Compositional Variability of Monazite–Cheralite–Huttonite Solid Solutions, Xenotime, and Uraninite in Geochemically Distinct Granites with Special Emphasis to the Strongly Fractionated Peraluminous Li–F–P-Rich Podlesí Granite System (Erzgebirge/Krušné Hory Mts., Central Europe)
Abstract
:1. Introduction
2. Geology
2.1. Variscan Granites of the Erzgebirge/Krušné Hory Area
- Low-F peraluminious biotite granites are abundant throughout the whole Erzgebirge (i.e., the Kirchberg pluton and a part of the Nejdek pluton in the west, Lesná granite in the central Erzgebirge, and the Fláje, Niederbobritsch, and Telnice plutons in the east);
- Low-F two-mica peraluminous granites represented by the Bergen massif in the western Erzgebirge; similar granites are more common in the adjacent Fichtelgebirge and Slavkovský Les areas;
- P–F–Li-rich peraluminous Li-mica granites form large outcropping bodies in the west (the Eibenstock–Nejdek pluton) and several, mostly hidden, bodies in the central Erzgebirge (Geyer, Ehrenfriedersdorf, Annaberg, Satzung). Micas in these granites evolved from Li-enriched biotite in less fractionated granites to zinnwaldite in later-formed, evolved granites forming small stocks; topaz is common;
- F–Li-rich and P-poor granites of A-type chemical signature which form less voluminous plutons, comprising the occurrences of strongly mineralized granite in the eastern Erzgebirge (Schellerhau, Sadisdorf, Altenberg, Zinnwald/Cínovec and Krupka). Micas in these granites are well evolved from Li-enriched biotite to zinnwaldite; magmatic fluorite is common;
- Medium-F and P-poor biotite-to-Li-mica granites of A-type chemical signature form dykes, stocks, and breccia pipes in the central (Seifen, Hora svaté Kateřiny) and western (the Gottesberg-Schneckenstein dyke swarm) parts of the province.

2.2. The Strongly Fractionated Podlesí Granite System
3. Methods
- The minerals designated as “monazite” or “xenotime” in this paper are, according to the IMA-CNMMN nomenclature, monazite-(Ce) and xenotime-(Y). We used abbreviated names in order to make the text more fluent.
- According to IMA-CNMNM rules, only endmember names should be used in the mica group of minerals; i.e., all Li–Fe mica varieties should be described either as annite/siderophyllite or as lepidolite. This is, from the petrological point of view, unsatisfactory. Similarly, as in the case of feldspars, a more elaborated classification is demand. Here, we used the traditional names protolithionite and zinnwaldite for micas with ideal compositions, namely K2LiFe4Al(Al2Si6O20) (F, OH)4 and K2Li2Fe2Al2(Al2Si6O20)F4, respectively.
4. Results
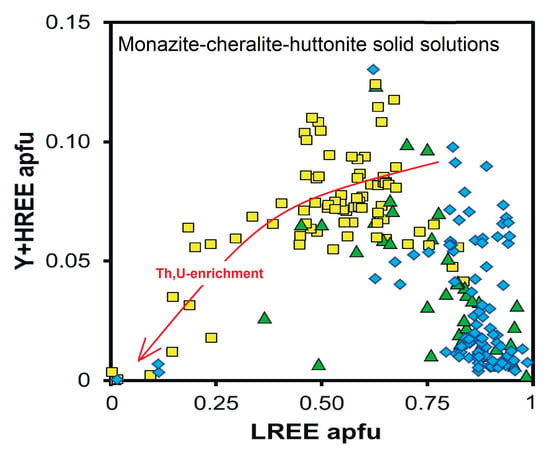
4.1. Monazite–Cheralite–Huttonite Solid Solutions
4.2. Xenotime-(Y)
4.3. Uraninite
5. Discussion
5.1. Evolution of Monazite and Xenotime Compositions during Magmatic/Hydrothermal Evolution of the Podlesí Granite System
5.2. Diversity of Monazite and Xenotime Compositions among Granite Groups
- Th-poor monazite with a steep but smooth REE distribution pattern gradually declining from La to Gd and devoid of anomalies, with a distinct negative Eu anomaly and very low contents of the HREE. This pattern characterizes less fractionated biotite granites of both S and A types (Nejdek–Eibenstock pluton, Schneckenstein, Gottesberg, lower part of the Cínovec cupola) and, occasionally, also less evolved intrusions of the peraluminous Li-mica granite group. This may be interpreted as a product of magmatic crystallization from relatively F-poor melt without or with only low-grade fluid–melt and/or fluid–crystals reaction.
- Th-poor monazite with REE patterns similar to previous type 1 but with maxima at Ce and Pr occurs in more evolved A-type granite facies at Cínovec and Hora Svaté Kateřiny.
- Th-rich monazite with relatively lower and flat LREE pattern from La to Sm, again with maxima at Ce and Pr, typifies peraluminous Li-mica granites including the most fractionated member of this group, the Podlesí stock. In these rocks, the relatively lower sum of REEs is compensated by enrichment in Th.

