Professional Documents
Culture Documents
Definition - What Does Electromotive Force Series (EMF
Uploaded by
Hassan Ali0 ratings0% found this document useful (0 votes)
54 views2 pagesThe electromotive force (EMF) series ranks metals based on their inherent reactivity, with the most noble metals at the top having the highest positive electrochemical potential and the most active metals at the bottom having the highest negative potential. This series determines a metal's tendency to release energy and corrode. The EMF values listed can predict if galvanic corrosion may occur between two metals in contact within an electrolyte, with the less noble metal corroding. Knowledge of the EMF series helps understand how corrosion happens and can be prevented or reduced, as galvanic corrosion will be fastest when the cathode is much larger than the anode.
Original Description:
Original Title
Electromotive Force Series
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe electromotive force (EMF) series ranks metals based on their inherent reactivity, with the most noble metals at the top having the highest positive electrochemical potential and the most active metals at the bottom having the highest negative potential. This series determines a metal's tendency to release energy and corrode. The EMF values listed can predict if galvanic corrosion may occur between two metals in contact within an electrolyte, with the less noble metal corroding. Knowledge of the EMF series helps understand how corrosion happens and can be prevented or reduced, as galvanic corrosion will be fastest when the cathode is much larger than the anode.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
54 views2 pagesDefinition - What Does Electromotive Force Series (EMF
Uploaded by
Hassan AliThe electromotive force (EMF) series ranks metals based on their inherent reactivity, with the most noble metals at the top having the highest positive electrochemical potential and the most active metals at the bottom having the highest negative potential. This series determines a metal's tendency to release energy and corrode. The EMF values listed can predict if galvanic corrosion may occur between two metals in contact within an electrolyte, with the less noble metal corroding. Knowledge of the EMF series helps understand how corrosion happens and can be prevented or reduced, as galvanic corrosion will be fastest when the cathode is much larger than the anode.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 2
BSME Applied Chemistry GC-103
Electromotive Force Series
(EMF Series)
Definition - What does Electromotive Force Series (EMF
Series) mean?
An electromotive force series (EMF series) is a metal's ranking
in respect to inherent reactivity. The metals located at the top of
the series are considered the most noble, with the highest level
of positive electrochemical potential. The metal that can be
found at the bottom is the most active and contains the highest
amount of negative electrochemical potential.
This series is helpful in determining the tendency of a metal to
release energy and corrode.
Corrosionpedia explains Electromotive Force Series (EMF
Series)
The material ranking in accordance to potential can be seen in
the EMF or galvanic series. For instance, those that have higher
EMF values include gold, copper and platinum. Materials that
have low EMF include zinc and magnesium. These EMF values
have been computed suited for standard cases, but the order may
differ according to the environment. Metals like aluminum and
titanium can build highly protective oxide layers at room
temperatures.
There can be different potentials in situations where two metals
are utilized within a single environment. If these metals are
connected electrically or come in contact with each other, an
adequate amount of potential difference might result in electron
flow between the metals. The more noble a metal is, the less
resistant it is to corrosion. This leads to heightened levels of
Dr. Islam Date: October 12, 2015 Page 1 of 2
BSME Applied Chemistry GC-103
corrosion of material that is anodic and less attack in cathodic
material. Knowledge of the electromagnetic force series helps in
understanding corrosion and how it can be reduced or prevented.
The EMF series can be most useful in evaluating the possible
occurrence of galvanic corrosion. It is essential that accurate
values are utilized or identified for the right temperature and
solution. Essentially, the anode’s relative area in comparison to
the cathode highly influences the rate of corrosion. The bigger
the cathode portion in relation to the anodic area, the faster the
rate of corrosion will be. For instance, bolts made of steel placed
through a copper sheet which is nobler will corrode faster than
the copper sheet in an identical environment. So, the principle is
that galvanic corrosion can take place when two alloys or metals
come in contact with each other within an electrolyte. Between
the two, the less noble metal will undergo corrosion.
