Neurotoxins Acting at Synaptic Sites: A Brief Review on Mechanisms and Clinical Applications
Abstract
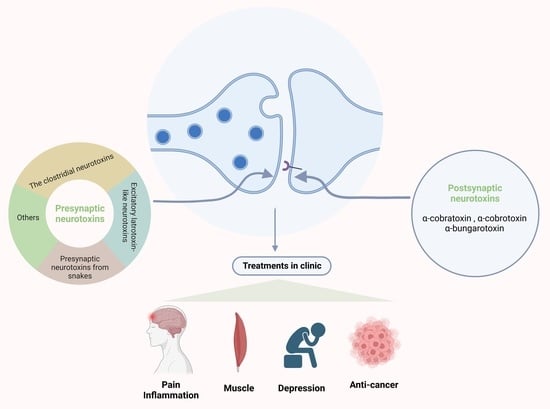
:1. Introduction
2. Presynaptic Neurotoxins
2.1. The Clostridial Neurotoxins
2.2. Excitatory Latrotoxin-like Neurotoxins
2.3. Presynaptic Neurotoxins from Snakes
2.4. Other Presynaptic Neurotoxins Acting on Ion Channels
3. Postsynaptic Neurotoxins
4. Clinical Applications of Neurotoxins
4.1. Clostridial Neurotoxins
4.1.1. Dystonic Muscle Contractions
4.1.2. Skin Diseases
4.1.3. Neuropathic Pain and Neuroinflammation
4.1.4. Depression
4.1.5. Headache
4.2. LaTXs
4.3. Snake Presynaptic Neurotoxins
4.3.1. Anticancer
4.3.2. Antibacterial
4.4. Other Presynaptic Neurotoxins Acting on Ion Channels
4.4.1. Analgesia
4.4.2. Neuroprotection
4.5. Postsynaptic Neurotoxins
4.5.1. Anticancer
4.5.2. Analgesia and Anti-Inflammation
5. Summary
5.1. Clinical Applications of FDA-Approved Neurotoxins
5.2. Neurotoxins in Preclinical Studies
5.3. Expectations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schiavo, G.; Matteoli, M.; Montecucco, C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 2000, 80, 717–766. [Google Scholar] [CrossRef] [Green Version]
- Ovsepian, S.V.; O’Leary, V.B.; Ayvazyan, N.M.; Al-Sabi, A.; Ntziachristos, V.; Dolly, J.O. Neurobiology and therapeutic applications of neurotoxins targeting transmitter release. Pharmacol. Ther. 2019, 193, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Hughes, B.W.; Kusner, L.L.; Kaminski, H.J. Molecular architecture of the neuromuscular junction. Muscle Nerve 2006, 33, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Ayvazyan, N.M.; O’Leary, V.B.; Dolly, J.O.; Ovsepian, S.V. Neurobiology and therapeutic utility of neurotoxins targeting postsynaptic mechanisms of neuromuscular transmission. Drug Discov. Today 2019, 24, 1968–1984. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Few, A.P. Calcium channel regulation and presynaptic plasticity. Neuron 2008, 59, 882–901. [Google Scholar] [CrossRef] [Green Version]
- Jensen, A.A.; Frølund, B.; Liljefors, T.; Krogsgaard-Larsen, P. Neuronal nicotinic acetylcholine receptors: Structural revelations, target identifications, and therapeutic inspirations. J. Med. Chem. 2005, 48, 4705–4745. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, E.X.; Pereira, E.F.R.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef] [Green Version]
- Wonnacott, S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997, 20, 92–98. [Google Scholar] [CrossRef]
- Chen, J.; Cheuk, I.W.Y.; Shin, V.Y.; Kwong, A. Acetylcholine receptors: Key players in cancer development. Surg. Oncol. 2019, 31, 46–53. [Google Scholar] [CrossRef]
- Schuller, H.M. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat. Rev. Cancer 2009, 9, 195–205. [Google Scholar] [CrossRef]
- Rossetto, O.; Rigoni, M.; Montecucco, C. Different mechanism of blockade of neuroexocytosis by presynaptic neurotoxins. Toxicol. Lett. 2004, 149, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Atlas, D. The voltage-gated calcium channel functions as the molecular switch of synaptic transmission. Annu. Rev. Biochem. 2013, 82, 607–635. [Google Scholar] [CrossRef] [PubMed]
- Tsetlin, V.I. Three-finger snake neurotoxins and Ly6 proteins targeting nicotinic acetylcholine receptors: Pharmacological tools and endogenous modulators. Trends Pharmacol. Sci. 2015, 36, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Dutertre, S.; Nicke, A.; Tsetlin, V.I. Nicotinic acetylcholine receptor inhibitors derived from snake and snail venoms. Neuropharmacology 2017, 127, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Barber, C.M.; Isbister, G.K.; Hodgson, W.C. Alpha neurotoxins. Toxicon Off. J. Int. Soc. Toxinology 2013, 66, 47–58. [Google Scholar] [CrossRef]
- Nirthanan, S. Snake three-finger α-neurotoxins and nicotinic acetylcholine receptors: Molecules, mechanisms and medicine. Biochem. Pharmacol. 2020, 181, 114168. [Google Scholar] [CrossRef]
- Lalli, G.; Bohnert, S.; Deinhardt, K.; Verastegui, C.; Schiavo, G. The journey of tetanus and botulinum neurotoxins in neurons. Trends Microbiol. 2003, 11, 431–437. [Google Scholar] [CrossRef]
- Dong, M.; Masuyer, G.; Stenmark, P. Botulinum and Tetanus Neurotoxins. Annu. Rev. Biochem. 2019, 88, 811–837. [Google Scholar] [CrossRef]
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum Neurotoxins: Biology, Pharmacology, and Toxicology. Pharmacol. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef] [Green Version]
- Montal, M. Translocation of botulinum neurotoxin light chain protease by the heavy chain protein-conducting channel. Toxicon Off. J. Int. Soc. Toxinology 2009, 54, 565–569. [Google Scholar] [CrossRef]
- Rossetto, O.; Pirazzini, M.; Montecucco, C. Botulinum neurotoxins: Genetic, structural and mechanistic insights. Nat. Rev. Microbiol. 2014, 12, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gardberg, A.S.; Edwards, T.E.; Sankaran, B.; Robinson, H.; Varnum, S.M.; Buchko, G.W. Structural insights into the functional role of the Hcn sub-domain of the receptor-binding domain of the botulinum neurotoxin mosaic serotype C/D. Biochimie 2013, 95, 1379–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, A.; Montal, M. Molecular dissection of botulinum neurotoxin reveals interdomain chaperone function. Toxicon Off. J. Int. Soc. Toxinology 2013, 75, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiavo, G.; Poulain, B.; Rossetto, O.; Benfenati, F.; Tauc, L.; Montecucco, C. Tetanus toxin is a zinc protein and its inhibition of neurotransmitter release and protease activity depend on zinc. EMBO J. 1992, 11, 3577–3583. [Google Scholar] [CrossRef] [PubMed]
- Breidenbach, M.A.; Brunger, A.T. Substrate recognition strategy for botulinum neurotoxin serotype A. Nature 2004, 432, 925–929. [Google Scholar] [CrossRef]
- Montal, M. Botulinum neurotoxin: A marvel of protein design. Annu. Rev. Biochem. 2010, 79, 591–617. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Zhou, P.; Wang, A.L.; Wu, D.; Zhao, M.; Südhof, T.C.; Brunger, A.T. The primed SNARE-complexin-synaptotagmin complex for neuronal exocytosis. Nature 2017, 548, 420–425. [Google Scholar] [CrossRef] [Green Version]
- Südhof, T.C.; Rothman, J.E. Membrane fusion: Grappling with SNARE and SM proteins. Science 2009, 323, 474–477. [Google Scholar] [CrossRef] [Green Version]
- Jahn, R.; Scheller, R.H. SNAREs--Engines for membrane fusion. Nat Rev Mol Cell Biol 2006, 7, 631–643. [Google Scholar] [CrossRef]
- Popoff, M.R.; Poulain, B. Bacterial toxins and the nervous system: Neurotoxins and multipotential toxins interacting with neuronal cells. Toxins 2010, 2, 683–737. [Google Scholar] [CrossRef]
- Poulain, B.; Popoff, M.R. Why Are Botulinum Neurotoxin-Producing Bacteria So Diverse and Botulinum Neurotoxins So Toxic? Toxins 2019, 11, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulain, B.; Lemichez, E.; Popoff, M.R. Neuronal selectivity of botulinum neurotoxins. Toxicon Off. J. Int. Soc. Toxinology 2020, 178, 20–32. [Google Scholar] [CrossRef]
- Luvisetto, S. Botulinum Neurotoxins beyond Neurons: Interplay with Glial Cells. Toxins 2022, 14, 704. [Google Scholar] [CrossRef]
- Zhang, S.; Masuyer, G.; Zhang, J.; Shen, Y.; Lundin, D.; Henriksson, L.; Miyashita, S.-I.; Martínez-Carranza, M.; Dong, M.; Stenmark, P. Identification and characterization of a novel botulinum neurotoxin. Nat. Commun. 2017, 8, 14130. [Google Scholar] [CrossRef] [PubMed]
- Simpson, L.L. Identification of the major steps in botulinum toxin action. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 167–193. [Google Scholar] [CrossRef]
- Peck, M.W.; Smith, T.J.; Anniballi, F.; Austin, J.W.; Bano, L.; Bradshaw, M.; Cuervo, P.; Cheng, L.W.; Derman, Y.; Dorner, B.G.; et al. Historical Perspectives and Guidelines for Botulinum Neurotoxin Subtype Nomenclature. Toxins 2017, 9, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgen, A.S.V.; Dickens, F.; Zatman, L.J. The action of botulinum toxin on the neuro-muscular junction. J. Physiol. 1949, 109, 10–24. [Google Scholar] [CrossRef]
- Van der Kloot, W.; Molgó, J. Quantal acetylcholine release at the vertebrate neuromuscular junction. Physiol. Rev. 1994, 74, 899–991. [Google Scholar] [CrossRef]
- Poulain, B.; Molgó, J.; Thesleff, S. Quantal neurotransmitter release and the clostridial neurotoxins’ targets. Curr. Top. Microbiol. Immunol. 1995, 195, 243–255. [Google Scholar]
- Cook, T.M.; Protheroe, R.T.; Handel, J.M. Tetanus: A review of the literature. Br. J. Anaesth. 2001, 87, 477–487. [Google Scholar] [CrossRef] [Green Version]
- Megighian, A.; Pirazzini, M.; Fabris, F.; Rossetto, O.; Montecucco, C. Tetanus and tetanus neurotoxin: From peripheral uptake to central nervous tissue targets. J. Neurochem. 2021, 158, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Brooks, V.B.; Curtis, D.R.; Eccles, J.C. Mode of action of tetanus toxin. Nature 1955, 175, 120–121. [Google Scholar] [CrossRef] [PubMed]
- Yen, L.M.; Thwaites, C.L. Tetanus. Lancet 2019, 393, 1657–1668. [Google Scholar] [CrossRef]
- Alfery, D.D.; Rauscher, L.A. Tetanus: A review. Crit. Care Med. 1979, 7, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Pellizzari, R.; Rossetto, O.; Schiavo, G.; Montecucco, C. Tetanus and botulinum neurotoxins: Mechanism of action and therapeutic uses. Philos. Trans. R Soc. Lond. B Biol. Sci. 1999, 354, 259–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, M.; Stenmark, P. The Structure and Classification of Botulinum Toxins. Handb. Exp. Pharmacol. 2021, 263, 11–33. [Google Scholar] [CrossRef]
