Depending on the extent to which the two components are soluble in each other in the solid state, different types of alloys result.
Introduction
In many technical fields, high demands are placed on the materials used, for example in aeronautical engineering. In some combustion chambers the temperature can exceed 2000 °C. The materials must therefore not only withstand high mechanical loads but also thermal stresses.
Simple metals often do not meet these requirements. For this reason, several metals are usually melted together in order to obtain completely new properties after solidification. Such mixtures of two or more metals are also known as alloys. On a chemical level, alloys are therefore characterized by their metallic bonds.
Mixtures of substances with metallic character are called alloys!
In order to specifically influence the desired material properties, profound knowledge of alloying is required. The basics of alloying will therefore be explained in this article. Due to their complexity, only alloys which are consist of two components are considered (also referred to as binary systems). The total of the possible mixture concentrations of a two-component alloy is also referred to as alloying system.
Binary systems are alloy systems consisting of two components!
In general, alloys are obtained by melting, mixturing and subsequent solidification. For this purpose, a certain amount of an alloying element B (solute) is added to a host material A (solvent) in the liquid state. In the liquid state, the atoms of the substances involved are only weakly bound to each other. In general, the substances can therefore be mixed relatively well.
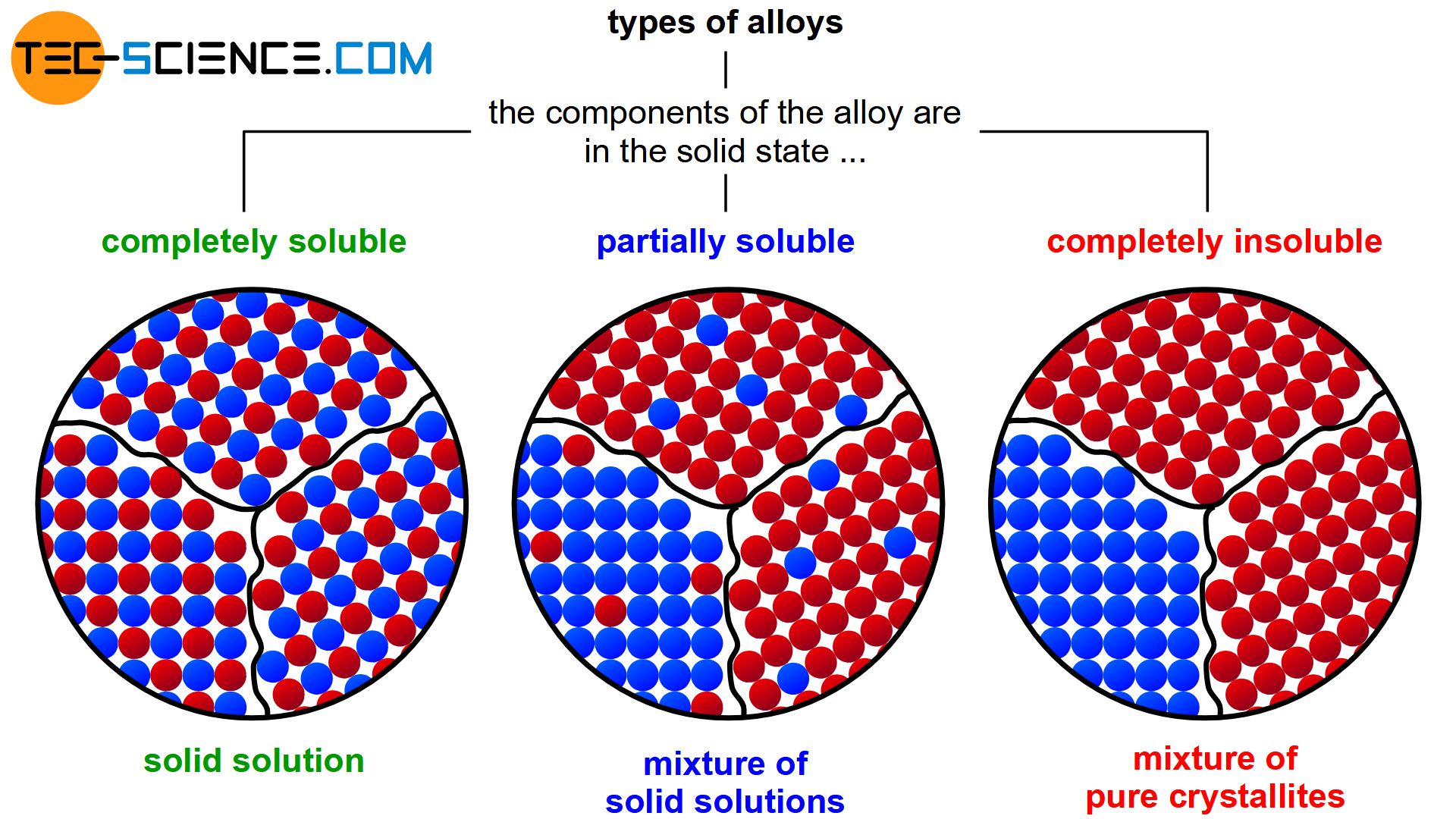
During solidification, this solubility can then either be retained completely (complete solid solution) or be completely lost (mixture of pure crystals). Partial solubilities of the substances can also occur during solidification (mixture of solid solutions).
