinale (Ginger). Int. J. Health Sci. Res. 2020, 10, 142–148. [Google Scholar]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Clinical Aspects and Health Benefits of Ginger (Zingiber officinale) in Both Traditional Chinese Medicine and Modern Industry. Acta Agric. Scand. Sect. B Soil Plant Sci. 2019, 69, 546–556. [Google Scholar] [CrossRef]
- Govindarajan, V.S.; Connell, D.W. Ginger—Chemistry, Technology, and Quality Evaluation: Part 1. C R C Crit. Rev. Food Sci. Nutr. 1983, 17, 1–96. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Sultan, M.T. Ginger and Its Health Claims: Molecular Aspects. Crit. Rev. Food Sci. Nutr. 2011, 51, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Munda, S.; Dutta, S.; Haldar, S.; Lal, M. Chemical Analysis and Therapeutic Uses of Ginger (Zingiber officinale Rosc.) Essential Oil: A Review. J. Essent. Oil Bear. Plants 2018, 21, 994–1002. [Google Scholar] [CrossRef]
- Tripathi, S.; Maier, K.; Bruch, D.; Kittur, D. Ginger and Its Active Ingredient 6-Gingerol down Regulate pro-Inflammatory Cytokine Release by Macrophages. J. Surg. Res. 2006, 130, 318. [Google Scholar] [CrossRef]
- Young, H.-Y.; Luo, Y.-L.; Cheng, H.-Y.; Hsieh, W.-C.; Liao, J.-C.; Peng, W.-H. Analgesic and Anti-Inflammatory Activities of [6]-Gingerol. J. Ethnopharmacol. 2005, 96, 207–210. [Google Scholar] [CrossRef]
- Jung, M.Y.; Lee, M.K.; Park, H.J.; Oh, E.-B.; Shin, J.Y.; Park, J.S.; Jung, S.Y.; Oh, J.-H.; Choi, D.-S. Heat-Induced Conversion of Gingerols to Shogaols in Ginger as Affected by Heat Type (Dry or Moist Heat), Sample Type (Fresh or Dried), Temperature and Time. Food Sci. Biotechnol. 2018, 27, 687–693. [Google Scholar] [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative Antioxidant and Anti-Inflammatory Effects of [6]-Gingerol, [8]-Gingerol, [10]-Gingerol and [6]-Shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef]
- Wu, H.; Hsieh, M.; Lo, C.; Liu, C.B.; Sang, S.; Ho, C.; Pan, M. 6-Shogaol Is More Effective than 6-gingerol and Curcumin in Inhibiting 12-O -tetradecanoylphorbol 13-acetate-induced Tumor Promotion in Mice. Mol. Nutr. Food Res. 2010, 54, 1296–1306. [Google Scholar] [CrossRef]
- Osae, R.; Apaliya, M.T.; Kwaw, E.; Chisepo, M.T.R.; Yarley, O.P.N.; Antiri, E.A.; Alolga, R.N. Drying Techniques Affect the Quality and Essential Oil Composition of Ghanaian Ginger (Zingiber officinale Roscoe). Ind. Crops Prod. 2021, 172, 114048. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.; Baghdadi, A.; Tayebi-Meigooni, A. Formation of 6-, 8- and 10-Shogaol in Ginger through Application of Different Drying Methods: Altered Antioxidant and Antimicrobial Activity. Molecules 2018, 23, 1646. [Google Scholar] [CrossRef]
- Ezez, D.; Tefera, M. Effects of Solvents on Total Phenolic Content and Antioxidant Activity of Ginger Extracts. J. Chem. 2021, 2021, 6635199. [Google Scholar] [CrossRef]
- Spiro, M.; Kandiah, M.; Price, W. Extraction of Ginger Rhizome: Kinetic Studies with Dichloromethane, Ethanol, 2-propanol and an Acetone—Water Mixture. Int. J. Food Sci. Tech. 1990, 25, 157–167. [Google Scholar] [CrossRef]
