Oxidizability of Oils Recovered from Olive Seeds by Isothermal Calorimetry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
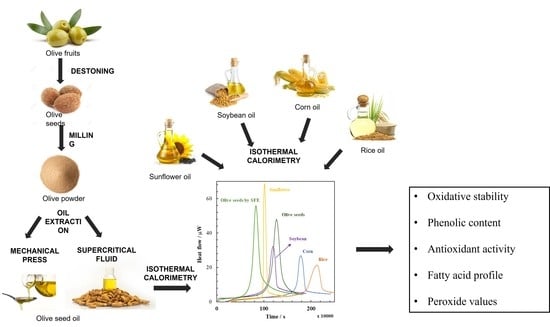
2.2. Olive Seeds Preparation
2.3. Mechanical Press Extraction
2.4. Supercritical Fluid Extraction
2.5. Fatty Acid Composition
2.6. Peroxide Value Measurement
2.7. Preparation of Extracts
2.7.1. Total Phenolic Content
2.7.2. DPPH Radical Scavenging Activity
2.8. Autoxidation Experiments
2.9. Statistical Analysis
3. Results and Discussion
3.1. Isothermal Calorimetry Properties of Olive Seed Oils
3.2. Kinetic and Thermodynamic Properties of Olive Seed Oils
3.2.1. Induction Time
3.2.2. Overall Heat of the Reaction
3.2.3. Rate of Initiation
3.2.4. Rate of the Inhibited Period
3.2.5. Rate of the Uninhibited Period
3.2.6. Oxidative Stability Index
3.3. Application to Bulk Edible Oils
3.4. Correlation between Isothermal Calorimetry Data and Chemical Properties of Bulk Oils
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leone, A.; Romaniello, R.; Zagaria, R.; Sabella, E.; De Bellis, L.; Tamborrino, A. Machining effects of different mechanical crushers on pit particle size and oil drop distribution in olive paste. Eur. J. Lipid Sci. Technol. 2015, 117, 1271–1279. [Google Scholar] [CrossRef]
- Ranalli, A.; Pollastri, L.; Contento, S.; Di Loreto, G.; Iannucci, E.; Lucera, L.; Russi, F. Acylglycerol and fatty acid components of pulp, seed, and whole olive fruit oils. Their use to characterize fruit variety by chemometrics. J. Agric. Food Chem. 2002, 50, 3775–3779. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, G.; Lama, A.; Rodríguez, R.; Jiménez, A.; Guillén, R.; Fernández-Bolaños, J. Olive stone an attractive source of bioactive and valuable compounds. Bioresour. Technol. 2008, 99, 5261–5269. [Google Scholar] [CrossRef] [PubMed]
- Barclay, L.R.; Ingold, K.U. Autoxidation of biological molecules. 2. Autoxidation of a model membrane. Comparison of the autoxidation of egg lecithin phosphatidylcholine in water and in chlorobenzene. J. Am. Chem. Soc. 1981, 103, 6478–6485. [Google Scholar] [CrossRef]
- Denisov, E.T.; Afanas’ ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology; CRC Press: Boca Raton, FL, USA, 2005; ISBN 042913164X. [Google Scholar]
- Méndez, E.; Sanhueza, J.; Speisky, H.; Valenzuela, A. Validation of the Rancimat test for the assessment of the relative stability of fish oils. J. Am. Oil Chem. Soc. 1996, 73, 1033–1037. [Google Scholar] [CrossRef]
- Hasenhuettl, G.L.; Wan, P.J. Temperature effects on the determination of oxidative stability with the Metrohm Rancimat. J. Am. Oil Chem. Soc. 1992, 69, 525–527. [Google Scholar] [CrossRef]