- Tzeng, M.C.; Siekevitz, P. The effect of the purified major protein factor (alpha-latrotoxin) of black widow spider venom on the release of acetylcholine and norepinephrine from mouse cerebral cortex slices. Brain Res. 1978, 139, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Grishin, E.V. Black widow spider toxins: The present and the future. Toxicon Off. J. Int. Soc. Toxinology 1998, 36, 1693–1701. [Google Scholar] [CrossRef]
- Krasnoperov, V.G.; Shamotienko, O.G.; Grishin, E.V. A crustacean-specific neurotoxin from the venom of the black widow spider Latrodectus mactans tredecimguttatus. Bioorg. Khim. 1990, 16, 1567–1569. [Google Scholar]
- Müller, G.J. Black and brown widow spider bites in South Africa. A series of 45 cases. S. Afr. Med. J. 1993, 83, 399–405. [Google Scholar]
- Zukowski, C.W. Black widow spider bite. J. Am. Board Fam. Pract. 1993, 6, 279–281. [Google Scholar] [PubMed]
- Petrenko, A.G.; Kovalenko, V.A.; Shamotienko, O.G.; Surkova, I.N.; Tarasyuk, T.A.; Ushkaryov Yu, A.; Grishin, E.V. Isolation and properties of the alpha-latrotoxin receptor. EMBO J. 1990, 9, 2023–2027. [Google Scholar] [CrossRef] [PubMed]
- Ushkaryov, Y.A.; Petrenko, A.G.; Geppert, M.; Südhof, T.C. Neurexins: Synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science 1992, 257, 50–56. [Google Scholar] [CrossRef]
- Davletov, B.A.; Krasnoperov, V.; Hata, Y.; Petrenko, A.G.; Südhof, T.C. High affinity binding of alpha-latrotoxin to recombinant neurexin I alpha. J. Biol. Chem. 1995, 270, 23903–23905. [Google Scholar] [CrossRef] [Green Version]
- Davletov, B.A.; Shamotienko, O.G.; Lelianova, V.G.; Grishin, E.V.; Ushkaryov, Y.A. Isolation and biochemical characterization of a Ca2+-independent alpha-latrotoxin-binding protein. J. Biol. Chem. 1996, 271, 23239–23245. [Google Scholar] [CrossRef] [Green Version]
- Krasnoperov, V.G.; Beavis, R.; Chepurny, O.G.; Little, A.R.; Plotnikov, A.N.; Petrenko, A.G. The calcium-independent receptor of alpha-latrotoxin is not a neurexin. Biochem. Biophys. Res. Commun. 1996, 227, 868–875. [Google Scholar] [CrossRef]
- Krasnoperov, V.; Bittner, M.A.; Mo, W.; Buryanovsky, L.; Neubert, T.A.; Holz, R.W.; Ichtchenko, K.; Petrenko, A.G. Protein-tyrosine phosphatase-sigma is a novel member of the functional family of alpha-latrotoxin receptors. J. Biol. Chem. 2002, 277, 35887–35895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMahon, H.T.; Rosenthal, L.; Meldolesi, J.; Nicholls, D.G. Alpha-latrotoxin releases both vesicular and cytoplasmic glutamate from isolated nerve terminals. J. Neurochem. 1990, 55, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, L.; Zacchetti, D.; Madeddu, L.; Meldolesi, J. Mode of action of alpha-latrotoxin: Role of divalent cations in Ca2(+)-dependent and Ca2(+)-independent effects mediated by the toxin. Mol. Pharmacol. 1990, 38, 917–923. [Google Scholar] [PubMed]
- Ushkaryov, Y.A.; Rohou, A.; Sugita, S. α-Latrotoxin and its receptors. In Pharmacology of Neurotransmitter Release; Handbook of Experimental Pharmacology book series; Springer: Berlin/Heidelberg, Germany, 2008; pp. 171–206. [Google Scholar]
- Grasso, A.; Alemà, S.; Rufini, S.; Senni, M.I. Black widow spider toxin-induced calcium fluxes and transmitter release in a neurosecretory cell line. Nature 1980, 283, 774–776. [Google Scholar] [CrossRef]
- Ashton, A.C.; Volynski, K.E.; Lelianova, V.G.; Orlova, E.V.; Van Renterghem, C.; Canepari, M.; Seagar, M.; Ushkaryov, Y.A. α-Latrotoxin, acting via two Ca2+-dependent pathways, triggers exocytosis of two pools of synaptic vesicles. J. Biol. Chem. 2001, 276, 44695–44703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.; Hodgson, W.C.; Isbister, G.K. Antivenom for Neuromuscular Paralysis Resulting from Snake Envenoming. Toxins 2017, 9, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.; Kuruppu, S.; Othman, I.; Goode, R.J.A.; Hodgson, W.C.; Isbister, G.K. Neurotoxicity in Sri Lankan Russell’s Viper (Daboia russelii) Envenoming is Primarily due to U1-viperitoxin-Dr1a, a Pre-Synaptic Neurotoxin. Neurotox. Res. 2017, 31, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, C.; Rossetto, O. How do presynaptic PLA2 neurotoxins block nerve terminals? Trends Biochem. Sci. 2000, 25, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Carredano, E.; Westerlund, B.; Persson, B.; Saarinen, M.; Ramaswamy, S.; Eaker, D.; Eklund, H. The three-dimensional structures of two toxins from snake venom throw light on the anticoagulant and neurotoxic sites of phospholipase A2. Toxicon Off. J. Int. Soc. Toxinology 1998, 36, 75–92. [Google Scholar] [CrossRef]
- Heinrikson, R.L.; Krueger, E.T.; Keim, P.S. Amino acid sequence of phospholipase A2-alpha from the venom of Crotalus adamanteus. A new classification of phospholipases A2 based upon structural determinants. J. Biol. Chem. 1977, 252, 4913–4921. [Google Scholar] [CrossRef]
- Andrião-Escarso, S.H.; Soares, A.M.; Rodrigues, V.M.; Angulo, Y.; Díaz, C.; Lomonte, B.; Gutiérrez, J.M.; Giglio, J.R. Myotoxic phospholipases A(2) in bothrops snake venoms: Effect of chemical modifications on the enzymatic and pharmacological properties of bothropstoxins from Bothrops jararacussu. Biochimie 2000, 82, 755–763. [Google Scholar] [CrossRef]
- Barbosa, P.S.F.; Martins, A.M.C.; Havt, A.; Toyama, D.O.; Evangelista, J.S.A.M.; Ferreira, D.P.P.; Joazeiro, P.P.; Beriam, L.O.S.; Toyama, M.H.; Fonteles, M.C.; et al. Renal and antibacterial effects induced by myotoxin I and II isolated from Bothrops jararacussu venom. Toxicon Off. J. Int. Soc. Toxinology 2005, 46, 376–386. [Google Scholar] [CrossRef]
- Costa Torres, A.F.; Dantas, R.T.; Toyama, M.H.; Diz Filho, E.; Zara, F.J.; Rodrigues de Queiroz, M.G.; Pinto Nogueira, N.A.; Rosa de Oliveira, M.; de Oliveira Toyama, D.; Monteiro, H.S.A.; et al. Antibacterial and antiparasitic effects of Bothrops marajoensis venom and its fractions: Phospholipase A2 and L-amino acid oxidase. Toxicon Off. J. Int. Soc. Toxinology 2010, 55, 795–804. [Google Scholar] [CrossRef]
- Evangelista, I.L.; Martins, A.M.C.; Nascimento, N.R.F.; Havt, A.; Evangelista, J.S.A.M.; de Norões, T.B.S.; Toyama, M.H.; Diz-Filho, E.B.; Toyama, D.d.O.; Fonteles, M.C.; et al. Renal and cardiovascular effects of Bothrops marajoensis venom and phospholipase A2. Toxicon Off. J. Int. Soc. Toxinology 2010, 55, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.B.; Grubb, B.D.; Maltin, C.A.; Dixon, R. The neurotoxicity of the venom phospholipases A(2), notexin and taipoxin. Exp. Neurol. 2000, 161, 517–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kini, R.M.; Evans, H.J. Structure-function relationships of phospholipases. The anticoagulant region of phospholipases A2. J. Biol. Chem. 1987, 262, 14402–14407. [Google Scholar] [CrossRef] [PubMed]
- Landucci, E.C.; de Castro, R.C.; Toyama, M.; Giglio, J.R.; Marangoni, S.; De Nucci, G.; Antunes, E. Inflammatory oedema induced by the lys-49 phospholipase A(2) homologue piratoxin-i in the rat and rabbit. Effect of polyanions and p-bromophenacyl bromide. Biochem. Pharmacol. 2000, 59, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Jacob-Ferreira, A.L.; Menaldo, D.L.; Sartim, M.A.; Riul, T.B.; Dias-Baruffi, M.; Sampaio, S.V. Antithrombotic activity of Batroxase, a metalloprotease from Bothrops atrox venom, in a model of venous thrombosis. Int. J. Biol. Macromol. 2017, 95, 263–267. [Google Scholar] [CrossRef]
- Hiu, J.J.; Yap, M.K.K. Cytotoxicity of snake venom enzymatic toxins: Phospholipase A2 and l-amino acid oxidase. Biochem. Soc. Trans. 2020, 48, 719–731. [Google Scholar] [CrossRef] [Green Version]
- Dixon, R.W.; Harris, J.B. Nerve terminal damage by beta-bungarotoxin: Its clinical significance. Am. J. Pathol. 1999, 154, 447–455. [Google Scholar] [CrossRef]
- Gopalakrishnakone, P.; Hawgood, B.J. Morphological changes induced by crotoxin in murine nerve and neuromuscular junction. Toxicon Off. J. Int. Soc. Toxinology 1984, 22, 791–804. [Google Scholar] [CrossRef]
- Cull-Candy, S.G.; Fohlman, J.; Gustavsson, D.; Lüllmann-Rauch, R.; Thesleff, S. The effects of taipoxin and notexin on the function and fine structure of the murine neuromuscular junction. Neuroscience 1976, 1, 175–180. [Google Scholar] [CrossRef]
- Chen, I.L.; Lee, C.Y. Ultrastructural changes in the motor nerve terminals caused by beta-bungarotoxin. Virchows Arch. B Cell Pathol 1970, 6, 318–325. [Google Scholar] [CrossRef]
- Lee, C.Y.; Tsai, M.C.; Chen, Y.M.; Ritonja, A.; Gubensek, F. Mode of neuromuscular blocking action of toxic phospholipases A2 from Vipera ammodytes venom. Arch. Int. Pharmacodyn. Ther. 1984, 268, 313–324. [Google Scholar]
- Belo, C.A.D.; Leite, G.B.; Toyama, M.H.; Marangoni, S.; Corrado, A.P.; Fontana, M.D.; Southan, A.; Rowan, E.G.; Hyslop, S.; Rodrigues-Simioni, L. Pharmacological and structural characterization of a novel phospholipase A2 from Micrurus dumerilii carinicauda venom. Toxicon Off. J. Int. Soc. Toxinology 2005, 46, 736–750. [Google Scholar] [CrossRef] [PubMed]