- Xenotime with a flat MREE–HREE pattern characterizes less fractionated biotite granites of both S- and A-type affiliation. This xenotime is mostly Y-rich, containing 0.80–0.85 apfu Y. Y/Ho ratios are generally near-chondritic (26–34) in less evolved rocks, but also may be superchondritic (up to 55) in more fractionated group 1–2 granites from Kirchberg and Bergen.
- Xenotime, with a smoothly inclining, Yb–Lu-dominated chondrite-normalized REE pattern defines fractionated A-type granites. At the same time, this type of xenotime is the most Y-poor one among all analyzed xenotime varieties. Xenotime exhibiting the lowest Y content (0.4–0.6 apfu Y) was discovered immediately below the upper contact of mineralized cupolas of A-type granites in the eastern Erzgebirge. Fractionated granites in the eastern Erzgebirge frequently contain xenotime possessing subchondritic Y/Ho ratios (down to 14).
- MREE-dominant (Gd–Tb–Dy) xenotime with 0.75–0.83 apfu Y is typical of peraluminous Li-mica granites of group #3. Most evolved representatives of this group are distinguished by suprachondritic Y/Ho ratios (up to 44).
5.3. Comparison with Other Areas
6. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bea, F. Residence of REE, Y, Th and U in granites and crustal protoliths; implications for the chemistry of crustal melts. J. Petrol. 1996, 7, 521–552. [Google Scholar] [CrossRef]
- Förster, H.-J. The Variscan Granites of the Erzgebirge and Their Accessory Minerals. Ph.D. Thesis, Technical University Bergakademie Freiberg, Freiberg, Germany, 1998. [Google Scholar]
- Förster, H.-J. The chemical composition of uraninite in Variscan granites of the Erzgebirge, Germany. Mineral. Mag. 1999, 63, 239–252. [Google Scholar] [CrossRef]
- Trumbull, R.; Förster, H.-J.; Rhede, D. REE–Y–Th–U bearing accessory minerals and their contribution to the lanthanide and actinide trace-element budget in an anorogenic granite from the Erongo complex, NW Namibia. Z. Geol. Wiss. 2010, 38, 145–165. [Google Scholar]
- Breiter, K. Monazite and zircon as major carriers of Th, U, and Y in peraluminous granites: Examples from the Bohemian Massif. Mineral. Petrol. 2016, 110, 767–785. [Google Scholar] [CrossRef]
- Förster, H.-J. The chemical composition of REE–Y–Th-U-rich accessory minerals in peraluminous granites of the Erzgebirge-Fichtelgebirge region, Germany, Part I: The monazite-(Ce)–brabantite solid solution series. Am. Mineral. 1998, 83, 259–272. [Google Scholar] [CrossRef]
- Förster, H.-J. The chemical composition of REE–Y–Th–U-rich accessory minerals in peraluminous granites of the Erzgebirge-Fichtelgebirge region, Germany, Part II: Xenotime. Am. Mineral. 1998, 83, 1302–1315. [Google Scholar] [CrossRef]
- Förster, H.-J. Composition and origin of intermediate solid solutions in the system thorite–xenotime–zircon–coffinite. Lithos 2006, 88, 35–55. [Google Scholar] [CrossRef]
- Förster, H.-J.; Gottesmann, B.; Tischendorf, G.; Siebel, W.; Rhede, D.; Seltmann, R.; Wasternack, J. Permo-Carboniferous subvolcanic rhyolitic dikes in the western Erzgebirge/Vogtland, Germany: A record of source heterogeneity of post-collisional felsic magmatism. N. J. Miner. Abh. 2007, 183, 123–147. [Google Scholar] [CrossRef]
- Breiter, K.; Čopjaková, R.; Škoda, R. The involvement of F, CO2-, and As in the alteration of Zr–Th–REE-bearing accessory minerals in the Hora Svaté Kateřiny A-type granite, Czech Republic. Can. Mineral. 2009, 47, 1375–1398. [Google Scholar] [CrossRef]