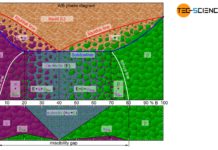
Depending on the solubility of the two components A and B in the solid state, alloys can thus be divided into three different types.
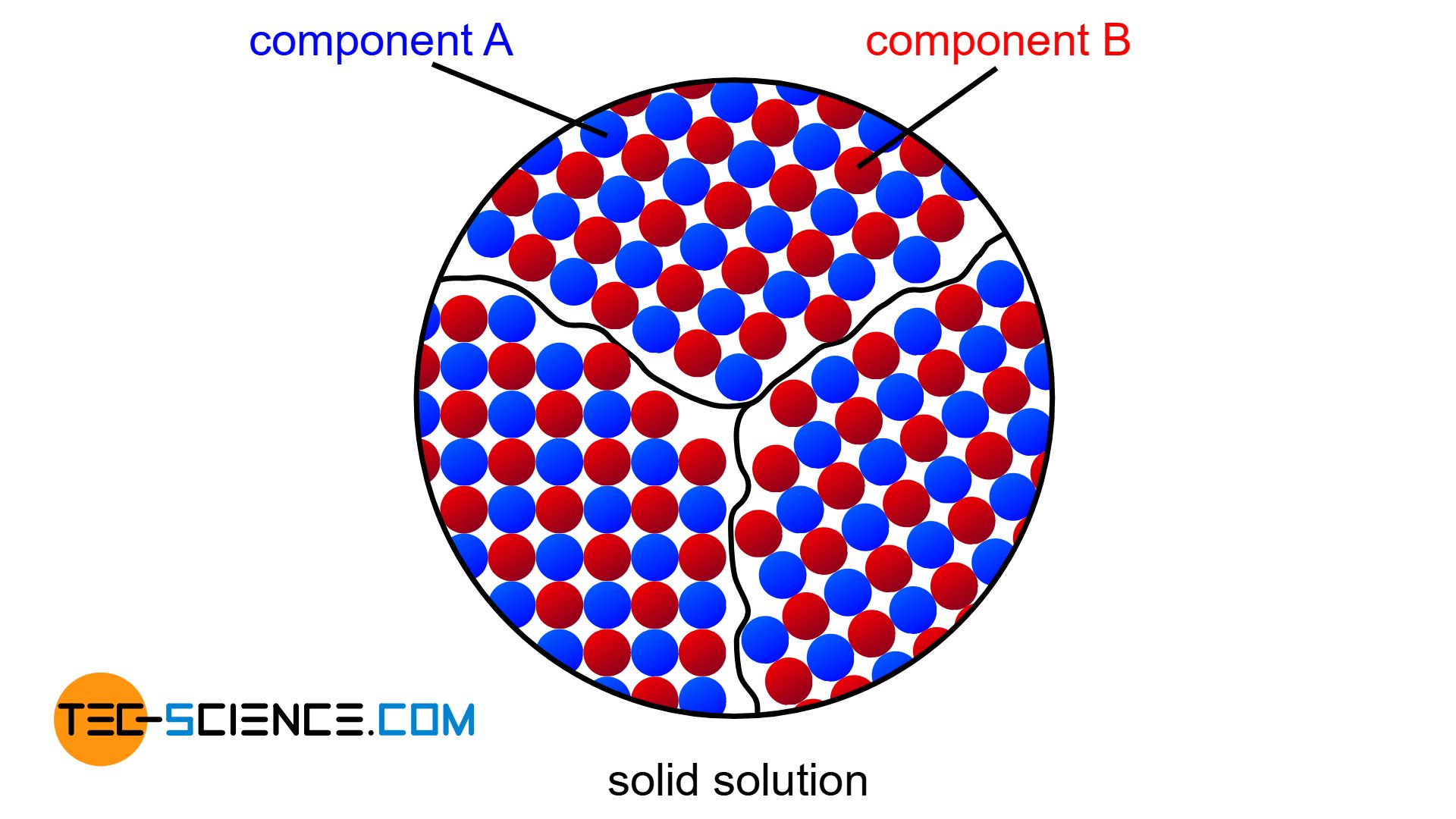
Solid solution
If the two components A and B of an alloy remain completely soluble in one another in the solid state, the atoms of the alloying component B are incorporated in the host crystal (host matrix) of the host component A. The atoms A and B then form a common lattice structure. Such a crystal structure of mixed atoms in a common lattice is called solid solution.