- Kilcast, D.; Subramaniam, P. The Stability and Shelf-life of Food; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Han, X.; Cheng, L.; Zhang, R.; Bi, J. Extraction of safflower seed oil by supercritical CO2. J. Food Eng. 2009, 92, 370–376. [Google Scholar] [CrossRef]
- Kiriamiti, H.K.; Rascol, E.; Marty, A.; Condoret, J.S. Extraction rates of oil from high oleic sunflower seeds with supercritical carbon dioxide. Chem. Eng. Process. Process Intensif. 2002, 41, 711–718. [Google Scholar] [CrossRef]
- Shantha, N.C.; Decker, E.A. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC Int. 1994, 77, 421–424. [Google Scholar] [CrossRef]
- Siger, A.; Nogala-kalucka, M.; Lampart-Szczapa, E. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J. Food Lipids 2008, 15, 137–149. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Koleva, I.I.; van Beek, T.A.; Linssen, J.P.H.; Groot, A.D.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Velasco, J.; Andersen, M.L.; Skibsted, L.H. Evaluation of oxidative stability of vegetable oils by monitoring the tendency to radical formation. A comparison of electron spin resonance spectroscopy with the Rancimat method and differential scanning calorimetry. Food Chem. 2004, 85, 623–632. [Google Scholar] [CrossRef]
- Sabolová, M.; Johanidesová, A.; Hasalíková, E.; Fišnar, J.; Doležal, M.; Réblová, Z. Relationship between the composition of fats and oils and their oxidative stability at different temperatures, determined using the Oxipres apparatus. Eur. J. Lipid Sci. Technol. 2017, 119, 1600454. [Google Scholar] [CrossRef]
- Valgimigli, L.; Pratt, D.A. Antioxidants in chemistry and biology. In Encyclopedia of Radicals in Chemistry, Biology and Materials; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Guo, Y.; Pizzol, R.; Gabbanini, S.; Baschieri, A.; Amorati, R.; Valgimigli, L. Absolute antioxidant activity of five phenol-rich essential oils. Molecules 2021, 26, 5237. [Google Scholar] [CrossRef]
- Lucarini, M.; Pedulli, G.F.; Valgimigli, L. Do peroxyl radicals obey the principle that kinetic solvent effects on H-atom abstraction are independent of the nature of the abstracting radical? J. Org. Chem. 1998, 63, 4497–4499. [Google Scholar] [CrossRef]
- Pryor, W.A.; Henderson, R.W.; Patsiga, R.A.; Carroll, N. Hydrogen secondary isotope effects on the radical polymerization of styrene1,2. J. Am. Chem. Soc. 1966, 88, 1199–1205. [Google Scholar] [CrossRef]
- Pryor, W.A.; Cornicelli, J.A.; Devall, L.J.; Tait, B.; Trivedi, B.K.; Witiak, D.T.; Wu, M. A rapid screening test to determine the antioxidant potencies of natural and synthetic antioxidants. J. Org. Chem. 1993, 58, 3521–3532. [Google Scholar] [CrossRef]