- Zick, S.M.; Djuric, Z.; Ruffin, M.T.; Litzinger, A.J.; Normolle, D.P.; Alrawi, S.; Feng, M.R.; Brenner, D.E. Pharmacokinetics of 6-Gingerol, 8-Gingerol, 10-Gingerol, and 6-Shogaol and Conjugate Metabolites in Healthy Human Subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, T.; Ohsawa, K. Metabolism of [6]-Gingerol in Rats. Life Sci. 2002, 70, 2165–2175. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zick, S.; Li, X.; Zou, P.; Wright, B.; Sun, D. Examination of the Pharmacokinetics of Active Ingredients of Ginger in Humans. AAPS J. 2011, 13, 417. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Naora, K.; Hayashibara, M.; Katagiri, Y.; Kano, Y.; Iwamoto, K. Pharmacokinetics of (6)-Gingerol after Intravenous Administration in Rats. Chem. Pharm. Bull. 1991, 39, 1612–1614. [Google Scholar] [CrossRef]
- Mukkavilli, R.; Yang, C.; Singh Tanwar, R.; Ghareeb, A.; Luthra, L.; Aneja, R. Absorption, Metabolic Stability, and Pharmacokinetics of Ginger Phytochemicals. Molecules 2017, 22, 553. [Google Scholar] [CrossRef]
- Oriani, V.B.; Alvim, I.D.; Consoli, L.; Molina, G.; Pastore, G.M.; Hubinger, M.D. Solid Lipid Microparticles Produced by Spray Chilling Technique to Deliver Ginger Oleoresin: Structure and Compound Retention. Food Res. Int. 2016, 80, 41–49. [Google Scholar] [CrossRef]
- Ekrami, M.; Babaei, M.; Fathi, M.; Abbaszadeh, S.; Nobakht, M. Ginger Essential Oil (Zingiber officinale) Encapsulated in Nanoliposome as Innovative Antioxidant and Antipathogenic Smart Sustained-Release System. Food Biosci. 2023, 53, 102796. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible Ginger-Derived Nanoparticles: A Novel Therapeutic Approach for the Prevention and Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Rambod, M.; Nazarinia, M.; Raieskarimian, F. The Prevalence and Predictors of Herbal Medicines Usage among Adult Rheumatoid Arthritis Patients: A Case-Control Study. Complement. Ther. Med. 2018, 41, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Casula, E.; Manca, M.L.; Perra, M.; Pedraz, J.L.; Lopez-Mendez, T.B.; Lozano, A.; Calvo, E.; Zaru, M.; Manconi, M. Nasal Spray Formulations Based on Combined Hyalurosomes and Glycerosomes Loading Zingiber Officinalis Extract as Green and Natural Strategy for the Treatment of Rhinitis and Rhinosinusitis. Antioxidants 2021, 10, 1109. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Zhou, D.; Xu, L.; Song, X. Curcumin Alleviates Rheumatoid Arthritis-Induced Inflammation and Synovial Hyperplasia by Targeting mTOR Pathway in Rats. Drug Des. Dev. Ther. 2018, 12, 4095–4105. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh, A.; Abbasi, F.; Ebrahimzadeh, A.; Jibril, A.T.; Milajerdi, A. Effects of Curcumin Supplementation on Inflammatory Biomarkers in Patients with Rheumatoid Arthritis and Ulcerative Colitis: A Systematic Review and Meta-Analysis. Complement. Ther. Med. 2021, 61, 102773. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Dong, Y.; Vu, S.; Yang, F.; Yarov-Yarovoy, V.; Tian, Y.; Zheng, J. Structural Mechanisms Underlying Activation of TRPV1 Channels by Pungent Compounds in Gingers. Br. J. Pharmacol. 2019, 176, 3364–3377. [Google Scholar] [CrossRef]
- Long, L.; Soeken, K.; Ernst, E. Herbal Medicines for the Treatment of Osteoarthritis: A Systematic Review. Rheumatology 2001, 40, 779–793. [Google Scholar] [CrossRef]