A complete solubility of the alloy components in the solid state is referred to as a solid solution!
Figuratively, the components of a solid solution behave like a mixture of water and alcohol, in which the alcohol particles can also be completely dissolved in the water.
In a solid solution, the alloying atoms B can accumulate in the host lattice of the host substance A in two different ways during crystallization. Accordingly, a distinction can be made between substitutional solid solutions and interstitial solid solutions. These are explained in more detail in the corresponding chapters.
Substitutional solid solution
If the atoms of the alloying element B occupy regular positions in the host lattice of the element A during crystallization, this is referred to as a substitutional solid solution. A comparison between the host lattice before melting down and the common crystal lattice after solidification shows that individual A atoms were simply substituted by B atoms.
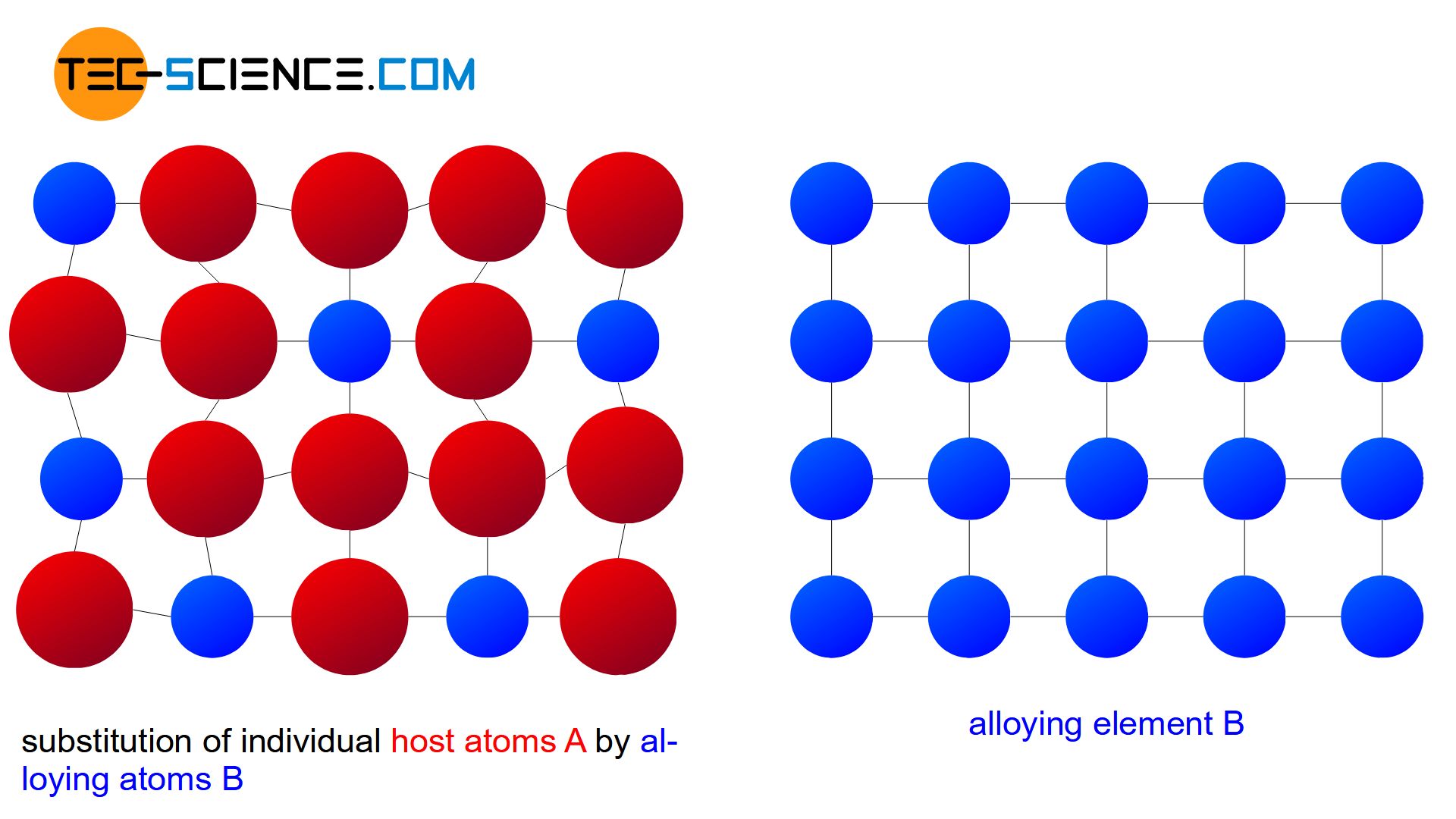