- Tischendorf, G. Silicic Magmatism and Metallogenesis of the Erzgebirge; Veröffentlichungen des Zentralinstituts für Physik der Erde: Potsdam, Germany, 1989; Volume 107, pp. 1–316. [Google Scholar]
- Förster, H.-J.; Tischendorf, G.; Trumbull, R.B.; Gottesmann, B. Late-collisional granites in the Variscan Erzgebirge, Germany. J. Petrol. 1999, 40, 1613–1645. [Google Scholar] [CrossRef]
- Förster, H.-J.; Romer, R.L. Carboniferous magmatism. In Pre-Mesozoic Geology of Saxo-Thuringia—From the Cadomian Active Margin to the Variscan Orogen; Linneman, U., Romer, R.L., Eds.; Schweizerbart: Stuttgart, Germany, 2010; pp. 287–308. [Google Scholar]
- Breiter, K. Nearly contemporaneous evolution of the A- and S-type fractionated granites in the Krušné hory/Erzgebirge Mts., Central Europe. Lithos 2012, 151, 105–121. [Google Scholar] [CrossRef]
- Laube, G.C. Geologie des Böhmischen Erzgebirges Teil I; Commissions-Verlag von Fr. Řivnáč: Prag, Czech Republic, 1876; p. 238. [Google Scholar]
- Teuscher, E.O. Primäre Bildungen des granitischen Magmas und seiner Restlösungen im Massif von Eibenstock-Neudeck. Miner. Petrogr. Mitt. 1936, 47, 211–262. [Google Scholar]
- Breiter, K.; Sokolová, M.; Sokol, A. Geochemical specialization of the tin-bearing granitoid massifs of NW Bohemia. Miner. Depos. 1991, 26, 298–306. [Google Scholar] [CrossRef]
- Tichomirowa, M.; Käßner, A.; Sperner, B.; Lapp, M.; Leonhardt, D.; Linnemann, U.; Münker, C.; Ovtcharova, M.; Pfänder, J.A.; Schaltegger, U.; et al. Dating multiply overprinted granites: The effect of protracted magmatism and fluid flow on dating sytems (zircon U–Pb: SHRIMP/SIMS, LA-ICP-MS, CA-ID-TIMS; and Rb–Sr, Ar–Ar) – Granites from the Western Erzgebirge (Bohemian Massif, Germany). Chem. Geol. 2019, 519, 11–38. [Google Scholar] [CrossRef]
- Förster, H.-J.; Rhede, D. The Be–Ta-rich granite of Seiffen (eastern Erzgebirge, Germany): Accessory-mineral chemistry, composition and age of a late-Variscan Li–F granite of A-type affinity. N. J. Miner. Abh. 2006, 182, 307–321. [Google Scholar] [CrossRef]
- Zhang, R.; Lehmann, B.; Seltmann, R.; Sun, W.; Li, C. Cassiterite U–Pb geochronology constrains magmatic-hydrothermal evolution in complex evolved granite systems: The classic Erzgebirge tin province (Saxony and Bohemia). Geology 2017, 45. [Google Scholar] [CrossRef]
- Tomek, F.; Žák, J.; Svojtka, M.; Finger, F.; Waitzinger, M. Emplacement dynamics of syn-collapse ring dykes: An example from the Altenberg-Teplice caldera, Bohemian Massif. Bull. GSA 2019, 131, 997–1016. [Google Scholar] [CrossRef]
- Breiter, K.; Frýda, J.; Seltmann, R.; Thomas, R. Mineralogical evidence for two magmatic stages in the evolution of an extremely fractionated P-rich rare-metal granite: The Podlesí stock. J. Petrol. 1997, 38, 1723–1739. [Google Scholar] [CrossRef]
- Breiter, K.; Müller, A.; Leichmann, J.; Gabašová, A. Textural and chemical evolution of a fractionated granitic system: The Podlesí stock, Czech Republic. Lithos 2005, 80, 323–345. [Google Scholar] [CrossRef]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Linthout, K. Tripartite division of the system 2REEPO4–CaTh(PO4)2–2ThSiO4, discredition of brabantite, and recognition of cheralite as the name for members dominated by CaTh(PO4)2. Can. Mineral. 2007, 45, 503–508. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Charoy, B.; Noronha, F. Multistage growth of a rare-element, volatile-rich microgranite at Argemela. J. Petrol. 1996, 37, 73–94. [Google Scholar] [CrossRef]
