Answer
284.1k+ views
Hint: Acrylonitrile is a chemical compound consisting of cyanide group, and double bond between the carbon and carbon atoms. This compound on the combination or repeating units forms a polymer known as acrilan. It is a hard, and a high melting material and can be used in the fabric of clothes.
Complete answer:
Chemical compounds consist of one or more functional groups. Alkenes are the unsaturated hydrocarbons consisting of double bonded carbon atoms. Cyanides are the compounds consisting of a cyanide group $ \left( {C \equiv N} \right) $ .
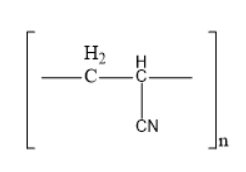
Acrylonitrile is a chemical compound consisting of cyanide group and a double bond. One end of the double bonded carbon is involved in the bond formation with another molecule of acrylonitrile consisting of a double bond. Like this acrylonitrile is involved in the repeating bond formation and forms an additional polymer known as acrilan.
Acrilan is also known as orlon, which is known as the additional polymer of acrylonitrile. It is generally known as poly acrylonitrile and simply abbreviated as PAN. Though it is a thermoplastic material it has a high melting point over $ {300^0}C $ .
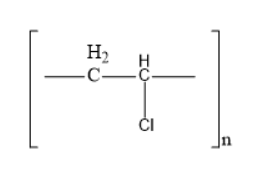
The structure of acrilan is
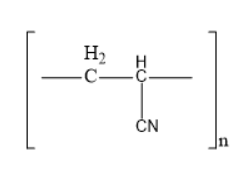
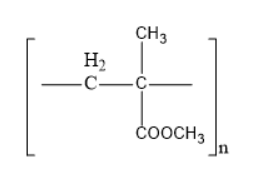
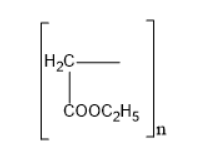
Thus, option B is the correct one.
Note:
Poly acrylonitrile has low density, and it degrades before melting, which is unusual for thermoplastics. It has so many applications in the fabric industry. It can be used more due to its inexpensiveness when compared to the natural fiber. As it degrades before its melting point, it was considered as a high melting material.
Complete answer:
Chemical compounds consist of one or more functional groups. Alkenes are the unsaturated hydrocarbons consisting of double bonded carbon atoms. Cyanides are the compounds consisting of a cyanide group $ \left( {C \equiv N} \right) $ .
Acrylonitrile is a chemical compound consisting of cyanide group and a double bond. One end of the double bonded carbon is involved in the bond formation with another molecule of acrylonitrile consisting of a double bond. Like this acrylonitrile is involved in the repeating bond formation and forms an additional polymer known as acrilan.
Acrilan is also known as orlon, which is known as the additional polymer of acrylonitrile. It is generally known as poly acrylonitrile and simply abbreviated as PAN. Though it is a thermoplastic material it has a high melting point over $ {300^0}C $ .
The structure of acrilan is

Thus, option B is the correct one.
Note:
Poly acrylonitrile has low density, and it degrades before melting, which is unusual for thermoplastics. It has so many applications in the fabric industry. It can be used more due to its inexpensiveness when compared to the natural fiber. As it degrades before its melting point, it was considered as a high melting material.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Three beakers labelled as A B and C each containing 25 mL of water were taken A small amount of NaOH anhydrous CuSO4 and NaCl were added to the beakers A B and C respectively It was observed that there was an increase in the temperature of the solutions contained in beakers A and B whereas in case of beaker C the temperature of the solution falls Which one of the following statements isarecorrect i In beakers A and B exothermic process has occurred ii In beakers A and B endothermic process has occurred iii In beaker C exothermic process has occurred iv In beaker C endothermic process has occurred

What is the stopping potential when the metal with class 12 physics JEE_Main

The momentum of a photon is 2 times 10 16gm cmsec Its class 12 physics JEE_Main

How do you arrange NH4 + BF3 H2O C2H2 in increasing class 11 chemistry CBSE

Is H mCT and q mCT the same thing If so which is more class 11 chemistry CBSE

Trending doubts
Difference between Prokaryotic cell and Eukaryotic class 11 biology CBSE

Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Fill the blanks with proper collective nouns 1 A of class 10 english CBSE

Which of the following is not a primary colour A Yellow class 10 physics CBSE

Change the following sentences into negative and interrogative class 10 english CBSE

What organs are located on the left side of your body class 11 biology CBSE

The Equation xxx + 2 is Satisfied when x is Equal to Class 10 Maths