Abstract
Within the past decade, multiple lines of evidence have converged to identify a critical role for activity-regulated myelination in tuning the function of neural networks. In this Review, we provide an overview of accumulating evidence that activity-regulated myelination is required for brain adaptation and learning across multiple domains. We then discuss dysregulation of activity-dependent myelination in the context of neurological disease, a novel frontier with the potential to uncover new mechanisms of disease pathogenesis and to develop new therapeutic strategies. Alterations in myelination and neural network function can result from deficient myelin plasticity that impairs neurological function or from maladaptive myelination, in which intact activity-dependent myelination contributes to the disease process by promoting pathological patterns of neuronal activity. These emerging mechanisms suggest new avenues for therapeutic intervention that could more fully address the complex interactions between neurons and oligodendroglia.
Key points
-
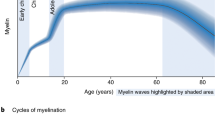
Activity-regulated myelin plasticity is a process in which myelin structure can change in response to neuronal activity, and can involve de novo myelination, remodelling of existing myelin, and both increases and decreases in myelination.
-
In the healthy brain, myelin plasticity is adaptive and supports brain function and cognition across multiple domains; adaptive changes in myelin are thought to tune neural circuit dynamics to promote coordinated circuit function.
-
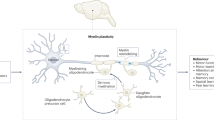
Mechanisms of neuron-to-oligodendrocyte progenitor cell communication include bona fide synapses, non-synaptic vesicle exocytosis, and paracrine signalling involving factors secreted by neurons; the key mechanisms underlying myelin plasticity are a topic of intense research.
-
In some diseases, neurological function is impaired by loss of activity-dependent myelination; a prominent example is cancer therapy-related cognitive impairment.
-
Maladaptive myelination is a distinct mechanism in which activity-dependent myelination is driven by, and subsequently promotes, pathological patterns of neuronal activity, such as seizures in generalized epilepsy.
-
Novel therapeutic strategies could address dysregulated neuron–oligodendroglial interactions in disease states by restoring deficient activity-dependent myelination or by modulating maladaptive myelination.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Huxley, A. F. & Stampfli, R. Evidence for saltatory conduction in peripheral myelinated nerve fibres. J. Physiol. 108, 315–339 (1949).
Funfschilling, U. et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521 (2012).
Bergles, D. E. & Richardson, W. D. Oligodendrocyte development and plasticity. Cold Spring Harb. Perspect. Biol. 8, a020453 (2015).
Hughes, E. G., Orthmann-Murphy, J. L., Langseth, A. J. & Bergles, D. E. Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat. Neurosci. 21, 696–706 (2018). This study demonstrated that sensory stimulation can increase oligodendrogenesis and myelin internode formation in the superficial somatosensory cortex.
Czopka, T., Ffrench-Constant, C. & Lyons, D. A. Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev. Cell 25, 599–609 (2013).
Yalcin, B. & Monje, M. Microenvironmental interactions of oligodendroglial cells. Dev. Cell 56, 1821–1832 (2021).
Zuchero, J. B. & Barres, B. A. Intrinsic and extrinsic control of oligodendrocyte development. Curr. Opin. Neurobiol. 23, 914–920 (2013).
Pajevic, S., Basser, P. J. & Fields, R. D. Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience 276, 135–147 (2014).
Scholz, J., Klein, M. C., Behrens, T. E. & Johansen-Berg, H. Training induces changes in white-matter architecture. Nat. Neurosci. 12, 1370–1371 (2009). This human neuroimaging study demonstrated microstructural changes in white matter regions relevant to hand–eye coordination after learning to juggle, suggesting motor learning-induced myelin plasticity.
Bengtsson, S. L. et al. Extensive piano practicing has regionally specific effects on white matter development. Nat. Neurosci. 8, 1148–1150 (2005).