- Raimbault, L.; Cuney, M.; Azencott, C.; Duthou, J.L.; Joron, J.L. Geochemical evidence for a multistage magmatic genesis of Ta–Sn–Li mineralization in the granite at Beauvoir, French Massif Central. Econ. Geol. 1995, 90, 548–596. [Google Scholar] [CrossRef]
- Hösel, G. Das Zinnerz-Lagerstättengebiet Ehrenfriedersdorf/Erzgebirge. Bergbaumonographie 1994, 1, 189. [Google Scholar]
- Huang, X.; Wang, R.C.; Chen, X.M.; Hu, H.; Liu, C.S. Vertical variation in the mineralogy of the Yichun topaz–lepidolite granite, Jiangxi province, Southern China. Can. Mineral. 2002, 40, 1047–1068. [Google Scholar] [CrossRef]
- Kirkham, R.V.; Sinclair, W.D. Comb quartz layers in felsic intrusions and their relationship to porphyry deposits. Recent advances in the geology of granite-related deposits. Can. Inst. Min. Metall. 1988, 39, 50–71. [Google Scholar]
- Breiter, K.; Škoda, R.; Uher, P. Nb–Ta–Ti–W–Sn-oxide minerals as indicators of a peraluminous P- and F-rich granitic system evolution: Podlesí, Czech Republic. Mineral. Petrol. 2007, 91, 225–248. [Google Scholar] [CrossRef]
- Mogilevsky, P. On the miscibility gap in monazite–xenotime systems. Chem. Geol. 2007, 34, 201–214. [Google Scholar] [CrossRef]
- Breiter, K. Mineral and textural evaluation of subvolcanic A-type granite: Hora sv. Kateřiny stock, Krušné Hory Mts., Czech Republic. Z. Geol. Wiss. 2008, 36, 365–382. [Google Scholar]
- Breiter, K.; Ďurišová, J.; Hrstka, T.; Korbelová, Z.; Hložková Vaňková, M.; Vašinová Galiová, M.; Kanický, V.; Rambousek, P.; Knésl, I.; Dobeš, P.; et al. Assessment of magmatic vs. metasomatic processes in rare-metal granites: A case study of the Cínovec/Zinnwald Sn–W–Li deposit, Central Europe. Lithos 2017, 292–293, 198–217. [Google Scholar] [CrossRef]
- Seydoux-Guillaume, A.M.; Wirth, R.; Heinrich, W.; Montel, J.-M. Experimental determination of thorium partitioning between monazite and xenotime using analytical electron microscopy and X-ray diffraction Rietveld analysis. Eur. J. Mineral. 2002, 14, 869–878. [Google Scholar] [CrossRef]
- Heinrich, W.; Andrehs, G.; Franz, G. Monazite–xenotime miscibility gap thermometry: I. An empirical calibration. J. Metamorph. Geol. 1997, 15, 3–17. [Google Scholar] [CrossRef]
- Lv, Z.-H.; Zhang, H.; Tang, Y. Lanthanide tetrads with implications for liquid immiscibility in an evolving magmatic-hydrothermal system: Evidence from rare earth elements in zircon from the No. 112 pegmatite, Kelumute, Chinese Altai. J. Asian Earth Sci. 2018, 164, 9–22. [Google Scholar] [CrossRef]
- Liu, C.-Q.; Zhang, H. The lanthanide tetrad effect in apatite from the Altay No. 3 pegmatite, Xingjiang, China: An intrinsic feature of the pegmatite magma. Chem. Geol. 2005, 214, 61–77. [Google Scholar] [CrossRef]
- Bau, M. Controls on the fractionation of isovalent trace elements in magmatic and aqueous systems: Evidence from Y/Ho, Zr/Hf, and lanthanide tetrad effect. Contrib. Mineral. Petrol. 1996, 123, 323–333. [Google Scholar] [CrossRef]
- Irber, W. The lanthanide tetrad effect and its correlation with K/Rb, Eu/Eu*, Sr/Eu, Y/Ho, and Zr/Hf of evolving peraluminous granite suites. Geochim. Cosmochim. Acta 1999, 63, 489–508. [Google Scholar] [CrossRef]
- Monecke, M.; Dulski, P.; Kempe, U. Origin of convex tetrads in rare earth element patterns of hydrothermally altered siliceous igneous rocks from the Zinnwald Sn–W deposit, Germany. Geochim. Cosmochim. Acta 2007, 71, 335–353. [Google Scholar] [CrossRef]
- Anenburg, M. Rare earth mineral diversity controlled by REE pattern shapes. Mineral. Mag. 2020, 84, 629–639. [Google Scholar] [CrossRef]