- Müller-Ladner, U.; Ospelt, C.; Gay, S.; Distler, O.; Pap, T. Cells of the Synovium in Rheumatoid Arthritis. Synovial Fibroblasts. Arthritis Res. Ther. 2007, 9, 223. [Google Scholar] [CrossRef]
- Ribel-Madsen, S.; Bartels, E.M.; Stockmarr, A.; Borgwardt, A.; Cornett, C.; Danneskiold-Samsøe, B.; Bliddal, H. A Synoviocyte Model for Osteoarthritis and Rheumatoid Arthritis: Response to Ibuprofen, Betamethasone, and Ginger Extract—A Cross-Sectional In Vitro Study. Arthritis 2012, 2012, 505842. [Google Scholar] [CrossRef] [PubMed]
- Frondoza, C.G.; Sohrabi, A.; Polotsky, A.; Phan, P.V.; Hungerford, D.S.; Lindmark, L. An in Vitro Screening Assay for Inhibitors of Proinflammatory Mediators in Herbal Extracts Using Human Synoviocyte Cultures. In Vitro Cell Dev. Biol. Anim. 2004, 40, 95. [Google Scholar] [CrossRef]
- Phan, P.V.; Sohrabi, A.; Polotsky, A.; Hungerford, D.S.; Lindmark, L.; Frondoza, C.G. Ginger Extract Components Suppress Induction of Chemokine Expression in Human Synoviocytes. J. Altern. Complement. Med. 2005, 11, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Ruangsuriya, J.; Budprom, P.; Viriyakhasem, N.; Kongdang, P.; Chokchaitaweesuk, C.; Sirikaew, N.; Chomdej, S.; Nganvongpanit, K.; Ongchai, S. Suppression of Cartilage Degradation by Zingerone Involving the P38 and JNK MAPK Signaling Pathway. Planta Med. 2016, 83, 268–276. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, C.; Liu, D.; Wei, X.; Tan, X.; Zhou, J.; Yu, X.; Liu, M. In Vitro Inhibitory Effect of Zingerone on TNFα-Stimulated Fibroblast-like Synoviocytes. In Vitro Cell. Dev. Biol.-Anim. 2023, 59, 615–623. [Google Scholar] [CrossRef]
- Li, N.; Li, X.; Deng, L.; Yang, H.; Gong, Z.; Wang, Q.; Pan, D.; Zeng, S.; Chen, J. 6-Shogaol Inhibits the Proliferation, Apoptosis, and Migration of Rheumatoid Arthritis Fibroblast-like Synoviocytes via the PI3K/AKT/NF-κB Pathway. Phytomedicine 2023, 109, 154562. [Google Scholar] [CrossRef]
- Chen, X.; Shen, J.; Zhao, J.; Guan, J.; Li, W.; Xie, Q.; Zhao, Y. Cedrol Attenuates Collagen-Induced Arthritis in Mice and Modulates the Inflammatory Response in LPS-Mediated Fibroblast-like Synoviocytes. Food Funct. 2020, 11, 4752–4764. [Google Scholar] [CrossRef]
- Kim, H.T.; Lo, M.Y.; Pillarisetty, R. Chondrocyte Apoptosis Following Intraarticular Fracture in Humans. Osteoarthr. Cartil. 2002, 10, 747–749. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Bahrampour Juybari, K.; Fatemi, M.J.; Kamarul, T.; Bagheri, A.; Tekiyehmaroof, N.; Sharifi, A.M. Protective Effect of Ginger (Zingiber officinale Roscoe) Extract against Oxidative Stress and Mitochondrial Apoptosis Induced by Interleukin-1β in Cultured Chondrocytes. Cells Tissues Organs 2017, 204, 241–250. [Google Scholar] [CrossRef]
- Shen, C.-L.; Hong, K.-J.; Kim, S.W. Comparative Effects of Ginger Root (Zingiber officinale Rosc.) on the Production of Inflammatory Mediators in Normal and Osteoarthrotic Sow Chondrocytes. J. Med. Food 2005, 8, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Villalvilla, A.; Da Silva, J.A.; Largo, R.; Gualillo, O.; Vieira, P.C.; Herrero-Beaumont, G.; Gómez, R. 6-Shogaol Inhibits Chondrocytes’ Innate Immune Responses and Cathepsin- K Activity. Mol. Nutr. Food Res 2014, 58, 256–266. [Google Scholar] [CrossRef]