Dziemian, S., Appenzeller, S., von Bastian, C. C., Jancke, L. & Langer, N. Working memory training effects on white matter integrity in young and older adults. Front. Hum. Neurosci. 15, 605213 (2021).
Schiller, R. M. et al. Training-induced white matter microstructure changes in survivors of neonatal critical illness: a randomized controlled trial. Dev. Cogn. Neurosci. 38, 100678 (2019).
Hofstetter, S., Tavor, I., Tzur Moryosef, S. & Assaf, Y. Short-term learning induces white matter plasticity in the fornix. J. Neurosci. 33, 12844–12850 (2013).
Alexander, D. C., Dyrby, T. B., Nilsson, M. & Zhang, H. Imaging brain microstructure with diffusion MRI: practicality and applications. NMR Biomed. 32, e3841 (2019).
Barres, B. A. & Raff, M. C. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature 361, 258–260 (1993).
Demerens, C. et al. Induction of myelination in the central nervous system by electrical activity. Proc. Natl Acad. Sci. USA 93, 9887–9892 (1996).
Stevens, B., Porta, S., Haak, L. L., Gallo, V. & Fields, R. D. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron 36, 855–868 (2002).
Gibson, E. M. et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304 (2014). In this study, in vivo optogenetic studies provided direct evidence that cortical projection neuronal activity promotes circuit-specific OPC proliferation, oligodendrogenesis and myelination, specifically in corticocallosal — but not corticospinal — projections of the premotor circuit; changes in motor behavioural performance depended on these oligodendroglial changes.
Mitew, S. et al. Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat. Commun. 9, 306 (2018). Chemogenetic stimulation of somatosensory axons stimulates OPC proliferation, oligodendrogenesis and increased myelination of the stimulated axons, while chemogenetic inhibition of axonal activity exerted opposite effects, demonstrating selective myelination of more active axons and bidirectional myelin plasticity.
Makinodan, M., Rosen, K. M., Ito, S. & Corfas, G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 337, 1357–1360 (2012). This study demonstrated that social isolation decreases prefrontal cortex myelination. If social isolation occurred during a critical period in adolescence, this deficit in prefrontal cortex myelination was irreversible upon social reintegration.
Liu, J. et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 15, 1621–1623 (2012).
Mensch, S. et al. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat. Neurosci. 18, 628–630 (2015). This study showed that synaptic vesicle release modulates axon selection during development in zebrafish.
Hines, J., Ravanelli, A., Schwindt, R., Scott, E. & Appel, B. Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci. 18, 683–689 (2015).
McKenzie, I. A. et al. Motor skill learning requires active central myelination. Science 346, 318–322 (2014). This study demonstrated that new oligodendrocyte generation is required for motor learning.
Xiao, L. et al. Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat. Neurosci. 19, 1210–1217 (2016).
Sampaio-Baptista, C. et al. Motor skill learning induces changes in white matter microstructure and myelination. J. Neurosci. 33, 19499–19503 (2013).
Steadman, P. E. et al. Disruption of oligodendrogenesis impairs memory consolidation in adult mice. Neuron 105, 150–164.e6 (2020). This study demonstrated that generation of new oligodendrocytes is required for spatial and fear memory consolidation, and provided experimental evidence that myelin plasticity promotes oscillatory synchrony between the hippocampus and frontal cortex.
Pan, S., Mayoral, S. R., Choi, H. S., Chan, J. R. & Kheirbek, M. A. Preservation of a remote fear memory requires new myelin formation. Nat. Neurosci. 23, 487–499 (2020).
Geraghty, A. C. et al. Loss of adaptive myelination contributes to methotrexate chemotherapy-related cognitive impairment. Neuron 103, 250–265.e8 (2019). This study demonstrated that activity-regulated BDNF signalling to TrkB in OPCs is required for activity-regulated OPC proliferation, oligodendrogenesis and myelination in cortical projection neurons, and that impaired myelin plasticity contributes to CRCI in mice.