- Belo, C.A.D.; Toyama, M.H.; Toyama, D.d.O.; Marangoni, S.; Moreno, F.B.; Cavada, B.S.; Fontana, M.D.; Hyslop, S.; Carneiro, E.M.; Boschero, A.C. Determination of the amino acid sequence of a new phospholipase A(2) (MIDCA1) isolated from Micrurus dumerilii carinicauda venom. Protein J. 2005, 24, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Schütter, N.; Barreto, Y.C.; Vardanyan, V.; Hornig, S.; Hyslop, S.; Marangoni, S.; Rodrigues-Simioni, L.; Pongs, O.; Dal Belo, C.A. Inhibition of Kv2.1 Potassium Channels by MiDCA1, A Pre-Synaptically Active PLA2-Type Toxin from Micrurus dumerilii carinicauda Coral Snake Venom. Toxins 2019, 11, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, D.T. The dawn of high-resolution structure for the queen of ion channels. Neuron 2004, 42, 357–359. [Google Scholar] [CrossRef]
- Pitt, G.S.; Matsui, M.; Cao, C. Voltage-Gated Calcium Channels in Nonexcitable Tissues. Annu. Rev. Physiol. 2021, 83, 183–203. [Google Scholar] [CrossRef]
- Nimmrich, V.; Gross, G. P/Q-type calcium channel modulators. Br. J. Pharmacol. 2012, 167, 741–759. [Google Scholar] [CrossRef] [Green Version]
- Dolphin, A.C.; Lee, A. Presynaptic calcium channels: Specialized control of synaptic neurotransmitter release. Nat. Rev. Neurosci. 2020, 21, 213–229. [Google Scholar] [CrossRef]
- Adams, M.E. Agatoxins: Ion channel specific toxins from the American funnel web spider, Agelenopsis aperta. Toxicon Off. J. Int. Soc. Toxinology 2004, 43, 509–525. [Google Scholar] [CrossRef]
- Pringos, E.; Vignes, M.; Martinez, J.; Rolland, V. Peptide neurotoxins that affect voltage-gated calcium channels: A close-up on ω-agatoxins. Toxins 2011, 3, 17–42. [Google Scholar] [CrossRef] [Green Version]
- Bindokas, V.P.; Venema, V.J.; Adams, M.E. Differential antagonism of transmitter release by subtypes of omega-agatoxins. J. Neurophysiol. 1991, 66, 590–601. [Google Scholar] [CrossRef]
- Santos, A.D.; Imperial, J.S.; Chaudhary, T.; Beavis, R.C.; Chait, B.T.; Hunsperger, J.P.; Olivera, B.M.; Adams, M.E.; Hillyard, D.R. Heterodimeric structure of the spider toxin omega-agatoxin IA revealed by precursor analysis and mass spectrometry. J. Biol. Chem. 1992, 267, 20701–20705. [Google Scholar] [CrossRef]
- Olivera, B.M.; Miljanich, G.P.; Ramachandran, J.; Adams, M.E. Calcium channel diversity and neurotransmitter release: The omega-conotoxins and omega-agatoxins. Annu. Rev. Biochem. 1994, 63, 823–867. [Google Scholar] [CrossRef] [PubMed]
- Mintz, I.M.; Venema, V.J.; Adams, M.E.; Bean, B.P. Inhibition of N- and L-type Ca2+ channels by the spider venom toxin omega-Aga-IIIA. Proc. Natl. Acad. Sci. USA 1991, 88, 6628–6631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mintz, I.M. Block of Ca channels in rat central neurons by the spider toxin omega-Aga-IIIA. J. Neurosci. 1994, 14, 2844–2853. [Google Scholar] [CrossRef] [PubMed]
- Bourinet, E.; Soong, T.W.; Sutton, K.; Slaymaker, S.; Mathews, E.; Monteil, A.; Zamponi, G.W.; Nargeot, J.; Snutch, T.P. Splicing of alpha 1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nat. Neurosci. 1999, 2, 407–415. [Google Scholar] [CrossRef] [PubMed]
- McDonough, S.I.; Mintz, I.M.; Bean, B.P. Alteration of P-type calcium channel gating by the spider toxin omega-Aga-IVA. Biophys. J. 1997, 72, 2117–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mintz, I.M.; Venema, V.J.; Swiderek, K.M.; Lee, T.D.; Bean, B.P.; Adams, M.E. P-type calcium channels blocked by the spider toxin omega-Aga-IVA. Nature 1992, 355, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.-H.; Dekan, Z.; Smout, M.J.; Wilson, D.; Dutertre, S.; Vetter, I.; Lewis, R.J.; Loukas, A.; Daly, N.L.; Alewood, P.F. Conotoxin Φ-MiXXVIIA from the Superfamily G2 Employs a Novel Cysteine Framework that Mimics Granulin and Displays Anti-Apoptotic Activity. Angew. Chem. Int. Ed. Engl. 2017, 56, 14973–14976. [Google Scholar] [CrossRef]
- Akondi, K.B.; Muttenthaler, M.; Dutertre, S.; Kaas, Q.; Craik, D.J.; Lewis, R.J.; Alewood, P.F. Discovery, synthesis, and structure-activity relationships of conotoxins. Chem. Rev. 2014, 114, 5815–5847. [Google Scholar] [CrossRef]
- Vetter, I.; Lewis, R.J. Therapeutic potential of cone snail venom peptides (conopeptides). Curr. Top. Med. Chem. 2012, 12, 1546–1552. [Google Scholar] [CrossRef]
- Adams, D.J.; Callaghan, B.; Berecki, G. Analgesic conotoxins: Block and G protein-coupled receptor modulation of N-type (Ca(V) 2.2) calcium channels. Br. J. Pharmacol. 2012, 166, 486–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boland, L.M.; Morrill, J.A.; Bean, B.P. omega-Conotoxin block of N-type calcium channels in frog and rat sympathetic neurons. J. Neurosci. 1994, 14, 5011–5027. [Google Scholar] [CrossRef] [PubMed]
- Ellinor, P.T.; Zhang, J.F.; Horne, W.A.; Tsien, R.W. Structural determinants of the blockade of N-type calcium channels by a peptide neurotoxin. Nature 1994, 372, 272–275. [Google Scholar] [CrossRef]
- Kerr, L.M.; Yoshikami, D. A venom peptide with a novel presynaptic blocking action. Nature 1984, 308, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Olivera, B.M.; Cruz, L.J.; de Santos, V.; LeCheminant, G.W.; Griffin, D.; Zeikus, R.; McIntosh, J.M.; Galyean, R.; Varga, J.; Gray, W.R. Neuronal calcium channel antagonists. Discrimination between calcium channel subtypes using omega-conotoxin from Conus magus venom. Biochemistry 1987, 26, 2086–2090. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef]
- Lin, Z.; Haus, S.; Edgerton, J.; Lipscombe, D. Identification of functionally distinct isoforms of the N-type Ca2+ channel in rat sympathetic ganglia and brain. Neuron 1997, 18, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Servent, D.; Winckler-Dietrich, V.; Hu, H.Y.; Kessler, P.; Drevet, P.; Bertrand, D.; Ménez, A. Only snake curaremimetic toxins with a fifth disulfide bond have high affinity for the neuronal alpha7 nicotinic receptor. J. Biol. Chem. 1997, 272, 24279–24286. [Google Scholar] [CrossRef] [Green Version]
- Changeux, J.P. The TiPS lecture. The nicotinic acetylcholine receptor: An allosteric protein prototype of ligand-gated ion channels. Trends Pharmacol. Sci. 1990, 11, 485–492. [Google Scholar] [CrossRef]
- Fruchart-Gaillard, C.; Gilquin, B.; Antil-Delbeke, S.; Le Novère, N.; Tamiya, T.; Corringer, P.-J.; Changeux, J.-P.; Ménez, A.; Servent, D. Experimentally based model of a complex between a snake toxin and the alpha 7 nicotinic receptor. Proc. Natl. Acad. Sci. USA 2002, 99, 3216–3221. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.C. Looking back on the discovery of alpha-bungarotoxin. J. Biomed. Sci. 1999, 6, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Nirthanan, S.; Gwee, M.C.E. Three-finger alpha-neurotoxins and the nicotinic acetylcholine receptor, forty years on. J. Pharmacol. Sci. 2004, 94, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nirthanan, S.; Gopalakrishnakone, P.; Gwee, M.C.E.; Khoo, H.E.; Kini, R.M. Non-conventional toxins from Elapid venoms. Toxicon Off. J. Int. Soc. Toxinology 2003, 41, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Tsetlin, V. Snake venom alpha-neurotoxins and other ‘three-finger’ proteins. Eur. J. Biochem. 1999, 264, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Modahl, C.M.; Mukherjee, A.K.; Mackessy, S.P. An analysis of venom ontogeny and prey-specific toxicity in the Monocled Cobra (Naja kaouthia). Toxicon Off. J. Int. Soc. Toxinology 2016, 119, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Tamiya, N. Current view on the structure-function relationship of postsynaptic neurotoxins from snake venoms. Pharmacol. Ther. 1987, 34, 403–451. [Google Scholar] [CrossRef]
- Tsetlin, V.; Utkin, Y.; Kasheverov, I. Polypeptide and peptide toxins, magnifying lenses for binding sites in nicotinic acetylcholine receptors. Biochem. Pharmacol. 2009, 78, 720–731. [Google Scholar] [CrossRef]
- Servent, D.; Antil-Delbeke, S.; Gaillard, C.; Corringer, P.J.; Changeux, J.P.; Ménez, A. Molecular characterization of the specificity of interactions of various neurotoxins on two distinct nicotinic acetylcholine receptors. Eur. J. Pharmacol. 2000, 393, 197–204. [Google Scholar] [CrossRef]
- Antil-Delbeke, S.; Gaillard, C.; Tamiya, T.; Corringer, P.J.; Changeux, J.P.; Servent, D.; Ménez, A. Molecular determinants by which a long chain toxin from snake venom interacts with the neuronal alpha 7-nicotinic acetylcholine receptor. J. Biol. Chem. 2000, 275, 29594–29601. [Google Scholar] [CrossRef] [Green Version]
- Papke, R.L.; Horenstein, N.A. Therapeutic Targeting of 7 Nicotinic Acetylcholine Receptors. Pharmacol. Rev. 2021, 73, 1118–1149. [Google Scholar] [CrossRef]
- Briggs, C.A.; Grønlien, J.H.; Curzon, P.; Timmermann, D.B.; Ween, H.; Thorin-Hagene, K.; Kerr, P.; Anderson, D.J.; Malysz, J.; Dyhring, T.; et al. Role of channel activation in cognitive enhancement mediated by alpha7 nicotinic acetylcholine receptors. Br. J. Pharmacol. 2009, 158, 1486–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieschl, R.L.; Miller, R.; Jones, K.M.; Post-Munson, D.J.; Chen, P.; Newberry, K.; Benitex, Y.; Molski, T.; Morgan, D.; McDonald, I.M.; et al. Effects of BMS-902483, an α7 nicotinic acetylcholine receptor partial agonist, on cognition and sensory gating in relation to receptor occupancy in rodents. Eur. J. Pharmacol. 2017, 807, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Giacobini, E.; Struble, R.G.; Zilles, K.; Maelicke, A. Nicotinic cholinoceptive neurons of the frontal cortex are reduced in Alzheimer’s disease. Neurobiol. Aging 1991, 12, 259–262. [Google Scholar] [CrossRef]
- Schröder, H.; Giacobini, E.; Struble, R.G.; Zilles, K.; Maelicke, A.; Luiten, P.G.; Strosberg, A.D. Cellular distribution and expression of cortical acetylcholine receptors in aging and Alzheimer’s disease. Ann. New York Acad. Sci. 1991, 640, 189–192. [Google Scholar] [CrossRef]