- Öz, B.; Orhan, C.; Tuzcu, M.; Şahin, N.; Özercan, İ.H.; Öner, P.D.; Koca, S.S.; Juturu, V.; Şahin, K. Ginger Extract Suppresses the Activations of NF-κB and Wnt Pathways and Protects Inflammatory Arthritis. Eur. J. Rheumatol. 2021, 8, 196–201. [Google Scholar] [CrossRef]
- Hwang, J.H.; Jung, H.W.; Oh, S.Y.; Kang, J.-S.; Kim, J.-P.; Park, Y.-K. Effects of Zingiber officinale Extract on Collagen-Induced Arthritis in Mice and IL-1β-Induced Inflammation in Human Synovial Fibroblasts. Eur. J. Inflamm. 2017, 15, 168–178. [Google Scholar] [CrossRef]
- Fouda, A.M.; Berika, M.Y. Evaluation of the Effect of Hydroalcoholic Extract of Zingiber officinale Rhizomes in Rat Collagen-induced Arthritis. Basic Clin. Pharma. Tox. 2009, 104, 262–271. [Google Scholar] [CrossRef]
- Funk, J.L.; Frye, J.B.; Oyarzo, J.N.; Timmermann, B.N. Comparative Effects of Two Gingerol-Containing Zingiber officinale Extracts on Experimental Rheumatoid Arthritis. J. Nat. Prod. 2009, 72, 403–407. [Google Scholar] [CrossRef]
- Funk, J.L.; Frye, J.B.; Oyarzo, J.N.; Chen, J.; Zhang, H.; Timmermann, B.N. Anti-Inflammatory Effects of the Essential Oils of Ginger (Zingiber officinale Roscoe) in Experimental Rheumatoid Arthritis. PharmaNutrition 2016, 4, 123–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, J.; Zhao, J.; Guan, J.; Wei, X.; Miao, D.; Li, W.; Xie, Y.; Zhao, Y. Cedrol from Ginger Ameliorates Rheumatoid Arthritis via Reducing Inflammation and Selectively Inhibiting JAK3 Phosphorylation. J. Agric. Food Chem. 2021, 69, 5332–5343. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.S.; Simon, O.; Shelly, J.; Gardener, M. 6-Shogaol Reduced Chronic Inflammatory Response in the Knees of Rats Treated with Complete Freund’s Adjuvant. BMC Pharmacol. 2006, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Niempoog, S.; Pawa, K.K.; Amatyakul, C. The Efficacy of Powdered Ginger in Osteoarthritis of the Knee. J. Med. Assoc. Thai 2012, 95, 59–64. [Google Scholar]
- Aryaeian, N.; Mahmoudi, M.; Shahram, F.; Poursani, S.; Jamshidi, F.; Tavakoli, H. The Effect of Ginger Supplementation on IL2, TNFα, and IL1β Cytokines Gene Expression Levels in Patients with Active Rheumatoid Arthritis: A Randomized Controlled Trial. Med. J. Islam. Repub. Iran 2019, 33, 154. [Google Scholar] [CrossRef] [PubMed]
- Aryaeian, N.; Shahram, F.; Mahmoudi, M.; Tavakoli, H.; Yousefi, B.; Arablou, T.; Jafari Karegar, S. The Effect of Ginger Supplementation on Some Immunity and Inflammation Intermediate Genes Expression in Patients with Active Rheumatoid Arthritis. Gene 2019, 698, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari-Khosravi, H.; Naderi, Z.; Dehghan, A.; Nadjarzadeh, A.; Fallah Huseini, H. Effect of Ginger Supplementation on Proinflammatory Cytokines in Older Patients with Osteoarthritis: Outcomes of a Randomized Controlled Clinical Trial. J. Nutr. Gerontol. Geriatr. 