- Duc-Tin, Q.; Keppler, H. Monazite and xenotime solubility in granitic melts and the origin of the lanthanide tetrad effect. Contrib. Mineral. Petrol. 2015, 169, 1–26. [Google Scholar] [CrossRef]
- Anenburg, M.; Burnham, A.D.; Mavrogenes, J.A. REE redistribution textures in altered fluorapatite: Symplectites, veins and phosphate–silicate–carbonate assemblages from the Nolans Bore P–REE–Th deposit, NT, Australia. Can. Mineral. 2018, 56, 331–354. [Google Scholar] [CrossRef]
- Hecht, L. Die Glimmer als Indikatoren für die magmatische und postmagmatische Entwicklung der Granite des Fichtelgebirges (NE Bayern). Münchner Geol. Hefte 1993, 10, 1–221. [Google Scholar]
- Göd, R.; Oberlercher, G.; Brandstatter, F. Zur Geochemie und Mineralogie eines Monazit-führenden Granitkörpers im Südbohmischen Pluton (Gutau, Oberösterreich). J. Geol. Bundesanst. 1996, 139, 445–452. [Google Scholar]
- Breiter, K. Short note on a thorium-rich granite in the Three Corner Area (Dreiländereck) of Austria, Czech Republic and Germany. J. Geol. Bundesanst. 2005, 145, 141–143. [Google Scholar]
- Broska, I.; Williams, C.T.; Janák, M.; Nagy, G. Alteration and brealdown of xenotime-(Y) and monazite-(Ce) in granitic rocks of the Western Carpathians, Slovakia. Lithos 2005, 82, 71–83. [Google Scholar] [CrossRef]
- Uher, P.; Plašienka, D.; Ondrejka, M.; Hraško, L.; Konečný, P. Uranium-rich monazite-(Ce) from the Krivá type granitic cobbles in conglomerates of the Pieniny Klippen Belt, Western Carpathians, Slovakia: Composition, age determination and possible source areas. Geol. Q. 2013, 57, 343–352. [Google Scholar]
- Broska, I.; Kubiš, M. Accessory minerals and evolution of tin-bearing S-type granites in the western segment of the Gemeric Unit (Western Carpathians). Geol. Carpath. 2018, 69, 483–497. [Google Scholar] [CrossRef] [Green Version]
- René, M. REE and Y mineralogy of the Krudum granite body (Saxothuringian zone). Minerals 2018, 8, 287. [Google Scholar] [CrossRef] [Green Version]
- Förster, H.-J.; Rhede, D.; Hecht, L. Chemical composition of radioactive accessory minerals: Implications for the evolution, alteration, age, and uranium fertility of the Fichtelgebirge granites (NE Bavaria, Germany). N. J. Miner. Abh. 2008, 185, 161–182. [Google Scholar] [CrossRef]
- Finger, F.; Broska, I.; Roberts, M.P.; Schermaier, A. Rerplacement of primary monazite by apatite–allanite–epidote coronas in an amphibolite facies granite gneiss from the eastern Alps. Am. Mineral. 1998, 83, 248–258. [Google Scholar] [CrossRef]
- Pérez-Soba, C.; Villaseca, C.; Orejana, D.; Jeffries, F. Uranium-rich accessory minerals in the peraluminous and perphosphorous Belvís de Monroy pluton (Iberian Variscan belt). Contrib. Mineral. Petrol. 2014, 167, 1008. [Google Scholar] [CrossRef]
- Hoshino, M.; Watanabe, Y.; Ishihara, S. Crystal chemistry of monazite from the granitic rocks of Japan: Petrogenetic implications. Can. Mineral. 2012, 50, 1331–1346. [Google Scholar] [CrossRef]









| Assemblage, Compare Figure 2 | Sample No. | Sample Location and Description | Analyzed Minerals and Number of Analyses |
|---|---|---|---|
| A | 3361 | stockscheider (marginal pegmatite) at the uppermost contact of the stock, thickness of the stockscheider layer is 20–40 cm, composed of red Kfs crystals up to 5 × 2 cm in size and fine-grained Qtz–Ab–protolithionite matrix | Mnz (10), Xnt (9) |
| B | 3385 | Protolithionite granite, outcrop in the upper part of the stock, fine-grained granite composed of Qtz, Kfs, Ab, protolithionite, and Toz | Mnz (10), Xnt (3) |