Yang, S. M., Michel, K., Jokhi, V., Nedivi, E. & Arlotta, P. Neuron class-specific responses govern adaptive myelin remodeling in the neocortex. Science 370, eabd2109 (2020).
Tomassy, G. S. et al. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 344, 319–324 (2014). This study demonstrated that cortical axons are discontinuously myelinated, illustrating that axonal territory is available for activity-regulated addition of myelin internodes.
Jito, J. et al. Maturational changes in diffusion anisotropy in the rat corpus callosum: comparison with quantitative histological evaluation. J. Magn. Reson. Imaging 28, 847–854 (2008).
Hughes, E. G., Kang, S. H., Fukaya, M. & Bergles, D. E. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci. 16, 668–676 (2013).
Hill, R. A., Li, A. M. & Grutzendler, J. Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat. Neurosci. 21, 683–695 (2018).
Tripathi, R. B. et al. Remarkable stability of myelinating oligodendrocytes in mice. Cell Rep. 21, 316–323 (2017).
Hughes, A. N. & Appel, B. Microglia phagocytose myelin sheaths to modify developmental myelination. Nat. Neurosci. 23, 1055–1066 (2020).
Stevens, B. et al. The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178 (2007).
Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012).
Swire, M., Kotelevtsev, Y., Webb, D. J., Lyons, D. A. & Ffrench-Constant, C. Endothelin signalling mediates experience-dependent myelination in the CNS. Elife 8, e49493 (2019). This study demonstrated that social experience-regulated myelination (the number of internodes per oligodendrocyte) is mediated by endothelin signalling from cerebral vessels to oligodendroglial endothelin B receptor in the superficial medial prefrontal cortex.
Osso, L. A., Rankin, K. A. & Chan, J. R. Experience-dependent myelination following stress is mediated by the neuropeptide dynorphin. Neuron 109, 3619–3632.e5 (2021).
Bergles, D. E., Roberts, J. D., Somogyi, P. & Jahr, C. E. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405, 187–191 (2000). This study demonstrated that electrophysiologically functional synapses exist between neurons and OPCs.
Karadottir, R., Cavelier, P., Bergersen, L. H. & Attwell, D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 438, 1162–1166 (2005).
De Biase, L. M., Nishiyama, A. & Bergles, D. E. Excitability and synaptic communication within the oligodendrocyte lineage. J. Neurosci. 30, 3600–3611 (2010).
Spitzer, S. O. et al. Oligodendrocyte progenitor cells become regionally diverse and heterogeneous with age. Neuron 101, 459–471.e5 (2019).
Marques, S. et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352, 1326–1329 (2016).
Mount, C. W., Yalcin, B., Cunliffe-Koehler, K., Sundaresh, S. & Monje, M. Monosynaptic tracing maps brain-wide afferent oligodendrocyte precursor cell connectivity. eLife 8, 49291 (2019).
De Biase, L. M. et al. NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. J. Neurosci. 31, 12650–12662 (2011).
Chen, T. J. et al. In vivo regulation of oligodendrocyte precursor cell proliferation and differentiation by the AMPA-receptor subunit GluA2. Cell Rep. 25, 852–861.e7 (2018).
Kougioumtzidou, E. et al. Signalling through AMPA receptors on oligodendrocyte precursors promotes myelination by enhancing oligodendrocyte survival. Elife 6, e28080 (2017).
Lundgaard, I. et al. Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol. 11, e1001743 (2013).
Kukley, M., Capetillo-Zarate, E. & Dietrich, D. Vesicular glutamate release from axons in white matter. Nat. Neurosci. 10, 311–320 (2007).
Ziskin, J. L., Nishiyama, A., Rubio, M., Fukaya, M. & Bergles, D. E. Vesicular release of glutamate from unmyelinated axons in white matter. Nat. Neurosci. 10, 321–330 (2007).
Lin, S. C. & Bergles, D. E. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat. Neurosci. 7, 24–32 (2004).