- Lange, K.W.; Wells, F.R.; Jenner, P.; Marsden, C.D. Altered muscarinic and nicotinic receptor densities in cortical and subcortical brain regions in Parkinson’s disease. J. Neurochem. 1993, 60, 197–203. [Google Scholar] [CrossRef]
- Freedman, R.; Hall, M.; Adler, L.E.; Leonard, S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol. Psychiatry 1995, 38, 22–33. [Google Scholar] [CrossRef]
- James, J.R.; Nordberg, A. Genetic and environmental aspects of the role of nicotinic receptors in neurodegenerative disorders: Emphasis on Alzheimer’s disease and Parkinson’s disease. Behav. Genet. 1995, 25, 149–159. [Google Scholar] [CrossRef]
- Perry, E.K.; Morris, C.M.; Court, J.A.; Cheng, A.; Fairbairn, A.F.; McKeith, I.G.; Irving, D.; Brown, A.; Perry, R.H. Alteration in nicotine binding sites in Parkinson’s disease, Lewy body dementia and Alzheimer’s disease: Possible index of early neuropathology. Neuroscience 1995, 64, 385–395. [Google Scholar] [CrossRef]
- Nordberg, A.; Lundqvist, H.; Hartvig, P.; Andersson, J.; Johansson, M.; Hellstrŏm-Lindahi, E.; Långström, B. Imaging of nicotinic and muscarinic receptors in Alzheimer’s disease: Effect of tacrine treatment. Dement. Geriatr. Cogn. Disord. 1997, 8, 78–84. [Google Scholar] [CrossRef]
- Spurden, D.P.; Court, J.A.; Lloyd, S.; Oakley, A.; Perry, R.; Pearson, C.; Pullen, R.G.; Perry, E.K. Nicotinic receptor distribution in the human thalamus: Autoradiographical localization of [3H]nicotine and [125I] alpha-bungarotoxin binding. J. Chem. Neuroanat. 1997, 13, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Gotti, C.; Moretti, M.; Bohr, I.; Ziabreva, I.; Vailati, S.; Longhi, R.; Riganti, L.; Gaimarri, A.; McKeith, I.G.; Perry, R.H.; et al. Selective nicotinic acetylcholine receptor subunit deficits identified in Alzheimer’s disease, Parkinson’s disease and dementia with Lewy bodies by immunoprecipitation. Neurobiol. Dis. 2006, 23, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef] [PubMed]
- van Westerloo, D.J.; Giebelen, I.A.; Florquin, S.; Bruno, M.J.; Larosa, G.J.; Ulloa, L.; Tracey, K.J.; van der Poll, T. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology 2006, 130, 1822–1830. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Ochani, M.; Yang, L.-H.; Gallowitsch-Puerta, M.; Ochani, K.; Lin, X.; Levi, J.; Parrish, W.R.; Rosas-Ballina, M.; Czura, C.J.; et al. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit. Care Med. 2007, 35, 1139–1144. [Google Scholar] [CrossRef]
- Rosas-Ballina, M.; Goldstein, R.S.; Gallowitsch-Puerta, M.; Yang, L.; Valdés-Ferrer, S.I.; Patel, N.B.; Chavan, S.; Al-Abed, Y.; Yang, H.; Tracey, K.J. The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol. Med. 2009, 15, 195–202. [Google Scholar] [CrossRef]
- Rosas-Ballina, M.; Tracey, K.J. Cholinergic control of inflammation. J. Intern. Med. 2009, 265, 663–679. [Google Scholar] [CrossRef]
- Wang, G.K.; Schmidt, J. Primary structure and binding properties of iodinated derivatives of alpha-bungarotoxin. J. Biol. Chem. 1980, 255, 11156–11162. [Google Scholar] [CrossRef]
- Azam, L.; McIntosh, J.M. Alpha-conotoxins as pharmacological probes of nicotinic acetylcholine receptors. Acta Pharmacol. Sin. 2009, 30, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Terlau, H.; Olivera, B.M. Conus venoms: A rich source of novel ion channel-targeted peptides. Physiol. Rev. 2004, 84, 41–68. [Google Scholar] [CrossRef] [Green Version]
- Janes, R.W. α-Conotoxins as selective probes for nicotinic acetylcholine receptor subclasses. Curr. Opin. Pharmacol. 2005, 5, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Wioland, L.; Dupont, J.-L.; Bossu, J.-L.; Popoff, M.R.; Poulain, B. Attack of the nervous system by Clostridium perfringens Epsilon toxin: From disease to mode of action on neural cells. Toxicon Off. J. Int. Soc. Toxinology 2013, 75, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Castagliuolo, I.; LaMont, J.T.; Letourneau, R.; Kelly, C.; O’Keane, J.C.; Jaffer, A.; Theoharides, T.C.; Pothoulakis, C. Neuronal involvement in the intestinal effects of Clostridium difficile toxin A and Vibrio cholerae enterotoxin in rat ileum. Gastroenterology 1994, 107, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Burleigh, D.E.; Banks, M.R. Stimulation of intestinal secretion by vasoactive intestinal peptide and cholera toxin. Auton. Neurosci. 2007, 133, 64–75. [Google Scholar] [CrossRef]
- Aktories, K. Bacterial protein toxins that modify host regulatory GTPases. Nat. Rev. Microbiol. 2011, 9, 487–498. [Google Scholar] [CrossRef]
- Aktories, K.; Schwan, C.; Papatheodorou, P.; Lang, A.E. Bidirectional attack on the actin cytoskeleton. Bacterial protein toxins causing polymerization or depolymerization of actin. Toxicon Off. J. Int. Soc. Toxinology 2012, 60, 572–581. [Google Scholar] [CrossRef]
- Blanchfield, J.T.; Gallagher, O.P.; Cros, C.; Lewis, R.J.; Alewood, P.F.; Toth, I. Oral absorption and in vivo biodistribution of alpha-conotoxin MII and a lipidic analogue. Biochem. Biophys. Res. Commun. 2007, 361, 97–102. [Google Scholar] [CrossRef]
- Oller-Salvia, B.; Teixidó, M.; Giralt, E. From venoms to BBB shuttles: Synthesis and blood-brain barrier transport assessment of apamin and a nontoxic analog. Biopolymers 2013, 100, 675–686. [Google Scholar] [CrossRef]
- Matak, I.; Bölcskei, K.; Bach-Rojecky, L.; Helyes, Z. Mechanisms of Botulinum Toxin Type A Action on Pain. Toxins 2019, 11, 459. [Google Scholar] [CrossRef] [Green Version]
- Hallett, M.; Benecke, R.; Blitzer, A.; Comella, C.L. Treatment of focal dystonias with botulinum neurotoxin. Toxicon Off. J. Int. Soc. Toxinology 2009, 54, 628–633. [Google Scholar] [CrossRef] [Green Version]
- Dressler, D. Botulinum toxin for treatment of dystonia. Eur. J. Neurol. 2010, 17 (Suppl. 1), 88–96. [Google Scholar] [CrossRef] [PubMed]
- Sloop, R.R.; Cole, B.A.; Escutin, R.O. Human response to botulinum toxin injection: Type B compared with type A. Neurology 1997, 49, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Meunier, F.A.; Schiavo, G.; Molgó, J. Botulinum neurotoxins: From paralysis to recovery of functional neuromuscular transmission. J. Physiol. Paris 2002, 96, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.R. Evidence for antinociceptive activity of botulinum toxin type A in pain management. Headache 2003, 43 (Suppl. 1), S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.-M.; Chung, M.E. Botulinum Toxin for Neuropathic Pain: A Review of the Literature. Toxins 2015, 7, 3127–3154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wollmer, M.A.; Magid, M.; Kruger, T.H.C.; Finzi, E. The Use of Botulinum Toxin for Treatment of Depression. Handb. Exp. Pharmacol. 2021, 263, 265–278. [Google Scholar] [CrossRef]
- Martina, E.; Diotallevi, F.; Radi, G.; Campanati, A.; Offidani, A. Therapeutic Use of Botulinum Neurotoxins in Dermatology: Systematic Review. Toxins 2021, 13, 120. [Google Scholar] [CrossRef]
- Simpson, L. The life history of a botulinum toxin molecule. Toxicon Off. J. Int. Soc. Toxinology 2013, 68, 40–59. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Kotiya, A.; Kiris, E.; Yang, M.; Bavari, S.; Tessarollo, L.; Oyler, G.A.; Weissman, A.M. Deubiquitinating enzyme VCIP135 dictates the duration of botulinum neurotoxin type A intoxication. Proc. Natl. Acad. Sci. USA 2017, 114, E5158–E5166. [Google Scholar] [CrossRef] [Green Version]
- Schurch, B.; Schmid, D.M.; Stöhrer, M. Treatment of neurogenic incontinence with botulinum toxin A. N. Engl. J. Med. 2000, 342, 665. [Google Scholar] [CrossRef]
- Schurch, B.; Stöhrer, M.; Kramer, G.; Schmid, D.M.; Gaul, G.; Hauri, D. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: A new alternative to anticholinergic drugs? Preliminary results. J. Urol. 2000, 164, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Restani, L.; Novelli, E.; Bottari, D.; Leone, P.; Barone, I.; Galli-Resta, L.; Strettoi, E.; Caleo, M. Botulinum neurotoxin A impairs neurotransmission following retrograde transynaptic transport. Traffic 2012, 13, 1083–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anandan, C.; Jankovic, J. Botulinum Toxin in Movement Disorders: An Update. Toxins 2021, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Safarpour, Y.; Jabbari, B. Botulinum toxin treatment of pain syndromes—An evidence based review. Toxicon Off. J. Int. Soc. Toxinology 2018, 147, 120–128. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef]
- Zychowska, M.; Rojewska, E.; Makuch, W.; Luvisetto, S.; Pavone, F.; Marinelli, S.; Przewlocka, B.; Mika, J. Participation of pro- and anti-nociceptive interleukins in botulinum toxin A-induced analgesia in a rat model of neuropathic pain. Eur. J. Pharmacol. 2016, 791, 377–388. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Liu, X.; Su, C.-J.; Zhang, Q.-L.; Wang, Z.-H.; Cao, L.-F.; Guo, X.-Y.; Huang, Y.; Luo, W.; et al. Antidepressant-Like Action of Single Facial Injection of Botulinum Neurotoxin A is Associated with Augmented 5-HT Levels and BDNF/ERK/CREB Pathways in Mouse Brain. Neurosci. Bull. 2019, 35, 661–672. [Google Scholar] [CrossRef]
- Yuan, H.; Silberstein, S.D. The Use of Botulinum Toxin in the Management of Headache Disorders. Handb. Exp. Pharmacol. 2021, 263, 227–249. [Google Scholar] [CrossRef]