2016, 35, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Naderi, Z.; Mozaffari-Khosravi, H.; Dehghan, A.; Nadjarzadeh, A.; Huseini, H.F. Effect of Ginger Powder Supplementation on Nitric Oxide and C-Reactive Protein in Elderly Knee Osteoarthritis Patients: A 12-Week Double-Blind Randomized Placebo-Controlled Clinical Trial. J. Tradit. Complement. Med. 2016, 6, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.C.; Mustafa, T. Ginger (Zingiber officinale) in Rheumatism and Musculoskeletal Disorders. Med. Hypotheses 1992, 39, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, Z.; Izadi, S.; Bari, Z.; Soltani, F.; Narouie, B.; Ghasemi-rad, M. Evaluating the Effects of Ginger Extract on Knee Pain, Stiffness and Difficulty in Patients with Knee Osteoarthritis. J. Med. Plants Res. 2011, 5, 3375–3379. [Google Scholar]
- Kordi Yoosefinejad, A.; Zarshenas, M.M.; Sadeghi Mazidi, J.; Tahmasbi, M. Phonophoresis of Semisolid Formulation of Zingiber officinale Roscoe Hydroalcoholic Extract Improves Quality of Life in Patients with Moderate Knee Osteoarthritis: A Randomized Clinical Trial. J. Herb. Med. 2021, 29, 100464. [Google Scholar] [CrossRef]
- Nagy, G.; Roodenrijs, N.M.T.; Welsing, P.M.J.; Kedves, M.; Hamar, A.; Van Der Goes, M.C.; Kent, A.; Bakkers, M.; Pchelnikova, P.; Blaas, E.; et al. EULAR Points to Consider for the Management of Difficult-to-Treat Rheumatoid Arthritis. Ann. Rheum. Dis. 2022, 81, 20–33. [Google Scholar] [CrossRef]
- Fuggle, N.; Bere, N.; Bruyère, O.; Rosa, M.M.; Prieto Yerro, M.C.; Dennison, E.; Dincer, F.; Gabay, C.; Haugen, I.K.; Herrero-Beaumont, G.; et al. Management of Hand Osteoarthritis: From an US Evidence-Based Medicine Guideline to a European Patient-Centric Approach. Aging Clin. Exp. Res. 2022, 34, 1985–1995. [Google Scholar] [CrossRef]
- Hunter, C.W.; Deer, T.R.; Jones, M.R.; Chiang Chien, G.; D’Souza, R.S.; Davis, T.; Eldon, E.R.; Esposito, M.F.; Goree, J.H.; Hewan-Lowe, L.; et al. Consensus Guidelines on Interventional Therapies for Knee Pain (STEP Guidelines) from the American Society of Pain and Neuroscience. J. Pain Res. 2022, 15, 2683–2745. [Google Scholar] [CrossRef]
- Sabha, M.; Hochberg, M.C. Non-Surgical Management of Hip and Knee Osteoarthritis; Comparison of ACR/AF and OARSI 2019 and VA/DoD 2020 Guidelines. Osteoarthr. Cartil. Open 2022, 4, 100232. [Google Scholar] [CrossRef]
- Altman, R.D.; Marcussen, K.C. Effects of a Ginger Extract on Knee Pain in Patients with Osteoarthritis. Arthritis Rheum. 2001, 44, 2531–2538. [Google Scholar] [CrossRef]
- Bliddal, H.; Rosetzsky, A.; Schlichting, P.; Weidner, M.S.; Andersen, L.A.; Ibfelt, H.-H.; Christensen, K.; Jensen, O.N.; Barslev, J. A Randomized, Placebo-Controlled, Cross-over Study of Ginger Extracts and Ibuprofen in Osteoarthritis. Osteoarthr. Cartil. 2000, 8, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Heidari-Beni, M.; Moravejolahkami, A.R.; Gorgian, P.; Askari, G.; Tarrahi, M.J.; Bahreini-Esfahani, N. Herbal Formulation “Turmeric Extract, Black Pepper, and Ginger” versus Naproxen for Chronic Knee Osteoarthritis: A Randomized, Double-blind, Controlled Clinical Trial. Phytother. Res. 2020, 34, 2067–2073. [Google Scholar] [CrossRef]
- Niempoog, S.; Siriarchavatana, P.; Kajsongkram, T. The Efficacy of Plygersic Gel for Use in the Treatment of Osteoarthritis of the Knee. J. Med. Assoc. Thai 2012, 95, 113–119. [Google Scholar]
