Caddisflies of the Yukon - Department of Biological Sciences ...
Caddisflies of the Yukon - Department of Biological Sciences ...
Caddisflies of the Yukon - Department of Biological Sciences ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong><br />
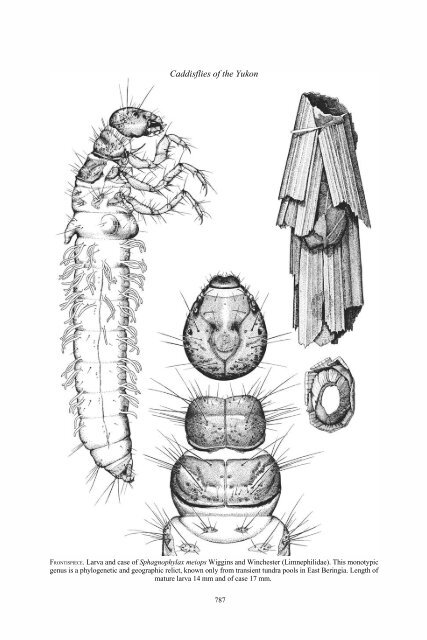
FRONTISPIECE. Larva and case <strong>of</strong> Sphagnophylax meiops Wiggins and Winchester (Limnephilidae). This monotypic<br />
genus is a phylogenetic and geographic relict, known only from transient tundra pools in East Beringia. Length <strong>of</strong><br />
mature larva 14 mm and <strong>of</strong> case 17 mm.<br />
787
<strong>Caddisflies</strong> (Trichoptera) <strong>of</strong> <strong>the</strong> <strong>Yukon</strong>,<br />
with Analysis <strong>of</strong> <strong>the</strong> Beringian and<br />
Holarctic Species <strong>of</strong> North America<br />
GLENN B. WIGGINS and CHARLES R. PARKER<br />
Centre for Biodiversity and Conservation Biology (formerly <strong>Department</strong> <strong>of</strong> Entomology)<br />
Royal Ontario Museum, 100 Queen’s Park, Toronto, Ontario, Canada M5S 2C6;<br />
and <strong>Department</strong> <strong>of</strong> Zoology, University <strong>of</strong> Toronto<br />
Current address <strong>of</strong> C.R. Parker:<br />
U.S. Geological Survey <strong>Biological</strong> Resources Division, Great Smokies Field Station<br />
1314 Cherokee Orchard Road, Gatlinburg, Tennessee 37738, U.S.A.<br />
Abstract. The Trichoptera recorded from <strong>the</strong> <strong>Yukon</strong> Territory now number 145 species, constituting 11 per cent<br />
<strong>of</strong> <strong>the</strong> North American fauna north <strong>of</strong> Mexico. Present distribution known for each species in <strong>the</strong> <strong>Yukon</strong> is outlined,<br />
and biological information at familial and generic levels is briefly summarized. For biogeographic analysis, evidence<br />
bearing on <strong>the</strong> distribution <strong>of</strong> <strong>the</strong> species is considered under 4 categories. Members <strong>of</strong> category I are wholly Nearctic<br />
in distribution (98 species, 68 per cent <strong>of</strong> <strong>Yukon</strong> Trichoptera) and, in <strong>the</strong> absence <strong>of</strong> evidence to <strong>the</strong> contrary, are<br />
considered to have repopulated <strong>the</strong> <strong>Yukon</strong> and o<strong>the</strong>r nor<strong>the</strong>rn areas from glacial refugia to <strong>the</strong> south <strong>of</strong> <strong>the</strong> Laurentide<br />
and Cordilleran ice sheets <strong>of</strong> Wisconsinan time.<br />
Species <strong>of</strong> category II are Holarctic, and are now more or less widely distributed in Eurasia and nor<strong>the</strong>rn North<br />
America (28 species, about 18 per cent <strong>of</strong> <strong>Yukon</strong> Trichoptera). These species could have passed <strong>the</strong> last glacial<br />
period in unglaciated Beringia, or to <strong>the</strong> south <strong>of</strong> <strong>the</strong> ice, or in both areas.<br />
Category III is composed <strong>of</strong> Palaearctic species which, from evidence available, are now confined in North<br />
America mainly to unglaciated Beringia or somewhat beyond (13 species, about 10 per cent <strong>of</strong> <strong>Yukon</strong> Trichoptera).<br />
Several represent a paradox <strong>of</strong> Beringian distribution—widely distributed Palaearctic species, evidently successful<br />
colonizers when <strong>the</strong>y entered North America but, with retreat <strong>of</strong> <strong>the</strong> ice, have not extended <strong>the</strong>ir Nearctic range.<br />
Geological and biological factors underlying this paradox are discussed. Two Palaearctic species are recorded from<br />
North America for <strong>the</strong> first time: Rhyacophila mongolica Schmid, Arefina and Levanidova and Limnephilus diphyes<br />
McLachlan.<br />
Category IV comprises 8 species (including 2 additional species expected from <strong>the</strong> <strong>Yukon</strong>), about 4 per cent <strong>of</strong><br />
<strong>the</strong> fauna, known mainly from <strong>the</strong> <strong>Yukon</strong> or from adjacent areas <strong>of</strong> Alaska or <strong>the</strong> Northwest Territories; <strong>the</strong>se<br />
species are considered to be Beringian endemics or glacial relicts. Finally, because almost all <strong>of</strong> <strong>the</strong> Holarctic<br />