- Baschieri, A.; Pizzol, R.; Guo, Y.; Amorati, R.; Valgimigli, L. Calibration of Squalene, p-Cymene, and sunflower oil as standard oxidizable substrates for quantitative antioxidant testing. J. Agric. Food Chem. 2019, 67, 6902–6910. [Google Scholar] [CrossRef]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.; Morozzi, G. Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspects of production that affect their occurrence in the oil. J. Chromatogr. A 2004, 1054, 113–127. [Google Scholar] [CrossRef]
- Xie, P.; Cecchi, L.; Bellumori, M.; Balli, D.; Giovannelli, L.; Huang, L.; Mulinacci, N. Phenolic compounds and triterpenes in different olive tissues and olive oil by-products, and cytotoxicity on human colorectal cancer cells: The case of frantoio, moraiolo and leccino cultivars (Olea europaea L.). Foods 2021, 10, 2823. [Google Scholar] [CrossRef]
- Laulloo, S.S.; Bhowon, M.G.; Hoolash, A. Influence of chemical refining processes on the total phenolics and antioxidant activity of sunflower oil. Int. J. Nutr. 2015, 1, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Romano, R.; Filosa, G.; Pizzolongo, F.; Durazzo, A.; Lucarini, M.; Severino, P.; Souto, E.B.; Santini, A. Oxidative stability of high oleic sunflower oil during deep-frying process of purple potato purple majesty. Heliyon 2021, 7, e06294. [Google Scholar] [CrossRef]
- Kozłowska, M.; Gruczyńska, E. Comparison of the oxidative stability of soybean and sunflower oils enriched with herbal plant extracts. Chem. Pap. 2018, 72, 2607–2615. [Google Scholar] [CrossRef] [Green Version]
- Martin-Rubio, A.S.; Sopelana, P.; Guillén, M.D. Assessment of soybean oil oxidative stability from rapid analysis of its minor component profile. Molecules 2020, 25, 4860. [Google Scholar] [CrossRef]
- Pratt, D.A.; Tallman, K.A.; Porter, N.A. Free radical oxidation of polyunsaturated lipids: New mechanistic insights and the development of peroxyl radical clocks. Acc. Chem. Res. 2011, 44, 458–467. [Google Scholar] [CrossRef] [Green Version]
- Karakaya, S.; Şimşek, Ş. Changes in total polar compounds, peroxide value, total phenols and antioxidant activity of various oils used in deep fat frying. J. Am. Oil Chem. Soc. 2011, 88, 1361–1366. [Google Scholar] [CrossRef]
- Maszewska, M.; Florowska, A.; Dłużewska, E.; Wroniak, M.; Marciniak-Lukasiak, K.; Żbikowska, A. Oxidative stability of selected edible oils. Molecules 2018, 23, 1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmania, H.; Kato, S.; Sawada, K.; Hayashi, C.; Hashimoto, H.; Nakajima, S.; Otoki, Y.; Ito, J.; Nakagawa, K. Revealing the thermal oxidation stability and its mechanism of rice bran oil. Sci. Rep. 2020, 10, 14091. [Google Scholar] [CrossRef] [PubMed]


| Oil | Folin–Ciocalteu | DPPH | Peroxide Value |
|---|---|---|---|
| g GAE/kg | g TE/kg | Millieq. Peroxide/kg | |
| Olive seed | 1.00 ± 0.2 | 0.60 ± 0.03 | 6.44 ± 0.3 |