- Zahmatkash, M.; Vafaeenasa, M.R. Comparing Analgesic Effects of a Topical Herbal Mixed Medicine with Salicylate in Patients with Knee Osteoarthritis. Pak. J. Biol. Sci. 2011, 14, 715–719. [Google Scholar] [CrossRef]
- Amorndoljai, P.; Taneepanichskul, S.; Niempoog, S.; Nimmannit, U. A Comparative of Ginger Extract in Nanostructure Lipid Carrier (NLC) and 1% Diclofenac Gel for Treatment of Knee Osteoarthritis (OA). J. Med. Assoc. Thai 2017, 100, 447–456. [Google Scholar] [PubMed]
- Zhang, Y. Studies on Compositions of Ginger Oleoresin by Su-Percritical CO2 Extraction Compare with Ultrasonic Solvent Extraction. Acad. J. Eng. Technol. Sci. 2021, 4, 22–25. [Google Scholar] [CrossRef]
- Bhattarai, S.; Tran, V.H.; Duke, C.C. Stability of [6]-Gingerol and [6]-Shogaol in Simulated Gastric and Intestinal Fluids. J. Pharm. Biomed. Anal. 2007, 45, 648–653. [Google Scholar] [CrossRef]
- Lete, I.; Alluέ, J. The Effectiveness of Ginger in the Prevention of Nausea and Vomiting during Pregnancy and Chemotherapy. Integr. Med. Insights 2016, 11, 36273. [Google Scholar] [CrossRef]
- Atencio, S.; Maestro, A.; Santamaría, E.; Gutiérrez, J.M.; González, C. Encapsulation of Ginger Oil in Alginate-Based Shell Materials. Food Biosci. 2020, 37, 100714. [Google Scholar] [CrossRef]
- Maleki, H.; Azadi, H.; Yousefpoor, Y.; Doostan, M.; Doostan, M.; Farzaei, M.H. Encapsulation of Ginger Extract in Nanoemulsions: Preparation, Characterization and In Vivo Evaluation in Rheumatoid Arthritis. J. Pharm. Sci. 2023, 112, 1687–1697. [Google Scholar] [CrossRef]
- Amit, C.; Kant, A.R.K.; Raj, P.G.; Bhawna, T. Formulation and Evaluation of Ginger Extract Loaded Nanoemulgel for the Treatment of Rheumatoid Arthritis. J. Drug Deliv. Ther. 2019, 9, 559–570. [Google Scholar] [CrossRef]
- Attri, D.S.; Rathour, A.; Ray, R.K.; Kumar, V. Formulation and Evaluation of Hydrogel for Topical Drug Delivery of Zingiber officinale Rosc. and Withania somnifera (L.) Dunal to Increase the Bioavailability of Oils for the Treatment of Arthritis. Ann. Phytomed. 2023, 12, 1–10. [Google Scholar] [CrossRef]
- Kravchenko, I.; Eberle, L.; Nesterkina, M.; Kobernik, A. Anti-Inflammatory and Analgesic Activity of Ointment Based on Dense Ginger Extract (Zingiber officinale). J. Herbmed. Pharmacol. 2019, 8, 126–132. [Google Scholar] [CrossRef]
- Londhe, V.Y.; Khogta, S.M.; Barve, K.H. Improved Anti-Arthritic Activity of Ginger Extract, a Traditional Medicine, Using Novel Drug Delivery Approach. J. Complement. Integr. Med. 2021, 18, 439–443. [Google Scholar] [CrossRef]



| Type of Extract/Active Compound | Dose | Route of Administration | Animal Model | Results | Ref. |
|---|---|---|---|---|---|
| Ginger extract (Gingever) | 50 mg/kg | Orally for 32 days | Rats with collagen-induced arthritis | ↓serum and tissue levels of TNF-α, IL-6, and IL-17; ↓synthesis of COX-2 and NF-κB in articular tissues; ↓peri-synovial inflammation, synovial hyperplasia, and cartilage destruction | [82] |
| Aqueous ginger extract | 100, 200 mg/kg | Orally for 14 days | Mice with collagen-induced arthritis | ↓concentration of IL-4, IL-17, and IFN-γ in mice serum; suppression of MMP-1, MMP-3, and MMP-13 expression in mouse paw tissues | [83] |