- Brooks, V.B.; Curtis, D.R.; Eccles, J.C. The action of tetanus toxin on the inhibition of motoneurones. J. Physiol. 1957, 135, 655–672. [Google Scholar] [CrossRef] [Green Version]
- Bizzini, B.; Stoeckel, K.; Schwab, M. An antigenic polypeptide fragment isolated from tetanus toxin: Chemical characterization, binding to gangliosides and retrograde axonal transport in various neuron systems. J. Neurochem. 1977, 28, 529–542. [Google Scholar] [CrossRef]
- Holz, G.G.; Habener, J.F. Black widow spider alpha-latrotoxin: A presynaptic neurotoxin that shares structural homology with the glucagon-like peptide-1 family of insulin secretagogic hormones. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998, 121, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.P.; Silva, V.A.O.; Silvestrini, A.V.P.; de Macedo, L.H.; Caetano, G.F.; Reis, R.M.; Mazzi, M.V. Crotoxin from Crotalus durissus terrificus venom: In vitro cytotoxic activity of a heterodimeric phospholipase A on human cancer-derived cell lines. Toxicon Off. J. Int. Soc. Toxinology 2018, 156, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, J.R.; Lancellotti, M.; Soares, A.M.; Calderon, L.A.; Ramírez, D.; González, W.; Marangoni, S.; Da Silva, S.L. CoaTx-II, a new dimeric Lys49 phospholipase A2 from Crotalus oreganus abyssus snake venom with bactericidal potential: Insights into its structure and biological roles. Toxicon Off. J. Int. Soc. Toxinology 2016, 120, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-L.; Han, R.; Gu, Z.-L.; Chen, Z.-X.; Chen, B.-W.; Reid, P.F.; Raymond, L.N.; Qin, Z.-H. A short-chain alpha-neurotoxin from Naja Naja atra produces potent cholinergic-dependent analgesia. Neurosci. Bull. 2006, 22, 103–109. [Google Scholar] [PubMed]
- Diaz, A.; Dickenson, A.H. Blockade of spinal N- and P-type, but not L-type, calcium channels inhibits the excitability of rat dorsal horn neurones produced by subcutaneous formalin inflammation. Pain 1997, 69, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Montagut-Bordas, C.; Dickenson, A.H. Calcium channel modulation as a target in chronic pain control. Br. J. Pharmacol. 2018, 175, 2173–2184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, K.M.; Binda, N.S.; Lavor, M.S.L.; Silva, C.M.O.; Rosado, I.R.; Gabellini, E.L.A.; Da Silva, J.F.; Oliveira, C.M.; Melo, M.M.; Gomez, M.V.; et al. Conotoxin MVIIA improves cell viability and antioxidant system after spinal cord injury in rats. PLoS ONE 2018, 13, e0204948. [Google Scholar] [CrossRef]
- Ovsepian, S.V.; LeBerre, M.; Steuber, V.; O’Leary, V.B.; Leibold, C.; Oliver Dolly, J. Distinctive role of KV1.1 subunit in the biology and functions of low threshold K(+) channels with implications for neurological disease. Pharmacol. Ther. 2016, 159, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Robbins, C.A.; Tempel, B.L. Kv1.1 and Kv1.2: Similar channels, different seizure models. Epilepsia 2012, 53 (Suppl. 1), 134–141. [Google Scholar] [CrossRef]
- Blacklow, B.; Kornhauser, R.; Hains, P.G.; Loiacono, R.; Escoubas, P.; Graudins, A.; Nicholson, G.M. α-Elapitoxin-Aa2a, a long-chain snake α-neurotoxin with potent actions on muscle (α1)(2)βγδ nicotinic receptors, lacks the classical high affinity for neuronal α7 nicotinic receptors. Biochem. Pharmacol. 2011, 81, 314–325. [Google Scholar] [CrossRef] [Green Version]
- Grozio, A.; Paleari, L.; Catassi, A.; Servent, D.; Cilli, M.; Piccardi, F.; Paganuzzi, M.; Cesario, A.; Granone, P.; Mourier, G.; et al. Natural agents targeting the alpha7-nicotinic-receptor in NSCLC: A promising prospective in anti-cancer drug development. Int. J. Cancer 2008, 122, 1911–1915. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.-n.; Liu, Y.-l.; Lin, H.-m.; Yang, S.-l.; Feng, Y.-l.; Reid, P.F.; Qin, Z.-h. Involvement of cholinergic system in suppression of formalin-induced inflammatory pain by cobratoxin. Acta Pharmacol. Sin. 2011, 32, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Webb, R.P. Engineering of Botulinum Neurotoxins for Biomedical Applications. Toxins 2018, 10, 231. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.B. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. J. Pediatr. Ophthalmol. Strabismus 1980, 17, 21–25. [Google Scholar] [CrossRef]
- Jankovic, J. Botulinum toxin in clinical practice. J. Neurol. Neurosurg. Psychiatry 2004, 75, 951–957. [Google Scholar] [CrossRef] [PubMed]
- O’Flynn, L.C.; Simonyan, K. Short- and Long-term Central Action of Botulinum Neurotoxin Treatment in Laryngeal Dystonia. Neurology 2022, 99, e1178–e1190. [Google Scholar] [CrossRef]
- Eaker, E.Y.; Gordon, J.M.; Vogel, S.B. Untoward effects of esophageal botulinum toxin injection in the treatment of achalasia. Dig. Dis. Sci. 1997, 42, 724–727. [Google Scholar] [CrossRef]
- Pasricha, P.J.; Miskovsky, E.P.; Kalloo, A.N. Intrasphincteric injection of botulinum toxin for suspected sphincter of Oddi dysfunction. Gut 1994, 35, 1319–1321. [Google Scholar] [CrossRef] [Green Version]
- Joo, J.S.; Agachan, F.; Wolff, B.; Nogueras, J.J.; Wexner, S.D. Initial North American experience with botulinum toxin type A for treatment of anismus. Dis. Colon Rectum 1996, 39, 1107–1111. [Google Scholar] [CrossRef]
- Zermann, D.h.; Ishigooka, M.; Schubert, J.; Schmidt, R.A. Perisphincteric injection of botulinum toxin type A. A treatment option for patients with chronic prostatic pain? Eur. Urol. 2000, 38, 393–399. [Google Scholar] [CrossRef]
- Naumann, M.; Jost, W.H.; Toyka, K.V. Botulinum toxin in the treatment of neurological disorders of the autonomic nervous system. Arch. Neurol. 1999, 56, 914–916. [Google Scholar] [CrossRef] [Green Version]
- Saadia, D.; Voustianiouk, A.; Wang, A.K.; Kaufmann, H. Botulinum toxin type A in primary palmar hyperhidrosis: Randomized, single-blind, two-dose study. Neurology 2001, 57, 2095–2099. [Google Scholar] [CrossRef]
- Naumann, M.K.; Hamm, H.; Lowe, N.J. Effect of botulinum toxin type A on quality of life measures in patients with excessive axillary sweating: A randomized controlled trial. Br. J. Dermatol. 2002, 147, 1218–1226. [Google Scholar] [CrossRef]
- Heckmann, M.; Ceballos-Baumann, A.O.; Plewig, G. Botulinum toxin A for axillary hyperhidrosis (excessive sweating). N. Engl. J. Med. 2001, 344, 488–493. [Google Scholar] [CrossRef]
- Frampton, J.E.; Easthope, S.E. Botulinum toxin A (Botox Cosmetic): A review of its use in the treatment of glabellar frown lines. Am. J. Clin. Dermatol. 2003, 4, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Matarasso, A.; Matarasso, S.L.; Brandt, F.S.; Bellman, B. Botulinum A exotoxin for the management of platysma bands. Plast. Reconstr. Surg. 1999, 103, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Hoorens, I.; Ongenae, K. Primary focal hyperhidrosis: Current treatment options and a step-by-step approach. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1–8. [Google Scholar] [CrossRef]
- Lakraj, A.-A.D.; Moghimi, N.; Jabbari, B. Hyperhidrosis: Anatomy, pathophysiology and treatment with emphasis on the role of botulinum toxins. Toxins 2013, 5, 821–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shon, U.; Kim, M.H.; Lee, D.Y.; Kim, S.H.; Park, B.C. The effect of intradermal botulinum toxin on androgenetic alopecia and its possible mechanism. J. Am. Acad. Dermatol. 2020, 83, 1838–1839. [Google Scholar] [CrossRef]
- Shackleton, T.; Ram, S.; Black, M.; Ryder, J.; Clark, G.T.; Enciso, R. The efficacy of botulinum toxin for the treatment of trigeminal and postherpetic neuralgia: A systematic review with meta-analyses. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 61–71. [Google Scholar] [CrossRef]
- Meng, F.; Peng, K.; Yang, J.-P.; Ji, F.-H.; Xia, F.; Meng, X.-W. Botulinum toxin-A for the treatment of neuralgia: A systematic review and meta-analysis. J. Pain Res. 2018, 11, 2343–2351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Park, H.J. Botulinum Toxin for the Treatment of Neuropathic Pain. Toxins 2017, 9, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitamura, Y.; Matsuka, Y.; Spigelman, I.; Ishihara, Y.; Yamamoto, Y.; Sonoyama, W.; Kamioka, H.; Yamashiro, T.; Kuboki, T.; Oguma, K. Botulinum toxin type a (150 kDa) decreases exaggerated neurotransmitter release from trigeminal ganglion neurons and relieves neuropathy behaviors induced by infraorbital nerve constriction. Neuroscience 2009, 159, 1422–1429. [Google Scholar] [CrossRef]
- Meng, J.; Dolly, J.O.; Wang, J. Selective cleavage of SNAREs in sensory neurons unveils protein complexes mediating peptide exocytosis triggered by different stimuli. Mol. Neurobiol. 2014, 50, 574–588. [Google Scholar] [CrossRef]
- Matak, I.; Rossetto, O.; Lacković, Z. Botulinum toxin type A selectivity for certain types of pain is associated with capsaicin-sensitive neurons. Pain 2014, 155, 1516–1526. [Google Scholar] [CrossRef] [Green Version]
- Mika, J.; Rojewska, E.; Makuch, W.; Korostynski, M.; Luvisetto, S.; Marinelli, S.; Pavone, F.; Przewlocka, B. The effect of botulinum neurotoxin A on sciatic nerve injury-induced neuroimmunological changes in rat dorsal root ganglia and spinal cord. Neuroscience 2011, 175, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Popiolek-Barczyk, K.; Pavone, F.; Mika, J. Comparison of the Expression Changes after Botulinum Toxin Type A and Minocycline Administration in Lipopolysaccharide-Stimulated Rat Microglial and Astroglial Cultures. Front. Cell. Infect. Microbiol. 2017, 7, 141. [Google Scholar] [CrossRef] [Green Version]