Trichoptera now recognized in North America are reviewed in <strong>the</strong> foregoing groups, <strong>the</strong> remaining Holarctic species<br />
that do not occur in Beringia are considered briefly in a fifth category, although <strong>the</strong>y have not been recorded from<br />
<strong>the</strong> <strong>Yukon</strong> and most do not appear to be species <strong>of</strong> far nor<strong>the</strong>rn latitudes. The origin <strong>of</strong> <strong>the</strong> Trichoptera <strong>of</strong> Greenland<br />
is also discussed.<br />
Ecological factors influencing <strong>the</strong> nor<strong>the</strong>rn penetration <strong>of</strong> <strong>Yukon</strong> and Beringian Trichoptera are considered with<br />
an analysis <strong>of</strong> lotic and lentic-dwelling species through a latitudinal gradient <strong>of</strong> 49° to 70°N—from <strong>the</strong> sou<strong>the</strong>rn<br />
border <strong>of</strong> British Columbia to <strong>the</strong> Arctic coastline <strong>of</strong> <strong>the</strong> <strong>Yukon</strong>. At latitude 60°N, <strong>the</strong> sou<strong>the</strong>rn boundary <strong>of</strong> <strong>the</strong><br />
<strong>Yukon</strong>, diversity has declined by almost 50 per cent from levels obtaining in British Columbia, 49° through 60°N.<br />
The main depletion occurs in <strong>the</strong> Spicipalpia and filter-feeding Annulipalpia; case-making caddisflies <strong>of</strong> <strong>the</strong><br />
Integripalpia show less reduction. Similar trends are continued through <strong>the</strong> <strong>Yukon</strong> from 60° to 70°N, where species<br />
diversity in <strong>the</strong> Trichoptera declines by ano<strong>the</strong>r 59 per cent. Although most North American Trichoptera occur in<br />
running waters, <strong>the</strong>re is a marked reduction <strong>of</strong> species in <strong>the</strong>se habitats with increasing latitude. Of 60 species<br />
recorded in <strong>the</strong> <strong>Yukon</strong> north <strong>of</strong> <strong>the</strong> Arctic Circle (67° – 70°N), 81 per cent are Integripalpia with case-making larvae<br />
living mainly in lentic habitats. Factors underlying <strong>the</strong> decline <strong>of</strong> lotic species, and <strong>the</strong> proportional increase <strong>of</strong><br />
lentic species at higher latitudes are considered. Trichoptera <strong>of</strong> lentic habitats were much more successful in crossing<br />
<strong>the</strong> Bering land bridge than were species dependent on lotic waters.<br />
Taxonomic changes resulting from this study include suppression <strong>of</strong> Grammotaulius subborealis Schmid as a<br />
junior subjective synonym <strong>of</strong> G. alascensis Schmid. The status <strong>of</strong> Limnephilus fumosus Banks is clarified as a<br />
species distinct from Limnephilus santanus Ross, and a lectotype is designated for L. fumosus; L. isobela Nimmo<br />
is recognized as a junior subjective synonym <strong>of</strong> L. fumosus Banks. Goera radissonica Harper and Méthot, described<br />
from nor<strong>the</strong>rn Quebec, is recognized as a junior subjective synonym <strong>of</strong> Goera tungusensis Martynov, originally<br />
described from Siberia. A morphological variant <strong>of</strong> Ceraclea nigronervosa (Retzius) is described. The distributional<br />
pp. 787 – 866 in H.V. Danks and J.A. Downes (Eds.), Insects <strong>of</strong> <strong>the</strong> <strong>Yukon</strong>. <strong>Biological</strong> Survey <strong>of</strong> Canada (Terrestrial Arthropods),<br />
Ottawa. 1034 pp. © 1997
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 789<br />
and taxonomic status <strong>of</strong> Mystacides interjectus (Banks) and M. sepulchralis (Walker) is reviewed and clarified.<br />
This study provides a taxonomic and conceptual framework for fur<strong>the</strong>r investigation <strong>of</strong> <strong>the</strong> Holarctic Trichoptera.<br />
Résumé. Les trichoptères (Trichoptera) du <strong>Yukon</strong>: inventaire et analyse des espèces béringiennes et holarctiques<br />
d’Amérique du Nord. Les trichoptères du <strong>Yukon</strong> comptent maintenant 145 espèces connues, soit 11 pourcent de la<br />
faune nord-américaine au nord du Mexique. On trouvera ici un aperçu de la répartition de chacune de ces espèces<br />
au <strong>Yukon</strong> et un sommaire de certains aspects de la biologie des familles et des genres. Une analyse biogéographique<br />