| Hydroalcoholic ginger extract | 50, 100, 200 mg/kg | i.p. for 25 days (from day 7 to day 32) | Rats with collagen-induced arthritis | Doses >50 mg/kg caused suppression of IL-2, IL-6, IL-1β, and TNF-α cytokines more effectively than 2 mg/kg of indometacin; a dose of 200 mg/kg was more effective in lowering the clinical, histological, and immunological parameters of rat joint inflammation than 2 mg/kg of indomethacin | [84] |
| Crude dichloromethane extract and a fraction with gingerols and their derivatives | 0.5–1 µL/g DMSO | i.p. (1) 4 days prior to arthritis induction and continuing until day 14 and then twice weekly; (2) from day 3 daily until day 10 and then twice weekly | Rats with streptococcal cell wall (SCW)-induced arthritis | Only the DCM extract inhibited joint destruction; the DCM extract was slightly more effective in reducing joint swelling than the gingerols-rich extract | [85] |
| Dichloromethane extract rich in sesquiterpenoids that are free of gingerols | 1 µL/g DMSO | i.p. 4 days prior to arthritis induction and continuing until day 14 and then five days per week | Rats with streptococcal cell wall (SCW)-induced arthritis | No effect on acute joint swelling (measured on third day), but it suppressed joint swelling during the chronic phase of arthritis (days 13–28); the extract did not affect the number of leukocytes, neutrophils, monocytes, and lymphocytes | [86] |
| Cedrol | 20, 40, 80 mg/kg | orally every 3 days from day 15 to day 27 | Rats with adjuvant-induced arthritis | Amelioration in paw edema volume and arthritis score; ↓levels of neutrophils, TNF-a, and IL-1β; inhibition of T-cell and B-cell activation; a higher dosage of 80 mg/kg diminished proliferation of the above cells in synovial tissue, inflammatory cell infiltration, and cartilage damage | [87] |
| 6-shogaol | 30 mg/kg, 60 mg/kg | i.p. for 21 days | Mice with collagen-induced arthritis | Reduction in joint inflammation, cartilage deterioration, and bone loss | [76] |
| 6-shogaol | 6.2 mg/kg | Orally for 28 days | Rats with adjuvant-induced arthritis | Reduction in knee edema after 28 days; ↓concentration of VCAM-1 and infiltration of leukocytes into the knee’s synovial cavity; the femurs of rats had well-preserved and morphologically intact cartilage lining | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymczak, J.; Grygiel-Górniak, B.; Cielecka-Piontek, J. Zingiber Officinale Roscoe: The Antiarthritic Potential of a Popular Spice—Preclinical and Clinical Evidence. Nutrients 2024, 16, 741. https://doi.org/10.3390/nu16050741
Szymczak J, Grygiel-Górniak B, Cielecka-Piontek J. Zingiber Officinale Roscoe: The Antiarthritic Potential of a Popular Spice—Preclinical and Clinical Evidence. Nutrients. 2024; 16(5):741. https://doi.org/10.3390/nu16050741
Chicago/Turabian StyleSzymczak, Joanna, Bogna Grygiel-Górniak, and Judyta Cielecka-Piontek. 2024. "Zingiber Officinale Roscoe: The Antiarthritic Potential of a Popular Spice—Preclinical and Clinical Evidence" Nutrients 16, no. 5: 741. https://doi.org/10.3390/nu16050741