Dr. Islam Date: October 12, 2015 Page 2 of 2
You might also like
- Assessment Formal AssessmentDocument7 pagesAssessment Formal Assessmentashish33% (3)
- The V8-5V Engine: Construction Features and FunctionsDocument52 pagesThe V8-5V Engine: Construction Features and Functionssheba1023100% (2)
- Is Globalization Today Really Different Than It Was A Hundred Years AgoDocument11 pagesIs Globalization Today Really Different Than It Was A Hundred Years Agonai09No ratings yet
- Anthropology Eportfolio EssayDocument2 pagesAnthropology Eportfolio Essayapi-267697848No ratings yet
- Why Is Innovation So Important For Firms To Compete in Many Industries?Document5 pagesWhy Is Innovation So Important For Firms To Compete in Many Industries?Gadha GadhaNo ratings yet
- What Is PotentiometerDocument19 pagesWhat Is PotentiometerVishvajeet PalNo ratings yet
- Japanese Occupation Turning Point SEA NationalismDocument2 pagesJapanese Occupation Turning Point SEA NationalismZachary Henry ChanNo ratings yet
- LBST 2102 Globalization EssayDocument5 pagesLBST 2102 Globalization EssayBrianBarrowsNo ratings yet
- Reflection Paper - MeadDocument3 pagesReflection Paper - MeadJOHN ANDREW EVIANo ratings yet
- John LockeDocument2 pagesJohn Lockereg100% (1)
- Electrical Engineering - Caltech CatalogDocument9 pagesElectrical Engineering - Caltech CatalogAnmol GuptaNo ratings yet
- Noise and Thermal PollutionDocument4 pagesNoise and Thermal PollutionSandra Magno100% (3)
- Calculus I Summary: Tabulated by Lambert Peng at Brown in Summer 2008Document3 pagesCalculus I Summary: Tabulated by Lambert Peng at Brown in Summer 2008Thuy TienNo ratings yet
- ElectrochemistryDocument22 pagesElectrochemistryRohit KumarNo ratings yet
- Application of Differential EquationDocument42 pagesApplication of Differential EquationabhishekNo ratings yet
- The Nitrogen Cycle Describes HowDocument2 pagesThe Nitrogen Cycle Describes HowNestor D'souzaNo ratings yet
- Nitrogen Cycling in EcosystemsDocument7 pagesNitrogen Cycling in EcosystemsMark Abion ValladolidNo ratings yet
- Why Save Endangered SpeciesDocument1 pageWhy Save Endangered SpeciesRuthNo ratings yet
- EssayDocument17 pagesEssayapi-360570932No ratings yet
- Communication Management - Shannon-Weaver Model ExampleDocument3 pagesCommunication Management - Shannon-Weaver Model ExampleBenj VasquezNo ratings yet
- Transient RLC Circuit AnalysisDocument7 pagesTransient RLC Circuit AnalysisAlamaskhan PathanNo ratings yet
- Electric Charge LectureDocument5 pagesElectric Charge LectureJohn Rudolf CatalanNo ratings yet
- Wallerstein's World Systems Theory and Global InequalityDocument2 pagesWallerstein's World Systems Theory and Global InequalityMustansar WazeerNo ratings yet
- An RLC CircuitDocument15 pagesAn RLC CircuitJeet SahooNo ratings yet
- Volunteer Functions InventoryDocument25 pagesVolunteer Functions InventoryClaudia BNo ratings yet
- 1) Atomic Mass Vs WeightDocument15 pages1) Atomic Mass Vs WeightShiva Manohar AnanthaNo ratings yet
- Environmental Science, 15e: Solid and Hazardous WasteDocument40 pagesEnvironmental Science, 15e: Solid and Hazardous WasteDiane DiazNo ratings yet
- Calculus: Sliding Ladder Rate Problem SolvedDocument1 pageCalculus: Sliding Ladder Rate Problem SolvedFrancisco De Real OndeNo ratings yet
- Emf (Electromotive Force)Document44 pagesEmf (Electromotive Force)shirley_ling_15No ratings yet
- Essay Culture 800 WordsDocument3 pagesEssay Culture 800 WordsMas BreezyNo ratings yet
- Renewable Energy: The Path To The FutureDocument10 pagesRenewable Energy: The Path To The FutureBuddhika LankaNo ratings yet