| C | 3365 | mica-rich greisen in the upper part of the stock, boulders, dark greisen composed of F- and Fe-rich Li-poor mica and Qtz (+ Toz +Ap), feldspars only in relicts | Mnz (19), Ur (2) |
| D | 3389 | quartz-rich greisen in the upper part of the stock, outcrop, a fully greisenized flat dyke of zinnwaldite granite composed of Qtz (+ Zinnwaldite +Toz +Wolframite) | Mnz (1), Ur (1) |
| E | 3413 | zinnwaldite granite, quarry, lower part of the main flat dyke with normal medium-grained granitic texture, composed of Qtz, P-rich Kfs, P-rich Ab, Zinnwaldite, and Toz | Cher (5) |
| F | 3436, 3443 | protolithionite granite, borehole PTP-1, depths 200 and 290 m, medium-grained granite composed of Qtz, Kfs, Ab, protolithionite, and Toz | Mnz (16), Xnt (4), Ur (13) |
| G | 4206, 4208 | Li-biotite granite, borehole PTP-5, depths 114 and 136 m, composed of Qtz, Kfs, Ab, Bt (+ Toz) | Mnz (12), Xnt (6) |
| Sample | 3361 | 3361 | 3365 | 3365 | 3385 | 3385 | 3389 | 3413 | 3413 | 3436 |
|---|---|---|---|---|---|---|---|---|---|---|
| P2O5 | 30.07 | 29.84 | 30.09 | 30.00 | 29.77 | 29.78 | 29.89 | 30.13 | 29.56 | 30.02 |
| SiO2 | 0.48 | 0.40 | 0.29 | 0.34 | 0.47 | 0.42 | 0.40 | 0.21 | 0.12 | 0.39 |
| ThO2 | 15.79 | 21.29 | 19.78 | 31.35 | 14.16 | 19.23 | 25.00 | 44.73 | 58.33 | 23.00 |
| UO2 | 3.89 | 2.91 | 5.25 | 3.61 | 2.16 | 2.80 | 5.02 | 6.32 | 0.05 | 3.38 |
| Y2O3 | 3.47 | 2.60 | 3.80 | 2.36 | 2.44 | 2.80 | 2.72 | 0.00 | 0.12 | 2.43 |
| La2O3 | 6.41 | 4.78 | 4.73 | 4.71 | 5.49 | 6.04 | 2.79 | 0.94 | 0.00 | 4.53 |
| Ce2O3 | 18.78 | 16.39 | 14.67 | 11.41 | 21.25 | 17.47 | 12.53 | 3.00 | 0.04 | 15.25 |
| Pr2O3 | 2.31 | 2.11 | 1.88 | 1.39 | 2.86 | 2.17 | 1.82 | 0.41 | 0.00 | 2.06 |
| Nd2O3 | 8.31 | 7.81 | 6.52 | 4.34 | 10.32 | 8.00 | 6.27 | 1.66 | 0.08 | 7.23 |
| Sm2O3 | 2.78 | 3.18 | 2.60 | 1.55 | 3.48 | 2.62 | 3.15 | 0.41 | 0.02 | 2.67 |
| Gd2O3 | 2.33 | 2.24 | 2.36 | 1.16 | 2.30 | 2.20 | 2.34 | 0.21 | 0.05 | 1.90 |
| Tb2O3 | 0.38 | 0.34 | 0.41 | 0.07 | 0.31 | 0.28 | 0.35 | 0.06 | 0.00 | 0.14 |
| Dy2O3 | 1.08 | 0.84 | 1.24 | 0.46 | 0.79 | 0.83 | 0.88 | 0.07 | 0.08 | 0.72 |
| Ho2O3 | 0.07 | 0.05 | 0.08 | 0.04 | 0.05 | 0.09 | 0.08 | 0.00 | 0.00 | 0.08 |
| Er2O3 | 0.26 | 0.19 | 0.24 | 0.04 | 0.16 | 0.10 | 0.11 | 0.05 | 0.00 | 0.10 |
| Yb2O3 | 0.00 | 0.03 | 0.05 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| CaO | 3.78 | 4.66 | 5.26 | 6.79 | 3.18 | 4.15 | 6.04 | 10.63 | 11.11 | 5.17 |
| PbO | 0.36 | 0.38 | 0.51 | 0.58 | 0.28 | 0.38 | 0.58 | 0.88 | 0.15 | 0.44 |
| Total | 100.52 | 100.05 | 99.75 | 100.21 | 99.46 | 99.35 | 99.97 | 99.71 | 99.70 | 99.51 |
| P | 0.984 | 0.985 | 0.988 | 0.986 | 0.987 | 0.987 | 0.985 | 0.994 | 0.986 | 0.991 |
| Si | 0.018 | 0.016 | 0.011 | 0.013 | 0.018 | 0.017 | 0.016 | 0.008 | 0.005 | 0.015 |
| Th | 0.139 | 0.189 | 0.175 | 0.277 | 0.126 | 0.171 | 0.222 | 0.397 | 0.523 | 0.204 |
| U | 0.033 | 0.025 | 0.045 | 0.031 | 0.019 | 0.024 | 0.043 | 0.055 | 0.000 | 0.029 |
| Y | 0.071 | 0.054 | 0.079 | 0.049 | 0.051 | 0.058 | 0.056 | 0.000 | 0.003 | 0.050 |
| La | 0.091 | 0.069 | 0.068 | 0.067 | 0.079 | 0.087 | 0.040 | 0.014 | 0.000 | 0.065 |
| Ce | 0.266 | 0.234 | 0.208 | 0.162 | 0.305 | 0.251 | 0.179 | 0.043 | 0.001 | 0.218 |
| Pr | 0.033 | 0.030 | 0.027 | 0.020 | 0.041 | 0.031 | 0.026 | 0.006 | 0.000 | 0.029 |
| Nd | 0.115 | 0.109 | 0.090 | 0.060 | 0.144 | 0.112 | 0.087 | 0.023 | 0.001 | 0.101 |
| Sm | 0.037 | 0.043 | 0.035 | 0.021 | 0.047 | 0.035 | 0.042 | 0.005 | 0.000 | 0.036 |