- Finzi, E.; Wasserman, E. Treatment of depression with botulinum toxin A: A case series. Dermatol. Surg. 2006, 32, 645–650. [Google Scholar] [CrossRef]
- Wollmer, M.A.; de Boer, C.; Kalak, N.; Beck, J.; Götz, T.; Schmidt, T.; Hodzic, M.; Bayer, U.; Kollmann, T.; Kollewe, K.; et al. Facing depression with botulinum toxin: A randomized controlled trial. J. Psychiatr. Res. 2012, 46, 574–581. [Google Scholar] [CrossRef] [Green Version]
- Wollmer, M.A.; Kalak, N.; Jung, S.; de Boer, C.; Magid, M.; Reichenberg, J.S.; Brand, S.; Holsboer-Trachsler, E.; Kruger, T.H.C. Agitation predicts response of depression to botulinum toxin treatment in a randomized controlled trial. Front. Psychiatry 2014, 5, 36. [Google Scholar] [CrossRef] [Green Version]
- Finzi, E.; Rosenthal, N.E. Treatment of depression with onabotulinumtoxinA: A randomized, double-blind, placebo controlled trial. J. Psychiatr. Res. 2014, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Magid, M.; Reichenberg, J.S.; Poth, P.E.; Robertson, H.T.; LaViolette, A.K.; Kruger, T.H.C.; Wollmer, M.A. Treatment of major depressive disorder using botulinum toxin A: A 24-week randomized, double-blind, placebo-controlled study. J. Clin. Psychiatry 2014, 75, 837–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamanian, A.; Ghanbari Jolfaei, A.; Mehran, G.; Azizian, Z. Efficacy of Botox versus Placebo for Treatment of Patients with Major Depression. Iran J. Public Health 2017, 46, 982–984. [Google Scholar] [PubMed]
- Finzi, E.; Rosenthal, N.E. Emotional proprioception: Treatment of depression with afferent facial feedback. J. Psychiatr. Res. 2016, 80, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Ibragić, S.; Matak, I.; Dračić, A.; Smajlović, A.; Muminović, M.; Proft, F.; Sofić, E.; Lacković, Z.; Riederer, P. Effects of botulinum toxin type A facial injection on monoamines and their metabolites in sensory, limbic and motor brain regions in rats. Neurosci. Lett. 2016, 617, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Caleo, M.; Schiavo, G. Central effects of tetanus and botulinum neurotoxins. Toxicon Off. J. Int. Soc. Toxinology 2009, 54, 593–599. [Google Scholar] [CrossRef]
- Marchand-Pauvert, V.; Aymard, C.; Giboin, L.-S.; Dominici, F.; Rossi, A.; Mazzocchio, R. Beyond muscular effects: Depression of spinal recurrent inhibition after botulinum neurotoxin A. J. Physiol. 2013, 591, 1017–1029. [Google Scholar] [CrossRef] [Green Version]
- Golden, S.A.; Christoffel, D.J.; Heshmati, M.; Hodes, G.E.; Magida, J.; Davis, K.; Cahill, M.E.; Dias, C.; Ribeiro, E.; Ables, J.L.; et al. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat. Med. 2013, 19, 337–344. [Google Scholar] [CrossRef] [Green Version]
- Molendijk, M.L.; Spinhoven, P.; Polak, M.; Bus, B.A.A.; Penninx, B.W.J.H.; Elzinga, B.M. Serum BDNF concentrations as peripheral manifestations of depression: Evidence from a systematic review and meta-analyses on 179 associations (N = 9484). Mol. Psychiatry 2014, 19, 791–800. [Google Scholar] [CrossRef]
- Guo, F.; Zhang, Q.; Zhang, B.; Fu, Z.; Wu, B.; Huang, C.; Li, Y. Burst-firing patterns in the prefrontal cortex underlying the neuronal mechanisms of depression probed by antidepressants. Eur. J. Neurosci. 2014, 40, 3538–3547. [Google Scholar] [CrossRef]
- Pilar-Cuéllar, F.; Vidal, R.; Díaz, A.; Castro, E.; dos Anjos, S.; Pascual-Brazo, J.; Linge, R.; Vargas, V.; Blanco, H.; Martínez-Villayandre, B.; et al. Neural plasticity and proliferation in the generation of antidepressant effects: Hippocampal implication. Neural Plast. 2013, 2013, 537265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gascón, S.; Ortega, F.; Götz, M. Transient CREB-mediated transcription is key in direct neuronal reprogramming. Neurogenesis 2017, 4, e1285383. [Google Scholar] [CrossRef] [PubMed]
- Einoch, R.; Weinreb, O.; Mandiuk, N.; Youdim, M.B.H.; Bilker, W.; Silver, H. The involvement of BDNF-CREB signaling pathways in the pharmacological mechanism of combined SSRI- antipsychotic treatment in schizophrenia. Eur. Neuropsychopharmacol. 2017, 27, 470–483. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1260–1344. [Google Scholar] [CrossRef] [PubMed]
- Vollesen, A.L.; Benemei, S.; Cortese, F.; Labastida-Ramírez, A.; Marchese, F.; Pellesi, L.; Romoli, M.; Ashina, M.; Lampl, C. Migraine and cluster headache—The common link. J. Headache Pain 2018, 19, 89. [Google Scholar] [CrossRef] [Green Version]
- Durham, P.L.; Cady, R.; Cady, R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: Implications for migraine therapy. Headache 2004, 44, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.J.; Purkiss, J.R.; Foster, K.A. Sensitivity of embryonic rat dorsal root ganglia neurons to Clostridium botulinum neurotoxins. Toxicon Off. J. Int. Soc. Toxinology 2000, 38, 245–258. [Google Scholar] [CrossRef]
- Cui, M.; Khanijou, S.; Rubino, J.; Aoki, K.R. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain 2004, 107, 125–133. [Google Scholar] [CrossRef]
- Xiao, L.; Cheng, J.; Dai, J.; Zhang, D. Botulinum toxin decreases hyperalgesia and inhibits P2X3 receptor over-expression in sensory neurons induced by ventral root transection in rats. Pain Med. 2011, 12, 1385–1394. [Google Scholar] [CrossRef] [Green Version]
- Hong, B.; Yao, L.; Ni, L.; Wang, L.; Hu, X. Antinociceptive effect of botulinum toxin A involves alterations in AMPA receptor expression and glutamate release in spinal dorsal horn neurons. Neuroscience 2017, 357, 197–207. [Google Scholar] [CrossRef]
- Shimizu, T.; Shibata, M.; Toriumi, H.; Iwashita, T.; Funakubo, M.; Sato, H.; Kuroi, T.; Ebine, T.; Koizumi, K.; Suzuki, N. Reduction of TRPV1 expression in the trigeminal system by botulinum neurotoxin type-A. Neurobiol. Dis. 2012, 48, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Strassman, A.M.; Novack, V.; Brin, M.F.; Burstein, R. Extracranial injections of botulinum neurotoxin type A inhibit intracranial meningeal nociceptors’ responses to stimulation of TRPV1 and TRPA1 channels: Are we getting closer to solving this puzzle? Cephalalgia 2016, 36, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Do, T.P.; Hvedstrup, J.; Schytz, H.W. Botulinum toxin: A review of the mode of action in migraine. Acta Neurol. Scand. 2018, 137, 442–451. [Google Scholar] [CrossRef]
- Dillingham, T.R. Musculoskeletal rehabilitation: Current understandings and future directions. Am. J. Phys. Med. Rehabil. 2007, 86, S19–S28. [Google Scholar] [CrossRef] [PubMed]
- Sasse, A.; Conduit, R.; Ryan, D.; Woods, W.; Tucker, A.P. A pharmacotherapy for obstructive sleep apnea. Sleep 2005, 28, 1015–1016. [Google Scholar] [CrossRef]
- Chen, C.; Baldwin, M.R.; Barbieri, J.T. Molecular basis for tetanus toxin coreceptor interactions. Biochemistry 2008, 47, 7179–7186. [Google Scholar] [CrossRef]
- Dimpfel, W.; Huang, R.T.; Habermann, E. Gangliosides in nervous tissue cultures and binding of 125I-labelled tetanus toxin, a neuronal marker. J. Neurochem. 1977, 29, 329–334. [Google Scholar] [CrossRef]
- Herreros, J.; Ng, T.; Schiavo, G. Lipid rafts act as specialized domains for tetanus toxin binding and internalization into neurons. Mol. Biol. Cell. 2001, 12, 2947–2960. [Google Scholar] [CrossRef] [Green Version]
- Rogers, T.B.; Snyder, S.H. High affinity binding of tetanus toxin to mammalian brain membranes. J. Biol. Chem. 1981, 256, 2402–2407. [Google Scholar] [CrossRef]
- Walton, K.M.; Sandberg, K.; Rogers, T.B.; Schnaar, R.L. Complex ganglioside expression and tetanus toxin binding by PC12 pheochromocytoma cells. J. Biol. Chem. 1988, 263, 2055–2063. [Google Scholar] [CrossRef]
- Lalli, G.; Gschmeissner, S.; Schiavo, G. Myosin Va and microtubule-based motors are required for fast axonal retrograde transport of tetanus toxin in motor neurons. J. Cell. Sci. 2003, 116, 4639–4650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deinhardt, K.; Salinas, S.; Verastegui, C.; Watson, R.; Worth, D.; Hanrahan, S.; Bucci, C.; Schiavo, G. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron 2006, 52, 293–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafezparast, M.; Klocke, R.; Ruhrberg, C.; Marquardt, A.; Ahmad-Annuar, A.; Bowen, S.; Lalli, G.; Witherden, A.S.; Hummerich, H.; Nicholson, S.; et al. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science 2003, 300, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Toivonen, J.M.; Oliván, S.; Osta, R. Tetanus toxin C-fragment: The courier and the cure? Toxins 2010, 2, 2622–2644. [Google Scholar] [CrossRef]
- Bayart, C.; Mularoni, A.; Hemmani, N.; Kerachni, S.; Jose, J.; Gouet, P.; Paladino, J.; Le Borgne, M. Tetanus Toxin Fragment C: Structure, Drug Discovery Research and Production. Pharmaceuticals 2022, 15, 756. [Google Scholar] [CrossRef]
- Moreno-Martinez, L.; de la Torre, M.; Muñoz, M.J.; Zaragoza, P.; Aguilera, J.; Calvo, A.C.; Osta, R. Neuroprotective Fragment C of Tetanus Toxin Modulates IL-6 in an ALS Mouse Model. Toxins 2020, 12, 330. [Google Scholar] [CrossRef]
- Moreno-Galarza, N.; Mendieta, L.; Palafox-Sánchez, V.; Herrando-Grabulosa, M.; Gil, C.; Limón, D.I.; Aguilera, J. Peripheral Administration of Tetanus Toxin Hc Fragment Prevents MPP Toxicity In Vivo. Neurotox. Res. 2018, 34, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Patricio-Martínez, A.; Mendieta, L.; Martínez, I.; Aguilera, J.; Limón, I.D. The recombinant C-terminal fragment of tetanus toxin protects against cholinotoxicity by intraseptal injection of β-amyloid peptide (25–35) in rats. Neuroscience 2016, 315, 18–30. [Google Scholar] [CrossRef]