Zonouzi, M. et al. GABAergic regulation of cerebellar NG2 cell development is altered in perinatal white matter injury. Nat. Neurosci. 18, 674–682 (2015).
Benamer, N., Vidal, M., Balia, M. & Angulo, M. C. Myelination of parvalbumin interneurons shapes the function of cortical sensory inhibitory circuits. Nat. Commun. 11, 5151 (2020).
Xiao, Y., Petrucco, L., Hoodless, L. J., Portugues, R. & Czopka, T. Oligodendrocyte precursor cells sculpt the visual system by regulating axonal remodeling. Nat. Neurosci. 25, 280–284 (2022).
Auguste, Y. S. S. et al. Oligodendrocyte precursor cells engulf synapses during circuit remodeling in mice. Nat. Neurosci. 25, 1273–1278 (2022).
Battefeld, A., Klooster, J. & Kole, M. H. Myelinating satellite oligodendrocytes are integrated in a glial syncytium constraining neuronal high-frequency activity. Nat. Commun. 7, 11298 (2016).
Kirby, L. et al. Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat. Commun. 10, 3887 (2019).
Waxman, S. G. Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve 3, 141–150 (1980).
Helfrich, R. F. & Knight, R. T. Oscillatory dynamics of prefrontal cognitive control. Trends Cogn. Sci. 20, 916–930 (2016).
Noori, R. et al. Activity-dependent myelination: a glial mechanism of oscillatory self-organization in large-scale brain networks. Proc. Natl Acad. Sci. USA 117, 13227–13237 (2020).
Seidl, A. H. & Rubel, E. W. Systematic and differential myelination of axon collaterals in the mammalian auditory brainstem. Glia 64, 487–494 (2016).
Salami, M., Itami, C., Tsumoto, T. & Kimura, F. Change of conduction velocity by regional myelination yields constant latency irrespective of distance between thalamus and cortex. Proc. Natl Acad. Sci. USA 100, 6174–6179 (2003).
Kato, D. et al. Motor learning requires myelination to reduce asynchrony and spontaneity in neural activity. Glia 68, 193–210 (2020).
Dubey, M. et al. Myelination synchronizes cortical oscillations by consolidating parvalbumin-mediated phasic inhibition. eLife 11, e73827 (2022).
Menning, S. et al. Changes in brain white matter integrity after systemic treatment for breast cancer: a prospective longitudinal study. Brain Imaging Behav. 12, 324–334 (2018).
Gibson, E. M. & Monje, M. Microglia in cancer therapy-related cognitive impairment. Trends Neurosci. 44, 441–451 (2021).
Gibson, E. M. et al. Methotrexate chemotherapy induces persistent tri-glial dysregulation that underlies chemotherapy-related cognitive impairment. Cell 176, 43–55.e13 (2019).
Fernandez-Castaneda, A. et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell 185, 2452–2468.e16 (2022).
Douaud, G. et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 604, 697–707 (2022).
Cecchetti, G. et al. Cognitive, EEG, and MRI features of COVID-19 survivors: a 10-month study. J. Neurol. 269, 3400–3412 (2022).
Chen, J. F. et al. Enhancing myelin renewal reverses cognitive dysfunction in a murine model of Alzheimer’s disease. Neuron 109, 2292–2307 (2021).
Mathys, H. et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570, 332–337 (2019).
Wang, F. et al. Myelin degeneration and diminished myelin renewal contribute to age-related deficits in memory. Nat. Neurosci. 23, 481–486 (2020).
Liu, J., Likhtik, E., Shereen, A. D., Dennis-Tiwary, T. A. & Casaccia, P. White matter plasticity in anxiety: disruption of neural network synchronization during threat-safety discrimination. Front. Cell Neurosci. 14, 587053 (2020).
Hsiao, E. Y. et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463 (2013).
Needham, B. D. et al. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature 602, 647–653 (2022).