| Gd | 0.030 | 0.029 | 0.030 | 0.015 | 0.030 | 0.029 | 0.030 | 0.003 | 0.001 | 0.025 |
| Tb | 0.005 | 0.004 | 0.005 | 0.001 | 0.004 | 0.004 | 0.005 | 0.001 | 0.000 | 0.002 |
| Dy | 0.013 | 0.011 | 0.015 | 0.006 | 0.010 | 0.010 | 0.011 | 0.001 | 0.001 | 0.009 |
| Ho | 0.001 | 0.001 | 0.001 | 0.000 | 0.001 | 0.001 | 0.001 | 0.000 | 0.000 | 0.001 |
| Er | 0.003 | 0.002 | 0.003 | 0.000 | 0.002 | 0.001 | 0.001 | 0.001 | 0.000 | 0.001 |
| Yb | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Ca | 0.156 | 0.194 | 0.219 | 0.282 | 0.133 | 0.174 | 0.252 | 0.444 | 0.469 | 0.216 |
| Pb | 0.004 | 0.004 | 0.005 | 0.006 | 0.003 | 0.004 | 0.006 | 0.009 | 0.002 | 0.005 |
| Sample | 3361 | 3361 | 3361 | 3385 | 3385 | 3436 | 3436 | 3443 | 3443 |
|---|---|---|---|---|---|---|---|---|---|
| P2O5 | 34.63 | 34.62 | 34.37 | 34.16 | 34.56 | 33.46 | 33.54 | 33.60 | 33.37 |
| SiO2 | 0.50 | 0.57 | 0.58 | 0.65 | 0.48 | 1.21 | 1.19 | 0.90 | 1.17 |
| ThO2 | 0.39 | 0.42 | 0.75 | 0.57 | 0.56 | 1.00 | 0.97 | 0.66 | 1.04 |
| UO2 | 4.87 | 5.13 | 3.67 | 3.26 | 2.93 | 5.39 | 4.98 | 5.18 | 3.73 |
| Y2O3 | 40.66 | 39.75 | 42.35 | 42.42 | 42.80 | 40.96 | 41.49 | 41.32 | 41.28 |
| Ce2O3 | 0.12 | 0.09 | 0.08 | 0.05 | 0.05 | 0.15 | 0.10 | 0.09 | 0.11 |
| Pr2O3 | 0.04 | 0.05 | 0.05 | 0.03 | 0.00 | 0.04 | 0.05 | 0.04 | 0.05 |
| Nd2O3 | 0.30 | 0.36 | 0.33 | 0.29 | 0.40 | 0.43 | 0.42 | 0.47 | 0.43 |
| Sm2O3 | 0.42 | 0.45 | 0.45 | 0.32 | 0.43 | 0.46 | 0.41 | 0.35 | 0.53 |
| Gd2O3 | 3.77 | 3.87 | 3.85 | 3.68 | 3.82 | 3.38 | 3.23 | 3.30 | 3.55 |
| Tb2O3 | 0.98 | 0.92 | 1.04 | 0.81 | 0.81 | 0.71 | 0.84 | 0.70 | 0.70 |
| Dy2O3 | 6.58 | 6.54 | 6.65 | 6.42 | 6.50 | 5.71 | 5.90 | 5.71 | 5.88 |
| Ho2O3 | 0.80 | 0.79 | 0.84 | 1.04 | 0.98 | 1.01 | 0.94 | 0.88 | 0.96 |
| Er2O3 | 2.20 | 2.03 | 2.23 | 2.72 | 2.87 | 2.71 | 2.77 | 2.62 | 2.65 |
| Tm2O3 a | 0.31 | 0.27 | 0.31 | 0.33 | 0.34 | 0.36 | 0.37 | 0.35 | 0.39 |
| Yb2O3 | 1.82 | 1.64 | 1.91 | 1.67 | 1.66 | 2.09 | 2.18 | 1.97 | 2.08 |
| Lu2O3 | 0.33 | 0.32 | 0.32 | 0.29 | 0.29 | 0.35 | 0.30 | 0.30 | 0.37 |
| CaO | 0.96 | 0.98 | 0.58 | 0.35 | 0.64 | 0.48 | 0.49 | 0.70 | 0.67 |
| PbO | 0.13 | 0.17 | 0.13 | 0.17 | 0.10 | 0.25 | 0.22 | 0.00 | 0.15 |
| Total | 99.81 | 98.97 | 100.49 | 99.23 | 100.22 | 100.17 | 100.40 | 99.14 | 99.11 |
| P | 0.988 | 0.993 | 0.977 | 0.979 | 0.981 | 0.962 | 0.961 | 0.970 | 0.963 |
| Si | 0.017 | 0.019 | 0.019 | 0.022 | 0.016 | 0.041 | 0.040 | 0.031 | 0.040 |
| Th | 0.003 | 0.003 | 0.006 | 0.004 | 0.004 | 0.008 | 0.007 | 0.005 | 0.008 |
| U | 0.037 | 0.039 | 0.027 | 0.025 | 0.022 | 0.041 | 0.038 | 0.039 | 0.028 |
| Y | 0.729 | 0.716 | 0.757 | 0.765 | 0.764 | 0.740 | 0.747 | 0.749 | 0.749 |
| Ce | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.002 | 0.001 | 0.001 | 0.001 |
| Pr | 0.000 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 |
| Nd | 0.004 | 0.004 | 0.004 | 0.004 | 0.005 | 0.005 | 0.005 | 0.006 | 0.005 |
| Sm | 0.005 | 0.005 | 0.005 | 0.004 | 0.005 | 0.005 | 0.005 | 0.004 | 0.006 |
| Gd | 0.042 | 0.043 | 0.043 | 0.041 | 0.042 | 0.038 | 0.036 | 0.037 | 0.040 |
| Tb | 0.011 | 0.010 | 0.011 | 0.009 | 0.009 | 0.008 | 0.009 | 0.008 | 0.008 |
| Dy | 0.071 | 0.071 | 0.072 | 0.070 | 0.070 | 0.062 | 0.064 | 0.063 | 0.065 |
| Ho | 0.009 | 0.009 | 0.009 | 0.011 | 0.010 | 0.011 | 0.010 | 0.010 | 0.010 |
| Er | 0.023 | 0.022 | 0.024 | 0.029 | 0.030 | 0.029 | 0.029 | 0.028 | 0.028 |
| Tm | 0.003 | 0.003 | 0.003 | 0.003 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 |
| Yb | 0.019 | 0.017 | 0.020 | 0.017 | 0.017 | 0.022 | 0.022 | 0.020 | 0.022 |