- Duggan, M.J.; Quinn, C.P.; Chaddock, J.A.; Purkiss, J.R.; Alexander, F.C.G.; Doward, S.; Fooks, S.J.; Friis, L.M.; Hall, Y.H.J.; Kirby, E.R.; et al. Inhibition of release of neurotransmitters from rat dorsal root ganglia by a novel conjugate of a Clostridium botulinum toxin A endopeptidase fragment and Erythrina cristagalli lectin. J. Biol. Chem. 2002, 277, 34846–34852. [Google Scholar] [CrossRef] [Green Version]
- Chaddock, J.A.; Purkiss, J.R.; Alexander, F.C.G.; Doward, S.; Fooks, S.J.; Friis, L.M.; Hall, Y.H.J.; Kirby, E.R.; Leeds, N.; Moulsdale, H.J.; et al. Retargeted clostridial endopeptidases: Inhibition of nociceptive neurotransmitter release in vitro, and antinociceptive activity in in vivo models of pain. Mov. Disord. 2004, 19 (Suppl. 8), S42–S47. [Google Scholar] [CrossRef]
- Mangione, A.S.; Obara, I.; Maiarú, M.; Geranton, S.M.; Tassorelli, C.; Ferrari, E.; Leese, C.; Davletov, B.; Hunt, S.P. Nonparalytic botulinum molecules for the control of pain. Pain 2016, 157, 1045–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arsenault, J.; Ferrari, E.; Niranjan, D.; Cuijpers, S.A.G.; Gu, C.; Vallis, Y.; O’Brien, J.; Davletov, B. Stapling of the botulinum type A protease to growth factors and neuropeptides allows selective targeting of neuroendocrine cells. J. Neurochem. 2013, 126, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zurawski, T.H.; Meng, J.; Lawrence, G.W.; Aoki, K.R.; Wheeler, L.; Dolly, J.O. Novel chimeras of botulinum and tetanus neurotoxins yield insights into their distinct sites of neuroparalysis. FASEB J. 2012, 26, 5035–5048. [Google Scholar] [CrossRef] [PubMed]
- Antoniazzi, C.; Belinskaia, M.; Zurawski, T.; Kaza, S.K.; Dolly, J.O.; Lawrence, G.W. Botulinum Neurotoxin Chimeras Suppress Stimulation by Capsaicin of Rat Trigeminal Sensory Neurons In Vivo and In Vitro. Toxins 2022, 14, 116. [Google Scholar] [CrossRef]
- Saez, N.J.; Senff, S.; Jensen, J.E.; Er, S.Y.; Herzig, V.; Rash, L.D.; King, G.F. Spider-venom peptides as therapeutics. Toxins 2010, 2, 2851–2871. [Google Scholar] [CrossRef] [Green Version]
- Gejl, K.D.; Ørtenblad, N.; Andersson, E.; Plomgaard, P.; Holmberg, H.-C.; Nielsen, J. Local depletion of glycogen with supramaximal exercise in human skeletal muscle fibres. J. Physiol. 2017, 595, 2809–2821. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.J.; Garcia, M.L. Therapeutic potential of venom peptides. Nat. Rev. Drug Discov. 2003, 2, 790–802. [Google Scholar] [CrossRef]
- Perry, T.; Greig, N.H. The glucagon-like peptides: A double-edged therapeutic sword? Trends Pharmacol. Sci. 2003, 24, 377–383. [Google Scholar] [CrossRef]
- Sobrinho, J.C.; Simões-Silva, R.; Holanda, R.J.; Alfonso, J.; Gomez, A.F.; Zanchi, F.B.; Moreira-Dill, L.S.; Grabner, A.N.; Zuliani, J.P.; Calderon, L.A.; et al. Antitumoral Potential of Snake Venom Phospholipases A2 and Synthetic Peptides. Curr. Pharm. Biotechnol. 2016, 17, 1201–1212. [Google Scholar] [CrossRef]
- DiPaola, R.S. To arrest or not to G(2)-M Cell-cycle arrest: Commentary re: A. K. Tyagi et al., Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth inhibition, G(2)-M arrest, and apoptosis. Clin. Cancer Res. 2002, 8, 3512–3519. [Google Scholar]
- Kato, E.E.; Sampaio, S.C. Crotoxin Modulates Events Involved in Epithelial-Mesenchymal Transition in 3D Spheroid Model. Toxins 2021, 13, 830. [Google Scholar] [CrossRef] [PubMed]
- Donato, N.J.; Martin, C.A.; Perez, M.; Newman, R.A.; Vidal, J.C.; Etcheverry, M. Regulation of epidermal growth factor receptor activity by crotoxin, a snake venom phospholipase A2 toxin. A novel growth inhibitory mechanism. Biochem. Pharmacol. 1996, 51, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qin, X.; Zhang, Z.; Chen, M.; Wang, Y.; Gao, B. Crotoxin suppresses the tumorigenic properties and enhances the antitumor activity of Iressa® (gefinitib) in human lung adenocarcinoma SPCA-1 cells. Mol. Med. Rep. 2014, 10, 3009–3014. [Google Scholar] [CrossRef] [Green Version]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Liang, H.; Qin, Z.-h.; Liu, C.-y. Crotoxin induces apoptosis and autophagy in human lung carcinoma cells in vitro via activation of the p38MAPK signaling pathway. Acta Pharmacol. Sin. 2014, 35, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Cura, J.E.; Blanzaco, D.P.; Brisson, C.; Cura, M.A.; Cabrol, R.; Larrateguy, L.; Mendez, C.; Sechi, J.C.; Silveira, J.S.; Theiller, E.; et al. Phase I and pharmacokinetics study of crotoxin (cytotoxic PLA(2), NSC-624244) in patients with advanced cancer. Clin. Cancer Res. 2002, 8, 1033–1041. [Google Scholar] [PubMed]
- Zhang, H.-L.; Han, R.; Chen, Z.-X.; Chen, B.-W.; Gu, Z.-L.; Reid, P.F.; Raymond, L.N.; Qin, Z.-H. Opiate and acetylcholine-independent analgesic actions of crotoxin isolated from Crotalus durissus terrificus venom. Toxicon Off. J. Int. Soc. Toxinology 2006, 48, 175–182. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, D.-C.; Zhou, X.-P.; Gong, S.; Cheng, B.-C.; Qin, Z.-H.; Reid, P.F.; Yin, Q.-Z.; Jiang, X.-H. Inhibitory effect of crotoxin on the pain-evoked discharge of neurons in thalamic parafascicular nucleus in rats. Toxicon Off. J. Int. Soc. Toxinology 2008, 51, 102–111. [Google Scholar] [CrossRef]
- Millan, M.J. N-methyl-D-aspartate receptor-coupled glycine B receptors in the pathogenesis and treatment of schizophrenia: A critical review. Curr. Drug Targets CNS Neurol. Disord. 2002, 1, 191–213. [Google Scholar] [CrossRef]
- Santos, I.A.; Shimizu, J.F.; de Oliveira, D.M.; Martins, D.O.S.; Cardoso-Sousa, L.; Cintra, A.C.O.; Aquino, V.H.; Sampaio, S.V.; Nicolau-Junior, N.; Sabino-Silva, R.; et al. Chikungunya virus entry is strongly inhibited by phospholipase A2 isolated from the venom of Crotalus durissus terrificus. Sci. Rep. 2021, 11, 8717. [Google Scholar] [CrossRef]
- Trevisan, G.; Oliveira, S.M. Animal Venom Peptides Cause Antinociceptive Effects by Voltage-gated Calcium Channels Activity Blockage. Curr. Neuropharmacol. 2022, 20, 1579–1599. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Luo, Z.D. Calcium channel functions in pain processing. Channels 2010, 4, 510–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malmberg, A.B.; Yaksh, T.L. Antinociception produced by spinal delivery of the S and R enantiomers of flurbiprofen in the formalin test. Eur. J. Pharmacol. 1994, 256, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Livett, B.G.; Gayler, K.R.; Khalil, Z. Drugs from the sea: Conopeptides as potential therapeutics. Curr. Med. Chem. 2004, 11, 1715–1723. [Google Scholar] [CrossRef]
- Twede, V.D.; Miljanich, G.; Olivera, B.M.; Bulaj, G. Neuroprotective and cardioprotective conopeptides: An emerging class of drug leads. Curr. Opin. Drug Discov. Devel. 2009, 12, 231–239. [Google Scholar]
- Schroeder, C.I.; Smythe, M.L.; Lewis, R.J. Development of small molecules that mimic the binding of omega-conotoxins at the N-type voltage-gated calcium channel. Mol. Divers 2004, 8, 127–134. [Google Scholar] [CrossRef]
- Schmidtko, A.; Lötsch, J.; Freynhagen, R.; Geisslinger, G. Ziconotide for treatment of severe chronic pain. Lancet 2010, 375, 1569–1577. [Google Scholar] [CrossRef]
- Niederberger, E.; Schmidtko, A.; Rothstein, J.D.; Geisslinger, G.; Tegeder, I. Modulation of spinal nociceptive processing through the glutamate transporter GLT-1. Neuroscience 2003, 116, 81–87. [Google Scholar] [CrossRef]
- Carlson, G.D.; Gorden, C. Current developments in spinal cord injury research. Spine J. 2002, 2, 116–128. [Google Scholar] [CrossRef]
- Hall, E.D.; Springer, J.E. Neuroprotection and acute spinal cord injury: A reappraisal. NeuroRx 2004, 1, 80–100. [Google Scholar] [CrossRef]
- Lu, J.; Ashwell, K.W.; Waite, P. Advances in secondary spinal cord injury: Role of apoptosis. Spine 2000, 25, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Isaac, L.; Pejic, L. Secondary mechanisms of spinal cord injury. Surg. Neurol. 1995, 43, 484–485. [Google Scholar] [CrossRef] [PubMed]
- Amar, A.P.; Levy, M.L. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery 1999, 44, 1027–1039. [Google Scholar] [CrossRef]
- Liu, W.-M.; Wu, J.-Y.; Li, F.-C.; Chen, Q.-X. Ion channel blockers and spinal cord injury. J. Neurosci. Res 2011, 89, 791–801. [Google Scholar] [CrossRef]
- Naderi, A.; Asgari, A.R.; Zahed, R.; Ghanbari, A.; Samandari, R.; Jorjani, M. Estradiol attenuates spinal cord injury-related central pain by decreasing glutamate levels in thalamic VPL nucleus in male rats. Metab. Brain Dis. 2014, 29, 763–770. [Google Scholar] [CrossRef]
- Gonçaves, J.M.; Ferreira, J.; Prado, M.A.M.; Cordeiro, M.N.; Richardson, M.; Pinheiro, A.C.d.N.; Silva, M.A.R.; Junior, C.J.d.C.; Souza, A.H.; Gomez, M.V. The effect of spider toxin PhTx3-4, ω-conotoxins MVIIA and MVIIC on glutamate uptake and on capsaicin-induced glutamate release and [Ca2+]i in spinal cord synaptosomes. Cell Mol. Neurobiol. 2011, 31, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, S.; Matsushima, K.; Fujita, H.; Nanri, K.; Ogawa, S.; Shinohara, Y. A selective N-type calcium channel antagonist reduces extracellular glutamate release and infarct volume in focal cerebral ischemia. J. Cereb. Blood Flow. Metab. 1995, 15, 611–618. [Google Scholar] [CrossRef] [Green Version]
- Verweij, B.H.; Muizelaar, J.P.; Vinas, F.C.; Peterson, P.L.; Xiong, Y.; Lee, C.P. Mitochondrial dysfunction after experimental and human brain injury and its possible reversal with a selective N-type calcium channel antagonist (SNX-111). Neurol. Res. 1997, 19, 334–339. [Google Scholar] [CrossRef]