In general, the two alloy components have different atom radii and chemical properties. Therefore, in reality, there is a lattice distortion within the crystalls. The lattice distortion increases with the number of substituted atoms and finally leads to the fact that the host atoms cannot be replaced to an unlimited extent by alloying atoms. The solubility of the alloying element in the host material is therefore generally limited (partial solubility of the components in the solid state).
Only under the conditions that the alloying component B, compared to the host component A, has
- the same lattice structure,
- similar atomic radii (differ less than 15 %) and
- similar chemical properties,
the alloying atoms B can occupy regular lattice sites of the host crystal A “unnoticed” over the entire mixing range. Any alloy concentration can ultimately be produced without a so-called miscibility gap by means of such a substitutional solid solution.
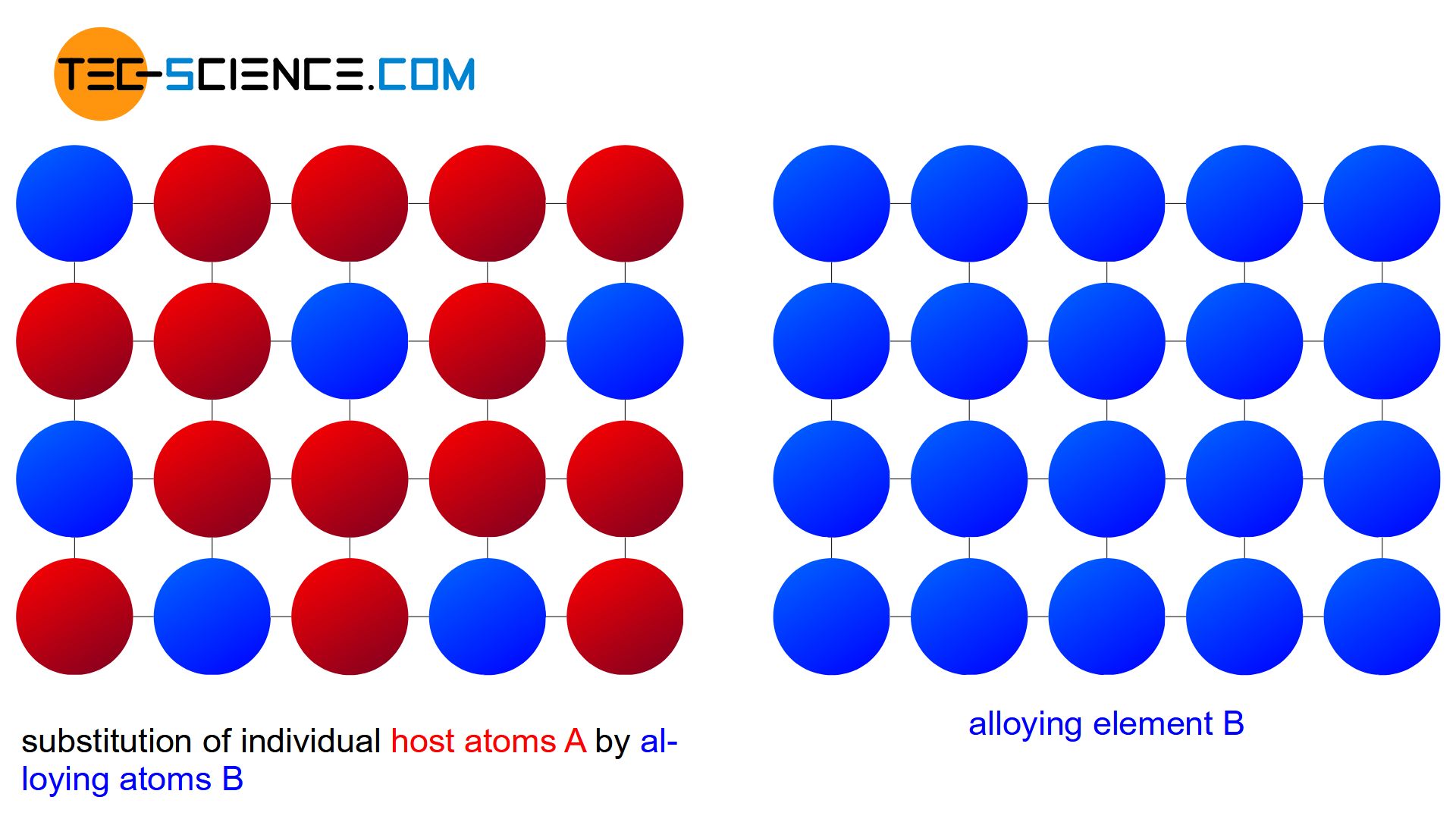
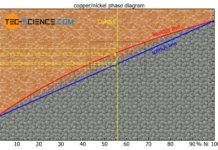
A solubility over the entire mixing range is also referred to as a complete solid solution series, i.e. a perfect solubility of the components in the solid state. In principle, the copper-nickel alloy system forms such a complete solid solution series.