| Olive seed from SFE | 0.36 ± 0.2 | 0.20 ± 0.01 | 6.88 ± 1.9 |
| Sunflower | 0.98 ± 0.1 | 0.48 ± 0.03 | 5.19 ± 2.8 |
| Soybean | 1.21 ± 0.5 | 0.65 ± 0.04 | 10.91 ± 1.5 |
| Corn | 1.54 ± 0.1 | 0.71 ± 0.05 | 6.67 ± 0.9 |
| Rice | 1.26 ± 0.1 | 1.00 ± 0.06 | 5.62 ± 1.3 |
| Oil | MUFA | PUFA | 16:0 | 16:1 | 18:0 | 18:1 | 18:2 | 18:3 |
|---|---|---|---|---|---|---|---|---|
| g/L | g/L | (%) | (%) | (%) | (%) | (%) | (%) | |
| Olive seed | 6.8 ± 0.6 | 1.7 ± 0.5 | 8.6 ± 0.8 | 0.21 ± 0.02 | 2.75 ± 0.9 | 71 ± 2.4 | 17.1 ± 1.5 | 0.32 ± 0.01 |
| Olive seed from SFE | 8.37 ± 1.1 | 2.1 ± 0.4 | 8.1 ± 1.1 | 0.11 ± 0.01 | 2.75 ± 0.4 | 71 ± 2.3 | 16.8 ± 2.5 | 0.31 ± 0.03 |
| Sunflower | 5.9 ± 0.7 | 12.4 ± 2.1 | 6.1 ± 0.9 | - | 2.96 ± 0.11 | 28 ± 1.5 | 62.4 ± 2.7 | - |
| Soybean | 6.3 ± 1.5 | 11.0 ± 1.5 | 10.6 ± 1.5 | - | 4.23 ± 0.5 | 28 ± 1.9 | 50.7 ± 2.8 | 5.62 ± 0.06 |
| Corn | 6.7 ± 1.0 | 12.5 ± 2.5 | 11.7 ± 1.6 | - | 1.55 ± 0.3 | 29 ± 2.5 | 56.6 ± 3.5 | 0.82 ± 0.05 |
| Rice | 9.8 ± 1.8 | 7.2 ± 1.7 | 20.2 ± 1.4 | 0.18 ± 0.01 | 2.11 ± 0.3 | 44 ± 3.2 | 32.5 ± 2.3 | 1.05 ± 0.6 |
| Oil | Qtot | τ | ||
|---|---|---|---|---|
| 10−7 s−1 | 10−6 s−1 | J | 106 s | |
| Olive seed | 2.94 ± 0.2 | 2.99 ± 0.6 | 15.1 ± 0.1 | 1.13 ± 0.5 |
| Olive seed from SFE | 4.18 ± 0.5 | 5.19 ± 1.2 | 14.1 ± 0.1 | 0.32 ± 0.1 |
| Sunflower | 3.02 ± 0.3 | 9.29 ± 1.7 | 6.1 ± 0.1 | 0.97 ± 0.3 |
| Soybean | 3.18 ± 0.2 | 2.71 ± 0.8 | 11.2 ± 0.1 | 1.11 ± 0.4 |
| Corn | 2.61 ± 1.1 | 2.21 ± 0.6 | 9.9 ± 0.1 | 1.70 ± 0.5 |
| Rice | 2.27 ± 0.4 | 2.01 ± 0.8 | 11.5 ± 0.1 | 1.91 ± 0.6 |
| Bulk Oil | Ri | Rinh | Runi | O.I. |
|---|---|---|---|---|
| 10−9 M/s | 10−8 M/s | 10−8 M/s | 10−3 (s/M)0.5 | |
| Olive seed | 8.7 ± 0.4 | 7.94 ± 0.15 | 0.86 ± 0.1 | 3.07 ± 0.3 |
| Olive seed from SFE | 15.3 ± 0.5 | 10.1 ± 0.25 | 1.61 ± 0.3 | 3.55 ± 0.4 |
| Sunflower | 9.5 ± 0.4 | 8.10 ± 0.45 | 2.17 ± 0.1 | 3.42 ± 0.8 |
| Soybean | 12.0 ± 0.5 | 8.42 ± 0.15 | 1.64 ± 0.1 | 2.44 ± 0.6 |
| Corn | 8.2 ± 0.4 | 7.06 ± 0.18 | 0.66 ± 0.4 | 1.11 ± 0.4 |
| Rice | 7.21 ± 0.5 | 6.08 ± 0.19 | 0.51 ± 0.1 | 0.98 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosibo, O.K.; Laopeng, S.; Ferrentino, G.; Scampicchio, M. Oxidizability of Oils Recovered from Olive Seeds by Isothermal Calorimetry. Foods 2022, 11, 1016. https://doi.org/10.3390/foods11071016
Mosibo OK, Laopeng S, Ferrentino G, Scampicchio M. Oxidizability of Oils Recovered from Olive Seeds by Isothermal Calorimetry. Foods. 2022; 11(7):1016. https://doi.org/10.3390/foods11071016
Chicago/Turabian StyleMosibo, Ornella Kongi, Siwawoot Laopeng, Giovanna Ferrentino, and Matteo Scampicchio. 2022. "Oxidizability of Oils Recovered from Olive Seeds by Isothermal Calorimetry" Foods 11, no. 7: 1016. https://doi.org/10.3390/foods11071016