- Verweij, B.H.; Muizelaar, J.P.; Vinas, F.C.; Peterson, P.L.; Xiong, Y.; Lee, C.P. Improvement in mitochondrial dysfunction as a new surrogate efficiency measure for preclinical trials: Dose-response and time-window profiles for administration of the calcium channel blocker Ziconotide in experimental brain injury. J. Neurosurg. 2000, 93, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Peterson, P.L.; Verweij, B.H.; Vinas, F.C.; Muizelaar, J.P.; Lee, C.P. Mitochondrial dysfunction after experimental traumatic brain injury: Combined efficacy of SNX-111 and U-101033E. J. Neurotrauma 1998, 15, 531–544. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Yu, S.; Liu, J.; Chen, R.; Zhang, Y.; Jiang, L.; Dai, Q. A novel -conotoxin Bu8 inhibiting N-type voltage-gated calcium channels displays potent analgesic activity. Acta Pharm. Sin. B 2021, 11, 2685–2693. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.R.; Richbart, S.D.; Merritt, J.C.; Brown, K.C.; Nolan, N.A.; Akers, A.T.; Lau, J.K.; Robateau, Z.R.; Miles, S.L.; Dasgupta, P. Acetylcholine signaling system in progression of lung cancers. Pharmacol. Ther. 2019, 194, 222–254. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Catassi, A.; Cesario, A.; Servent, D. Development of novel therapeutic strategies for lung cancer: Targeting the cholinergic system. Curr. Med. Chem. 2006, 13, 3493–3512. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Cesario, A.; Margaritora, S.; Granone, P.; Motta, G.; Falugi, C.; Russo, P. Alpha7-nicotinic acetylcholine receptors affect growth regulation of human mesothelioma cells: Role of mitogen-activated protein kinase pathway. Cancer Res. 2004, 64, 135–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesario, A.; Russo, P.; Viaggi, S.; Trombino, S.; Imperatori, A.; Margaritora, S.; Dominioni, L.; Festi, L.; Porziella, V.; Granone, P. Malignant pleural mesothelioma: Time for translational research. Lancet Oncol. 2004, 5, 591. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Bisio, A.; Catassi, A.; Cesario, A.; Falugi, C.; Russo, P. Role of the non-neuronal human cholinergic system in lung cancer and mesothelioma: Possibility of new therapeutic strategies. Curr. Med. Chem. Anticancer. Agents 2004, 4, 535–542. [Google Scholar] [CrossRef] [PubMed]
- West, K.A.; Brognard, J.; Clark, A.S.; Linnoila, I.R.; Yang, X.; Swain, S.M.; Harris, C.; Belinsky, S.; Dennis, P.A. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J. Clin. Invest. 2003, 111, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, P.; Kinkade, R.; Joshi, B.; Decook, C.; Haura, E.; Chellappan, S. Nicotine inhibits apoptosis induced by chemotherapeutic drugs by up-regulating XIAP and survivin. Proc. Natl. Acad. Sci. USA 2006, 103, 6332–6337. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, Y.; Jiang, D.; Reid, P.F.; Jiang, X.; Qin, Z.; Tao, J. Alpha-cobratoxin inhibits T-type calcium currents through muscarinic M4 receptor and Gο-protein βγ subunits-dependent protein kinase A pathway in dorsal root ganglion neurons. Neuropharmacology 2012, 62, 1062–1072. [Google Scholar] [CrossRef]
- Xu, L.; Wang, S.; Zhang, L.; Liu, B.; Zheng, S.; Yao, M. Cobratoxin Alleviates Cancer-Induced Bone Pain in Rats via Inhibiting CaMKII Signaling Pathway after Acting on M4 Muscarinic Cholinergic Receptors. ACS Chem. Neurosci. 2022, 13, 1422–1432. [Google Scholar] [CrossRef]
- Gazerani, P.; Cairns, B.E. Venom-based biotoxins as potential analgesics. Expert Rev. Neurother. 2014, 14, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Huang, J.; Wang, S.-Z.; Qin, Z.-H.; Lin, F. Cobrotoxin extracted from Naja atra venom relieves arthritis symptoms through anti-inflammation and immunosuppression effects in rat arthritis model. J. Ethnopharmacol. 2016, 194, 1087–1095. [Google Scholar] [CrossRef]
- Bordon, K.d.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.-Q.; Qian, X.-Y.; An, J.-X.; Liu, C.-C.; Fang, Q.-W.; Wang, Y.; Jiang, Y.-D.; Cope, D.K.; Williams, J.P. Rat Model of Trigeminal Neuralgia Using Cobra Venom Mimics the Electron Microscopy, Behavioral, and Anticonvulsant Drug Responses Seen in Patients. Pain Physician 2015, 18, E1083–E1090. [Google Scholar] [PubMed]
- Zhao, C.; Zhao, J.; Yang, Q.; Ye, Y. Cobra neurotoxin produces central analgesic and hyperalgesic actions via adenosine A and A receptors. Mol. Pain 2017, 13, 1744806917720336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, F.; Reid, P.F.; Qin, Z.-H. Cobrotoxin could be an effective therapeutic for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1258–1260. [Google Scholar] [CrossRef]
- Park, M.H.; Song, H.S.; Kim, K.H.; Son, D.J.; Lee, S.H.; Yoon, D.Y.; Kim, Y.; Park, I.Y.; Song, S.; Hwang, B.Y.; et al. Cobrotoxin inhibits NF-kappa B activation and target gene expression through reaction with NF-kappa B signal molecules. Biochemistry 2005, 44, 8326–8336. [Google Scholar] [CrossRef]


| Types of Neurotoxins | Trade Name (FDA Approved) | Indications (FDA Approved) | Clinical Applications | Reference | |
|---|---|---|---|---|---|
| BoNT | Botox (BoNT/A) Xeomin (BoNT/A) Dysport (BoNT/A) Myobloc/Neurobloc (BoNT/B) | Botox | Overactive bladder (OAB) with symptoms of urge urinary; Urinary incontinence due to detrusor overactivity associated with a neurologic condition (e.g., spinal cord injury (SCI), multiple sclerosis (MS)) in adults who have an inadequate response to or are intolerant of anticholinergic medication; Neurogenic detrusor overactivity (NDO) in pediatric patients 5 years of age and older who have an inadequate response to or are intolerant of anticholinergic medication; Prophylaxis of headaches in adult patients with chronic migraine (≥15 days per month with headache lasting 4 h a day or longer); Spasticity in patients 2 years of age and older; Cervical dystonia in adult patients; Severe axillary hyperhidrosis that is inadequately managed by topical agents in adult patients; Blepharospasm associated with dystonia in patients 12 years of age and older; Strabismus in patients 12 years of age and older. | Dystonic muscle contractions Neuropathic pain Neuroinflammation Depression (under investigation) Skin diseases Headache | [164] [155,156,165,166] [167] [168] [158] [169] |
| Xeomin | Chronic sialorrhea in patients 2 years of age and older; Upper limb spasticity in adults; Upper limb spasticity in pediatric patients 2 to 17 years of age, excluding spasticity caused by cerebral palsy; Cervical dystonia in adults; Blepharospasm in adults; Temporary improvement in the appearance of moderate-to-severe glabellar lines with corrugator and/or procerus muscle activity in adults. | ||||
| Dysport | Cervical dystonia in adults; Temporary improvement in the appearance of moderate-to-severe glabellar lines associated with procerus and corrugator muscle activity in adults < 65 years of age; Spasticity in patients 2 years of age and older. | ||||
| Myobloc/Neurobloc | Cervical dystonia to reduce the severity of abnormal head position and neck pain associated with cervical dystonia in adults; Chronic sialorrhea in adults. | ||||
| TeNT | Improve the motor functions Carrier to deliver into the CNS | [170] [171] | |||
| α-LTX | Type I diabetes (expected) | [172] | |||
| Snake Presynaptic Neurotoxins | Anticancer Antibacterial Antinociception | [173] [174] [175] | |||
| ω-agatoxin | Modulate the nociceptive process | [176] | |||
| Conotoxins | Prialt™ (Ziconitide) (a form of ω-conotoxin MVIIA) | Management of severe chronic pain in patients for whom intrathecal therapy is warranted and who are intolerant of or refractory to other treatment, such as systemic analgesics, adjunctive therapies, or intrathecal morphine. | Chronic pain (cancer- or AIDS-related neuropathy) Spinal cord injury | [177] [178] | |
| DTX | Diagnosis of neurodegenerative diseases (potential) | [179] [180] | |||
| Postsynaptic Neurotoxins | Cobratide | Disorders linked to NMJ dysfunction Anticancer Anti-inflammation Analgesic effect | [181] [182] [183] [183] | ||
| Applications | ||
|---|---|---|
| Botox | Muscle | Blepharospasm hemifacial spasm Strabismus cervical dystonia Upper limb and lower limb (adults) spasticity Bladder (neurogenic detrusor overactive (DO), overactive bladder (OB)) Forehead wrinkles |
| Other | Migraine | |
| Xeomin | Muscle | Cervical dystonia frown lines Blepharospasm upper limb spasticity |
| Other | Sialorrhea in adults | |
| Dysport | Muscle | Cervical dystonia Upper limb (adults) and lower limb (children + adults) spasticity Frown lines and wrinkles |
| Myobloc/Neurobloc | Cervical dystonia | |
| Prialt™ | Severe chronic pain | |
| Cobratide | Chronic pain | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, K.; Luo, W.; Liu, T.; Ni, Y.; Qin, Z. Neurotoxins Acting at Synaptic Sites: A Brief Review on Mechanisms and Clinical Applications. Toxins 2023, 15, 18. https://doi.org/10.3390/toxins15010018
Zhou K, Luo W, Liu T, Ni Y, Qin Z. Neurotoxins Acting at Synaptic Sites: A Brief Review on Mechanisms and Clinical Applications. Toxins. 2023; 15(1):18. https://doi.org/10.3390/toxins15010018
Chicago/Turabian StyleZhou, Kunming, Weifeng Luo, Tong Liu, Yong Ni, and Zhenghong Qin. 2023. "Neurotoxins Acting at Synaptic Sites: A Brief Review on Mechanisms and Clinical Applications" Toxins 15, no. 1: 18. https://doi.org/10.3390/toxins15010018