- Electromagnetics Course OutlineDocument40 pagesElectromagnetics Course OutlineGerard JimenezNo ratings yet
- Practical Applications of Electrical ConductorsDocument12 pagesPractical Applications of Electrical ConductorsHans De Keulenaer100% (5)
- UNIT Conversion FactorsDocument22 pagesUNIT Conversion FactorsGuillermo Lopez-FloresNo ratings yet
- Related Rates ShadeDocument7 pagesRelated Rates ShadeJohnNo ratings yet
- Unit Conversion ChartDocument5 pagesUnit Conversion Chartrosid_alhusnaNo ratings yet
- Feminism EssayDocument2 pagesFeminism EssayEliasNo ratings yet
- Globaloney DraftDocument3 pagesGlobaloney DraftGeorge Anthony NuarinNo ratings yet
- EssayDocument10 pagesEssaySyahirun NissaNo ratings yet
- Energy Conservation & EfficiencyDocument6 pagesEnergy Conservation & EfficiencymihirthakkarNo ratings yet
- Rizal's Life, Trial and DeathDocument3 pagesRizal's Life, Trial and DeathLester AmadoNo ratings yet
- Chapter One: Introductory Concepts and DefinitionsDocument43 pagesChapter One: Introductory Concepts and DefinitionsxixoNo ratings yet
- Rizal'S View Morga'S ViewDocument1 pageRizal'S View Morga'S ViewJm BalessNo ratings yet
- Rizal's Causes of Filipino IndolenceDocument3 pagesRizal's Causes of Filipino Indolencecarl pacanaNo ratings yet
- Galvanic Cell ExperimentDocument10 pagesGalvanic Cell ExperimentAsep Ridwan Setiawan0% (1)
- How Does ATP Couple Endergonic and Exergonic ReactionsDocument2 pagesHow Does ATP Couple Endergonic and Exergonic ReactionsmuradmajidNo ratings yet
- Insights from Rizal's Essays on Women and IndolenceDocument3 pagesInsights from Rizal's Essays on Women and IndolenceJochebed MirandaNo ratings yet
- Noli Me Tangere and El Filibusterismo: Padre DamasoDocument1 pageNoli Me Tangere and El Filibusterismo: Padre DamasoShailani HossainNo ratings yet
- Maximum and Minimum Function ValuesDocument18 pagesMaximum and Minimum Function ValuesCarlo CaniedoNo ratings yet
- Reflective Essay Assignment #8 - Managing Emotions and Coping With StressDocument2 pagesReflective Essay Assignment #8 - Managing Emotions and Coping With StressRej GarbosaNo ratings yet
- Current, Resistance and Electromotive ForceDocument20 pagesCurrent, Resistance and Electromotive ForceClarence LimNo ratings yet
- Conductors & InsulatorDocument14 pagesConductors & InsulatorSocias CyrusNo ratings yet
- RZL110 Individual Thought PaperDocument3 pagesRZL110 Individual Thought PaperfreyaNo ratings yet
- Conversions and FormulasDocument14 pagesConversions and FormulasBehroozRaadNo ratings yet
- Biochemistry Chapter 1Document115 pagesBiochemistry Chapter 1habteNo ratings yet
- All Unit Cells Consist of The: Ideal Arrangement of Atoms or Molecules Specific OrderDocument75 pagesAll Unit Cells Consist of The: Ideal Arrangement of Atoms or Molecules Specific Orderkhare_girishNo ratings yet
- Good Members of SocietyDocument2 pagesGood Members of SocietyAnh PhươngNo ratings yet
- LeadDocument60 pagesLeadechoechoNo ratings yet
- 2019 18-Day Campaign To End Violence Against Women - Ver2Document16 pages2019 18-Day Campaign To End Violence Against Women - Ver2Dorothea NavarroNo ratings yet
- Essay On Solar EnergyDocument4 pagesEssay On Solar Energyparthiv22191No ratings yet
- Structures of SolidsDocument53 pagesStructures of SolidsAndreas Vivaldi100% (1)
- Practical Importance of Electrochemical and Galvanic Series in Corrosion StudiesDocument2 pagesPractical Importance of Electrochemical and Galvanic Series in Corrosion StudiesYudhisthiraNo ratings yet