An alloy system which shows a complete solubility of the components in the solid state over the entire concentration range is also referred to as a “complete solid solution series”!
The conditions mentioned above to form a solid solution are also called Hume-Rothery rules.
Interstitial solid solution
If the atoms B of the alloying element are relatively small compared to the atoms A of the host material (maximum diameter ratio 0.4), there is another possibility of atomic arrangement in the lattice. Due to their small size, the alloying atoms B can then also be placed in interstitial lattices sites of the host crystal structure.
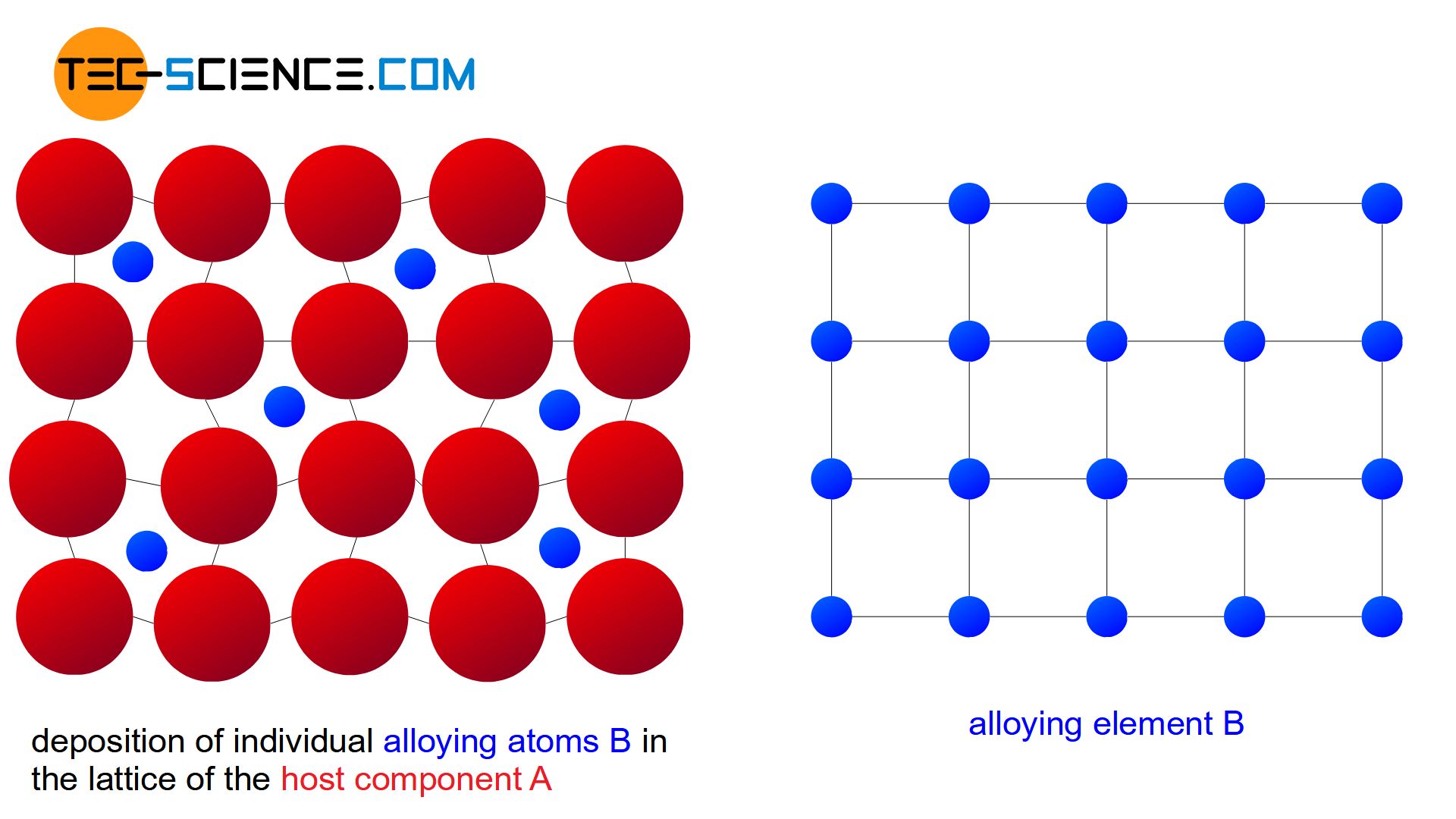
Such a mixture of atoms at intermediate lattice sites is also known as an interstitial solid solution. Due to their dominant role, the regular lattice sites are reserved exclusively for the atoms of the host lattice.
Since only the intermediate lattice sites are available in an interstitial solid solution, a complete solubility of the components only occurs within a strongly limited concentration range (usually only a few percent). If alloying is carried out beyond this solubility limit, the “excess” of alloying atoms B will precipitate and form its own crystal in the microstructure. This crystal can in turn partly contain atoms of the host component A. Therefore, only a partial solubility of the components in the solid state is obtained for interstitial solid solutions.
Mixture of pure crystals
If the conditions for the formation of a solid solution are not met, the alloying atoms B may not be able to occupy regular lattice sites or intermediate lattice sites as well. This is the case when the alloying component B, in comparison to the host component A, has
- another lattice structure or
- has very different chemical properties.
The atoms are then practically displaced from the other lattice structure during solidification and are forced to form their own (“pure”) crystals. Each type of atom then forms its own crystal structure, so that no alloying atoms can be found in the host lattice and no host atoms ca be found in the alloying lattice (complete insolubility of the components in the solid state). The microstructure consists of a mixture of completely different crystallites (grains).
If the components of an alloy are not soluble, each component will form its own crystal structure!