Knowles, J. K. et al. Maladaptive myelination promotes generalized epilepsy progression. Nat. Neurosci. 25, 596–606 (2022). This study demonstrated that activity-regulated myelination within the seizure network in rodent models of generalized epilepsy promotes synchrony between nodes in the seizure network and contributes to the progressive increase in seizure burden that occurs in untreated epilepsy, illustrating the concept of maladaptive myelination.
Sharma, P. et al. Differences in white matter structure between seizure prone (FAST) and seizure resistant (SLOW) rat strains. Neurobiol. Dis. 104, 33–40 (2017).
Alam, M. M. et al. Deficiency of microglial autophagy increases the density of oligodendrocytes and susceptibility to severe forms of seizures. eNeuro 8, ENEURO.0183-20.2021 (2021).
Goldsberry, G., Mitra, D., MacDonald, D. & Patay, Z. Accelerated myelination with motor system involvement in a neonate with immediate postnatal onset of seizures and hemimegalencephaly. Epilepsy Behav. 22, 391–394 (2011).
Duprez, T. et al. Focal seizure-induced premature myelination: speculation from serial MRI. Neuroradiology 40, 580–582 (1998).
Sandoval Karamian, A. G., Wusthoff, C. J., Boothroyd, D., Yeom, K. W. & Knowles, J. K. Neonatal genetic epilepsies display convergent white matter microstructural abnormalities. Epilepsia 61, e192–e197 (2020).
Bonilha, L. et al. Structural white matter abnormalities in patients with idiopathic dystonia. Mov. Disord. 22, 1110–1116 (2007).
Kim, J. H., Kim, D. W., Kim, J. B., Suh, S. I. & Koh, S. B. Thalamic involvement in paroxysmal kinesigenic dyskinesia: a combined structural and diffusion tensor MRI analysis. Hum. Brain Mapp. 36, 1429–1441 (2015).
Atkinson-Clement, C. et al. Structural and functional abnormalities within sensori-motor and limbic networks underpin intermittent explosive symptoms in Tourette disorder. J. Psychiatr. Res. 125, 1–6 (2020).
Bacmeister, C. M. et al. Motor learning promotes remyelination via new and surviving oligodendrocytes. Nat. Neurosci. 23, 819–831 (2020).
Gautier, H. O. et al. Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nat. Commun. 6, 8518 (2015).
Ortiz, F. C. et al. Neuronal activity in vivo enhances functional myelin repair. JCI Insight 4, e123434 (2019).
Li, C. et al. A functional role of NMDA receptor in regulating the differentiation of oligodendrocyte precursor cells and remyelination. Glia 61, 732–749 (2013).
Azoulay, D., Vachapova, V., Shihman, B., Miler, A. & Karni, A. Lower brain-derived neurotrophic factor in serum of relapsing remitting MS: reversal by glatiramer acetate. J. Neuroimmunol. 167, 215–218 (2005).
Frota, E. R. et al. Increased plasma levels of brain derived neurotrophic factor (BDNF) after multiple sclerosis relapse. Neurosci. Lett. 460, 130–132 (2009).
Naegelin, Y. et al. Levels of brain-derived neurotrophic factor in patients with multiple sclerosis. Ann. Clin. Transl. Neurol. 7, 2251–2261 (2020).
Tsiperson, V. et al. Brain-derived neurotrophic factor deficiency restricts proliferation of oligodendrocyte progenitors following cuprizone-induced demyelination. ASN Neuro 7, 1759091414566878 (2015).
Huang, Y. et al. Tropomyosin receptor kinase B expressed in oligodendrocyte lineage cells functions to promote myelin following a demyelinating lesion. ASN Neuro 12, 1759091420957464 (2020).
Makar, T. K. et al. Brain-derived neurotrophic factor gene delivery in an animal model of multiple sclerosis using bone marrow stem cells as a vehicle. J. Neuroimmunol. 210, 40–51 (2009).
Mei, F. et al. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat. Med. 20, 954–960 (2014).
Liu, J. et al. Clemastine enhances myelination in the prefrontal cortex and rescues behavioral changes in socially isolated mice. J. Neurosci. 36, 957–962 (2016).