| Lu | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.004 | 0.003 | 0.003 | 0.004 |
| Ca | 0.035 | 0.036 | 0.021 | 0.013 | 0.023 | 0.017 | 0.018 | 0.026 | 0.024 |
| Pb | 0.001 | 0.001 | 0.001 | 0.002 | 0.001 | 0.002 | 0.002 | 0.000 | 0.001 |
| Sample | 3365 | 3365 | 3389 | 3436 | 3436 | 3436 | 3443 | 3443 |
|---|---|---|---|---|---|---|---|---|
| SiO2 | 0.07 | b.d.l. | b.d.l. | 0.06 | 0.06 | 0.00 | 0.06 | b.d.l. |
| ThO2 | 5.23 | 5.32 | 4.47 | 1.80 | 3.78 | 4.48 | 2.47 | 2.34 |
| UO2 | 89.72 | 88.66 | 89.77 | 92.48 | 90.72 | 90.08 | 91.16 | 91.48 |
| Y2O3 | 0.12 | 0.11 | 0.14 | 0.10 | 0.13 | 0.18 | 0.19 | 0.22 |
| Ce2O3 | b.d.l. | 0.05 | b.d.l. | 0.08 | b.d.l. | 0.05 | b.d.l. | 0.02 |
| Nd2O3 | b.d.l. | b.d.l. | 0.05 | b.d.l. | 0.02 | b.d.l. | 0.01 | b.d.l. |
| Sm2O3 | b.d.l. | 0.15 | 0.10 | b.d.l. | b.d.l. | 0.06 | 0.03 | 0.05 |
| Gd2O3 | b.d.l. | 0.07 | 0.05 | 0.10 | 0.02 | b.d.l. | b.d.l. | 0.09 |
| Tb2O3 | b.d.l. | 0.01 | 0.04 | 0.05 | b.d.l. | 0.10 | b.d.l. | b.d.l. |
| Dy2O3 | 0.05 | 0.06 | 0.09 | 0.05 | b.d.l. | 0.09 | 0.09 | 0.08 |
| Er2O3 | 0.05 | 0.11 | 0.10 | 0.06 | 0.04 | b.d.l. | 0.03 | 0.10 |
| Yb2O3 | 0.03 | 0.04 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| PbO | 4.13 | 4.07 | 4.05 | 4.11 | 4.07 | 3.97 | 3.97 | 3.98 |
| Total | 99.41 | 98.65 | 98.86 | 98.88 | 98.83 | 99.01 | 98.01 | 98.37 |
| Si | 0.003 | 0.000 | 0.000 | 0.003 | 0.003 | 0.000 | 0.003 | 0.000 |
| Th | 0.054 | 0.056 | 0.047 | 0.019 | 0.040 | 0.047 | 0.026 | 0.025 |
| U | 0.913 | 0.911 | 0.920 | 0.948 | 0.929 | 0.922 | 0.941 | 0.942 |
| Y | 0.003 | 0.003 | 0.003 | 0.002 | 0.003 | 0.004 | 0.005 | 0.005 |
| Ce | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 | 0.000 |
| Nd | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Sm | 0.000 | 0.002 | 0.002 | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 |
| Gd | 0.000 | 0.001 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.001 |
| Tb | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.002 | 0.000 | 0.000 |
| Dy | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 0.001 | 0.001 | 0.001 |
| Er | 0.001 | 0.002 | 0.001 | 0.001 | 0.001 | 0.000 | 0.000 | 0.001 |
| Yb | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Pb | 0.051 | 0.051 | 0.050 | 0.051 | 0.050 | 0.049 | 0.050 | 0.050 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breiter, K.; Förster, H.-J. Compositional Variability of Monazite–Cheralite–Huttonite Solid Solutions, Xenotime, and Uraninite in Geochemically Distinct Granites with Special Emphasis to the Strongly Fractionated Peraluminous Li–F–P-Rich Podlesí Granite System (Erzgebirge/Krušné Hory Mts., Central Europe). Minerals 2021, 11, 127. https://doi.org/10.3390/min11020127
Breiter K, Förster H-J. Compositional Variability of Monazite–Cheralite–Huttonite Solid Solutions, Xenotime, and Uraninite in Geochemically Distinct Granites with Special Emphasis to the Strongly Fractionated Peraluminous Li–F–P-Rich Podlesí Granite System (Erzgebirge/Krušné Hory Mts., Central Europe). Minerals. 2021; 11(2):127. https://doi.org/10.3390/min11020127
Chicago/Turabian StyleBreiter, Karel, and Hans-Jürgen Förster. 2021. "Compositional Variability of Monazite–Cheralite–Huttonite Solid Solutions, Xenotime, and Uraninite in Geochemically Distinct Granites with Special Emphasis to the Strongly Fractionated Peraluminous Li–F–P-Rich Podlesí Granite System (Erzgebirge/Krušné Hory Mts., Central Europe)" Minerals 11, no. 2: 127. https://doi.org/10.3390/min11020127