- The Scalar-Transport EquationDocument83 pagesThe Scalar-Transport EquationHassan AliNo ratings yet
- Title The 4Document5 pagesTitle The 4Hassan AliNo ratings yet
- MET 648 - LAB 3 - Literature Review ReportDocument1 pageMET 648 - LAB 3 - Literature Review ReportHassan AliNo ratings yet
- FEM Heat Transfer Node TemperaturesDocument4 pagesFEM Heat Transfer Node TemperaturesHassan AliNo ratings yet
- MET 648 - LAB 3 - Literature Review ReportDocument1 pageMET 648 - LAB 3 - Literature Review ReportHassan AliNo ratings yet
- Approximations and Simplified EquationsDocument19 pagesApproximations and Simplified EquationsHassan AliNo ratings yet
- Engineering Statics SolutionDocument9 pagesEngineering Statics SolutionHassan AliNo ratings yet
- Title The 4Document5 pagesTitle The 4Hassan AliNo ratings yet
- Project Report Submitted By:: Mehancis of Materials 2Document8 pagesProject Report Submitted By:: Mehancis of Materials 2Hassan AliNo ratings yet
- Fluid-Flow EquationsDocument28 pagesFluid-Flow EquationsHassan AliNo ratings yet
- Computational Fluid Dynamics: The Finite-Volume Method: David ApsleyDocument29 pagesComputational Fluid Dynamics: The Finite-Volume Method: David ApsleyHassan AliNo ratings yet
- Hassan Ali 722-BSME-FET-F17B: Door SR# Dimensions D1 D2 D3 D4 D5 D6 D7 Window SR# Dimensions Sill W1 W2 W3Document2 pagesHassan Ali 722-BSME-FET-F17B: Door SR# Dimensions D1 D2 D3 D4 D5 D6 D7 Window SR# Dimensions Sill W1 W2 W3Hassan AliNo ratings yet
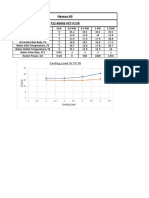
- Hassan Ali 722-BSME-FET-F17B: Cooling Load Vs T5 T6Document1 pageHassan Ali 722-BSME-FET-F17B: Cooling Load Vs T5 T6Hassan AliNo ratings yet
- Cooling Load Vs T5 T6: Hassan Ali 722-BSME-FET-F17BDocument1 pageCooling Load Vs T5 T6: Hassan Ali 722-BSME-FET-F17BHassan AliNo ratings yet
- Fakher Abbas 703-BSME-FET-F17B: Cooling Load Vs T5 T6Document1 pageFakher Abbas 703-BSME-FET-F17B: Cooling Load Vs T5 T6Hassan AliNo ratings yet
- Analyzing Bernoulli Equation ApplicationsDocument1 pageAnalyzing Bernoulli Equation ApplicationsHassan AliNo ratings yet
- Inverse Laplace Transformation Ex 11 2 Umer Asghar MethodDocument34 pagesInverse Laplace Transformation Ex 11 2 Umer Asghar MethodSikandar Khan100% (1)
- Lecture 3 Gears Machine Design - CAD-II ..Example... Contact Ratio... Interference...Document17 pagesLecture 3 Gears Machine Design - CAD-II ..Example... Contact Ratio... Interference...Hassan AliNo ratings yet
- CS Lect.2 Modeling in The Frequency DomainDocument60 pagesCS Lect.2 Modeling in The Frequency DomainHassan AliNo ratings yet
- Machine Design & CAD-II: Text Book Reference BooksDocument18 pagesMachine Design & CAD-II: Text Book Reference BooksHassan AliNo ratings yet
- Machine Design & CAD-II Lecture 02gears TerminologiesDocument10 pagesMachine Design & CAD-II Lecture 02gears TerminologiesUmar ChNo ratings yet
- Chap 01 Solutions Ex 1 2 Method PDFDocument38 pagesChap 01 Solutions Ex 1 2 Method PDFHassan AliNo ratings yet
- Control Engineering and Instrumentation (LAB) : ClassDocument12 pagesControl Engineering and Instrumentation (LAB) : ClassHassan AliNo ratings yet
- Chap 01 Solutions Ex 1 1 MethodDocument16 pagesChap 01 Solutions Ex 1 1 MethodMuhammad SalmanNo ratings yet
- Laplace Transformation Umer Asghar Method PDFDocument36 pagesLaplace Transformation Umer Asghar Method PDFSikandar Khan100% (3)
- Chap 01 Solutions Ex 1 1 MethodDocument16 pagesChap 01 Solutions Ex 1 1 MethodMuhammad SalmanNo ratings yet
- Assignment CH 4Document5 pagesAssignment CH 4Punjabi FootballNo ratings yet
- Causes of The Decline of MUghal EmpireDocument4 pagesCauses of The Decline of MUghal EmpireHassan AliNo ratings yet