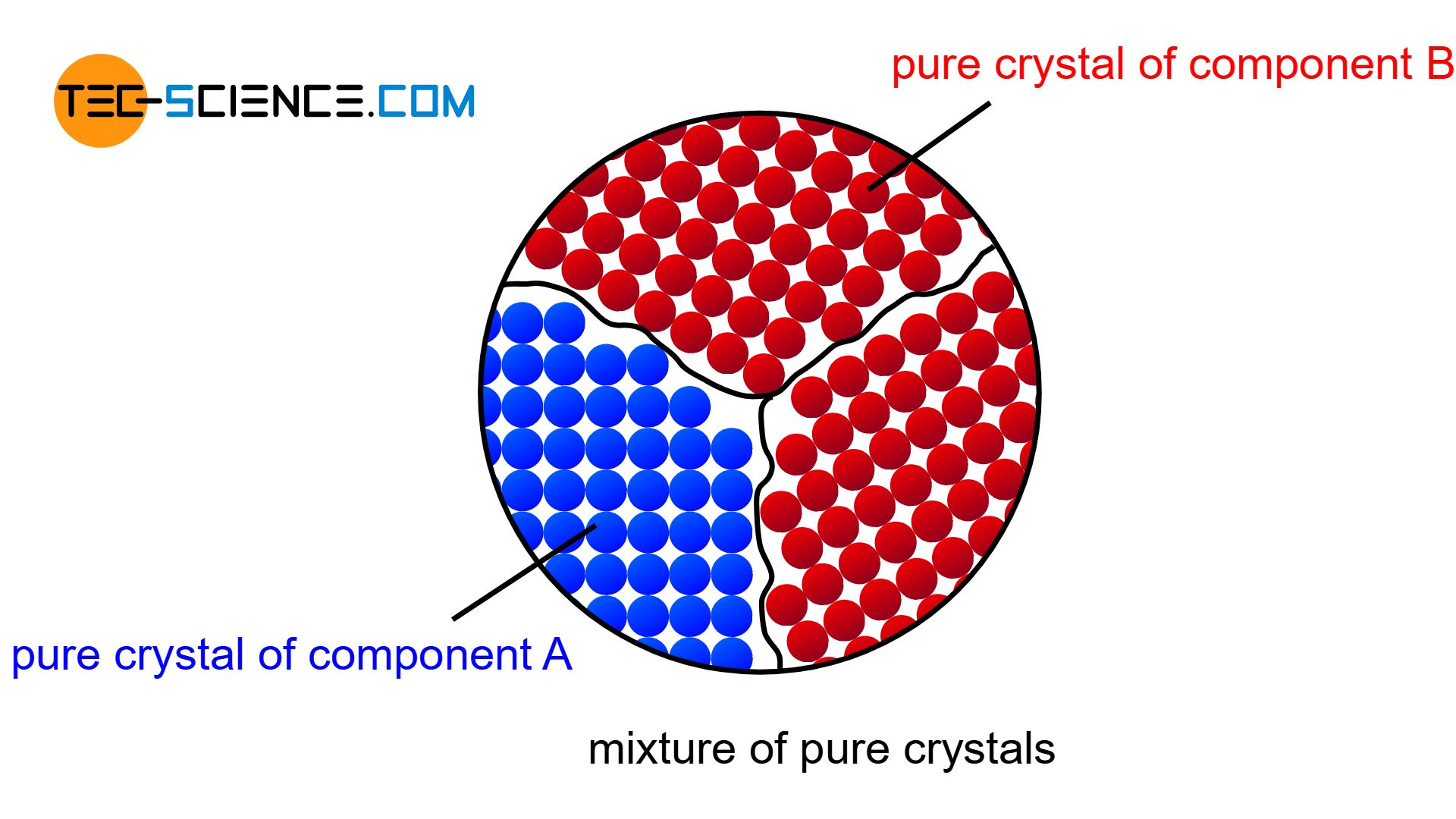
Figuratively, the components of such a alloy behave like a mixture of water and oil, whose components cannot be mixed either – neither the water particles in oil nor the oil particles in water.
Note that just because the atoms of a mixed crystal alloy cannot be mixed in a common lattice structure, this does not mean that the alloy is less stable than a solid solution alloy! Very high interatomic forces also act between the pure crystals of such an alloy to ensure cohesion.
A mixture of pure crystallites will not be found in reality, since the components can always be mixed to a certain degree, even if the solubility is very low. But in the bismuth-cadmium alloy system, for example, the solubility is so low that a complete insolubility of the components can be assumed for simplicity.
Mixture of solid solutions
Perfect solubility (solid solution) or complete insolubility of the components (crystal mixture) are only special cases. In general, the components are neither completely miscible nor immiscible.
In reality, an alloying component B can always be dissolved to a certain degree in the host component A and vice versa. In general, therefore, a limited solubility of the components in the solid state is always obtained.
Figuratively, partial solubility can be compared with a water-sugar mixture in which the solubility of sugar in water is also limited. The water can only dissolve the sugar in it to a certain extent, the undissolved sugar will eventually settle.