Marin-Husstege, M., Muggironi, M., Liu, A. & Casaccia-Bonnefil, P. Histone deacetylase activity is necessary for oligodendrocyte lineage progression. J. Neurosci. 22, 10333–10345 (2002).
Citraro, R. et al. Effects of histone deacetylase inhibitors on the development of epilepsy and psychiatric comorbidity in WAG/Rij rats. Mol. Neurobiol. 57, 408–421 (2020).
Ceolin, L. et al. A novel anti-epileptic agent, perampanel, selectively inhibits AMPA receptor-mediated synaptic transmission in the hippocampus. Neurochem. Int. 61, 517–522 (2012).
Cullen, C. L. et al. Low-intensity transcranial magnetic stimulation promotes the survival and maturation of newborn oligodendrocytes in the adult mouse brain. Glia 67, 1462–1477 (2019).
Cullen, C. L. et al. Periaxonal and nodal plasticities modulate action potential conduction in the adult mouse brain. Cell Rep. 34, 108641 (2021).
Gross, D. W., Concha, L. & Beaulieu, C. Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia 47, 1360–1363 (2006).
Rodriguez-Cruces, R. & Concha, L. White matter in temporal lobe epilepsy: clinico-pathological correlates of water diffusion abnormalities. Quant. Imaging Med. Surg. 5, 264–278 (2015).
Luo, Y. et al. Alterations in hippocampal myelin and oligodendrocyte precursor cells during epileptogenesis. Brain Res. 1627, 154–164 (2015).
Vezzani, A., Balosso, S. & Ravizza, T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat. Rev. Neurol. 15, 459–472 (2019).
Larson, V. A. et al. Oligodendrocytes control potassium accumulation in white matter and seizure susceptibility. Elife 7, e34829 (2018).
Nagy, B., Hovhannisyan, A., Barzan, R., Chen, T. J. & Kukley, M. Different patterns of neuronal activity trigger distinct responses of oligodendrocyte precursor cells in the corpus callosum. PLoS Biol. 15, e2001993 (2017).
Author information
Authors and Affiliations
Contributions
J.K.K. and M.M. researched the literature for the article, contributed substantially to discussion of the content and reviewed and/or edited the manuscript before submission. All authors wrote the article.
Corresponding authors
Ethics declarations
Competing interests
M.M. holds equity in MapLight Therapeutics and Syncopation Life Sciences. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks B. Emery and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Fractional anisotropy
-
A measure that reflects the relative constraint (anisotropy) of water diffusion in specific directions or eigenvectors; in brain tissue, it is thought to correlate with myelin integrity as well as myelin-independent axonal factors, such as axon size, number and orientation.
- g-ratio
-
The ratio of the axon diameter to the total diameter of the axon and its myelin sheath; a lower g-ratio indicates a thicker myelin sheath relative to the axon diameter. The g-ratio is a major determinant of conduction velocity, in addition to axon diameter.
- Mean diffusivity
-
A measure that indicates the overall degree of water diffusion, independent of the direction of diffusion; it is thought to be inversely proportional to myelin integrity.
- Ocular dominance column
-
A stripe-like group of neurons across multiple cortical layers in the primary visual cortex that responds preferentially to thalamic input originating from one eye or the other.
- Optomotor responses
-
A reflexive motor response in zebrafish that stabilizes body position after perceived motion and that can be induced by videos of moving gratings.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Knowles, J.K., Batra, A., Xu, H. et al. Adaptive and maladaptive myelination in health and disease. Nat Rev Neurol 18, 735–746 (2022). https://doi.org/10.1038/s41582-022-00737-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-022-00737-3
This article is cited by
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)
-
Oligodendrocyte progenitor cells in Alzheimer’s disease: from physiology to pathology
Translational Neurodegeneration (2023)
-
Neuron–oligodendroglial interactions in health and malignant disease
Nature Reviews Neuroscience (2023)