- Assignment CH 4Document5 pagesAssignment CH 4Punjabi FootballNo ratings yet
- Strauss Dental Catalog 2013Document74 pagesStrauss Dental Catalog 2013d3xt3rokNo ratings yet
- Analisis Dampak Reklamasi Teluk Banten Terhadap Kondisi Lingkungan Dan Sosial EkonomiDocument10 pagesAnalisis Dampak Reklamasi Teluk Banten Terhadap Kondisi Lingkungan Dan Sosial EkonomiSYIFA ABIYU SAGITA 08211840000099No ratings yet
- LH 11 180 190 220 230 270 280 390 400 Breaker Safety & Operating InstructionsDocument304 pagesLH 11 180 190 220 230 270 280 390 400 Breaker Safety & Operating InstructionshadensandorNo ratings yet
- g21 Gluta MsdsDocument3 pagesg21 Gluta Msdsiza100% (1)
- Insurance Principles, Types and Industry in IndiaDocument10 pagesInsurance Principles, Types and Industry in IndiaAroop PalNo ratings yet
- Nutrition During PregnancyDocument8 pagesNutrition During PregnancyHalliahNo ratings yet
- Dr. Namrata Misra Head of Bioinnovations at KIIT UniversityDocument1 pageDr. Namrata Misra Head of Bioinnovations at KIIT Universitymanisha maniNo ratings yet
- AYUSHMAN BHARAT Operationalizing Health and Wellness CentresDocument34 pagesAYUSHMAN BHARAT Operationalizing Health and Wellness CentresDr. Sachendra Raj100% (1)
- Emission of Volatile Organic Compounds (Vocs) From Dispersion and Cementitious Waterproofing ProductsDocument16 pagesEmission of Volatile Organic Compounds (Vocs) From Dispersion and Cementitious Waterproofing ProductsKrishna KusumaNo ratings yet
- COVID 19 Impacts On The Construction IndustryDocument46 pagesCOVID 19 Impacts On The Construction IndustryAlemayehu DargeNo ratings yet
- Pet - WikipediaDocument12 pagesPet - Wikipediabdalcin5512No ratings yet
- 07 Chapter2Document16 pages07 Chapter2Jigar JaniNo ratings yet
- Proper Operating Room Decorum: Lee, Sullie Marix P. Maderal, Ma. Hannah Isabelle JDocument15 pagesProper Operating Room Decorum: Lee, Sullie Marix P. Maderal, Ma. Hannah Isabelle Jjoannamhay ceraldeNo ratings yet
- Alternate Mekton Zeta Weapon CreationDocument7 pagesAlternate Mekton Zeta Weapon CreationJavi BuenoNo ratings yet
- Quality Control Plan Static EquipmentDocument1 pageQuality Control Plan Static EquipmentdhasdjNo ratings yet
- 57882d4608ae21394a0c7b00 PDFDocument574 pages57882d4608ae21394a0c7b00 PDFtualaNo ratings yet
- HierbasDocument25 pagesHierbasrincón de la iohNo ratings yet
- Space Analysis in Orthodontic: University of GlasgowDocument16 pagesSpace Analysis in Orthodontic: University of GlasgowNizam Muhamad100% (1)
- Retail Management PPT1Document14 pagesRetail Management PPT1Srilekha GubbalaNo ratings yet
- QRF HD785-7Document2 pagesQRF HD785-7Ralf MaurerNo ratings yet
- Auramo Oy spare parts listsDocument12 pagesAuramo Oy spare parts listsYavuz ErcanliNo ratings yet
- To The OneDocument8 pagesTo The OnePizzaCowNo ratings yet
- AZ ATTR Concept Test Clean SCREENERDocument9 pagesAZ ATTR Concept Test Clean SCREENEREdwin BennyNo ratings yet
- A. Kumar Aswamy Job Offer LetterDocument1 pageA. Kumar Aswamy Job Offer LetterHimanshu PatelNo ratings yet
- Cap 716 PDFDocument150 pagesCap 716 PDFjanhaviNo ratings yet
- FileDocument284 pagesFileJesse GarciaNo ratings yet
- Formularium ApotekDocument12 pagesFormularium ApotekNurul Evi kurniatiNo ratings yet
- MR23002 D Part Submission Warrant PSWDocument1 pageMR23002 D Part Submission Warrant PSWRafik FafikNo ratings yet
- Hydrogeological Characterization of Karst Areas in NW VietnamDocument152 pagesHydrogeological Characterization of Karst Areas in NW VietnamCae Martins100% (1)