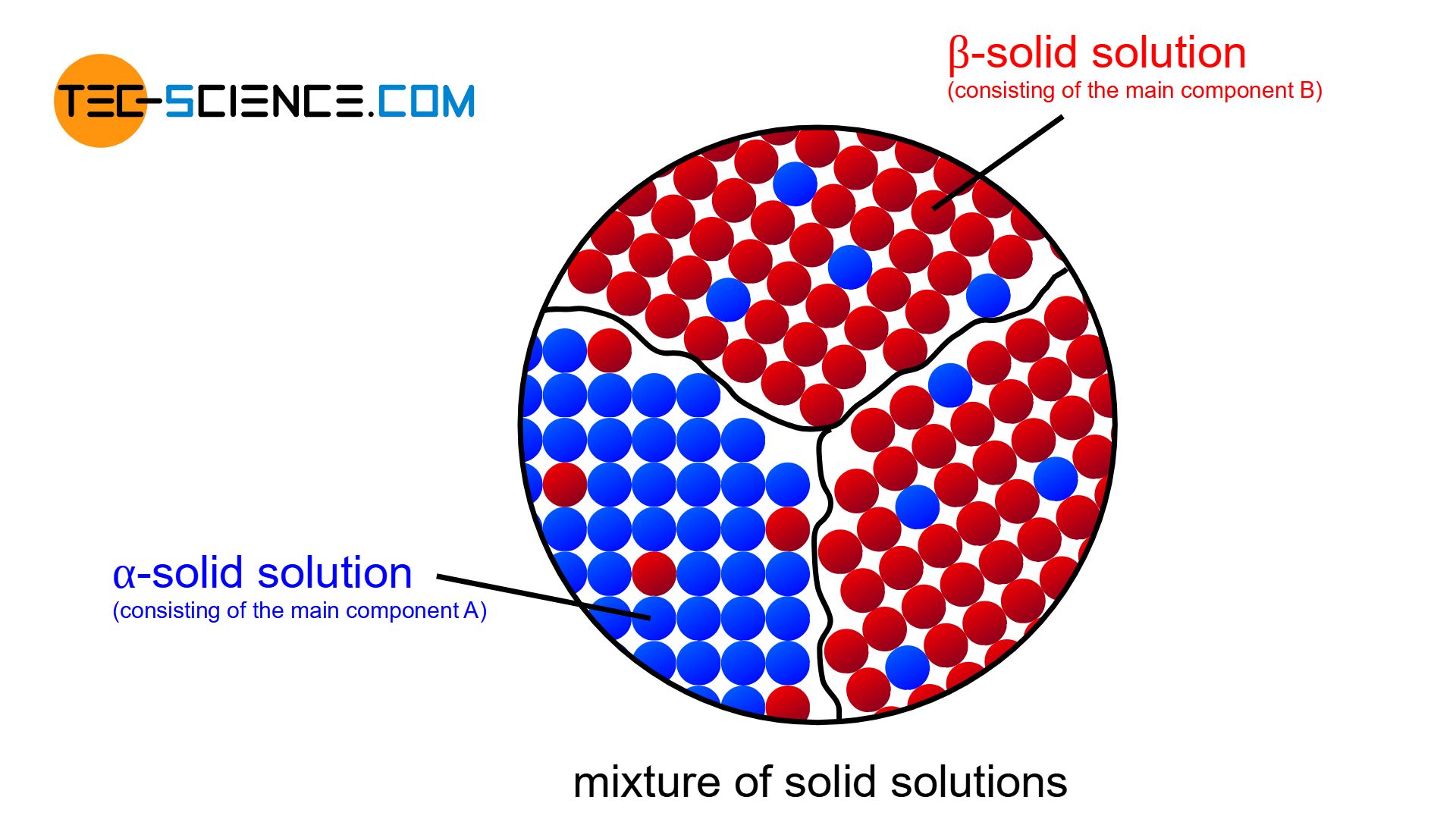
In the lattice structure of the host component A (solvent), B atoms (solute) will also be found to a certain extent. Depending on their chemical properties, the B atoms can either occupy regular lattice sites in the host lattice A or be stored at intermediate lattice sites. It is then either a substitutional solid solution or an interstitial solid solution.
Such a solid solution, which primarily consists of the host element A (in particular of the lattice structure of component A) and contains only small amounts of alloying element B, is also referred to as \(\alpha\)-solid solution (alpha solid solution).
Conversely, at very high concentrations of B atoms, the crystallites consist mainly of the lattice structure of component B (solvent), while small amounts of atoms A (solute) will be deposited therein. In such a case one speaks of a \(\beta\)-solid solution (beta solid solution).