de la répartition des espèces a donné lieu à quatre grandes catégories: les membres de la catégorie I ont une répartition<br />
essentiellement néarctique (98 espèces, 68% des trichoptères du <strong>Yukon</strong>) et, jusqu’à preuve du contraire, sont<br />
estimées avoir recolonisé le <strong>Yukon</strong> et les autres zones nordiques à partir de refuges glaciaires situés au sud des<br />
glaciations laurentidiennes et cordillériennes au cours du Wisconsinien.<br />
Les espèces de la catégorie II sont holarctiques et sont maintenant généralement bien répandues en Eurasie et<br />
dans le nord de l’Amérique du Nord (28 espèces, environ 18% des trichoptères du <strong>Yukon</strong>). Ces espèces ont<br />
probablement passé la dernière période glaciaire dans la partie non englacée de la Béringie, ou alors au sud des<br />
glaces, ou ont occupé les deux endroits.<br />
La catégorie III se compose d’espèces paléarctiques qui semblent confinées, en Amérique du Nord, à la partie<br />
non englacée de la Béringie ou un peu au-delà (13 espèces, environ 10% des trichoptères du <strong>Yukon</strong>). Plusieurs ont<br />
une répartition béringienne un peu énigmatique—ce sont des espèces paléarctiques bien répandues qui ont colonisé<br />
l’Amérique du Nord avec succès, mais qui, au retrait des glaces, ne se sont pas répandues davantage dans la zone<br />
néarctique. Les facteurs géologiques et biologiques qui pourraient expliquer ce paradoxe font l’objet d’une<br />
discussion. Deux espèces paléarctiques sont mentionnées en Amérique du Nord pour la première fois, Rhyacophila<br />
mongolica Schmid, Arefina et Levanidova et Limnephilus diphyes McLachlan.<br />
La catégorie IV comprend 8 espèces (dont 2 qui n’ont pas encore été trouvées au <strong>Yukon</strong>), soit environ 4% de<br />
la faune, connues surtout au <strong>Yukon</strong> ou dans les zones adjacentes en Alaska et dans les Territoires du Nord-Ouest;<br />
ces espèces sont considérées comme endémiques en Béringie ou comme des espèces relictes des glaciations. Enfin,<br />
comme la plupart des trichoptères holarctiques reconnus en Amérique du Nord appartiennent aux catégories<br />
précédentes, les autres espèces holarctiques qui n’ont jamais été trouvées en Béringie sont examinées brièvement<br />
et forment une cinquième catégorie d’espèces jamais rencontrées au <strong>Yukon</strong> et dont la plupart ne semblent pas être<br />
des espèces très nordiques. L’origine des trichoptères du Groenland est également examinée.<br />
Les facteurs écologiques qui ont pu influencer la dispersion vers le nord des trichoptères du <strong>Yukon</strong> et de la<br />
Béringie sont étudiés et une analyse des espèces lotiques et lénitiques présentes le long d’un gradient latitudinal du<br />
49 e au 70 e parallèle, du sud de la Colombie-Britannique à la côte arctique du <strong>Yukon</strong>, donne un aperçu global de la<br />
situation. A la latitude 60°N, le long de la frontière australe du <strong>Yukon</strong>, la diversité est déjà réduite de près de 50%<br />
par rapport à la situation qui prévaut en Colombie-Britannique, soit entre les latitudes 49°N et 60°N, diminution<br />
qui affecte surtout les Spicipalpia et les Annulipalpia filtreurs; les Integripalpia constructeurs de fourreaux sont<br />
encore présents en assez grand nombre. La tendance se poursuit vers le nord, entre les parallèles 60 et 70, et la<br />
diversité est réduite d’un autre 59%. Bien que la plupart des trichoptères nord-américains soient des espèces d’eau<br />
courante, le nombre de ces espèces diminue à mesure que la latitude augmente. Des 60 espèces rencontrées au<br />
<strong>Yukon</strong> au nord du cercle arctique (67° – 70°N), 81% sont des Integripalpia dont les larves vivent dans des fourreaux<br />
en milieu lénitique. Les facteurs susceptibles d’expliquer le déclin des espèces lotiques et l’augmentation proportionnelle<br />
des espèces lénitiques aux latitudes plus élevées sont examinés. Les trichoptères des milieux lénitiques<br />
semblent avoir réussi à traverser le pont continental de Bering plus facilement que les espèces des milieux lotiques.<br />
Cette étude a donné lieu à certains remaniements taxonomiques: Grammotaulius subborealis Schmid est<br />
considéré comme un synonyme subjectif récent de G. alascensis Schmid. Le statut de Limnephilus fumosus Banks<br />
est redéfini et l’espèce est distincte de Limnephilus santanus Ross; un lectotype a été désigné pour représenter<br />
L. fumosus; L. isobela Nimmo est reconnu comme un synonyme subjectif récent de L. fumosus Banks. Goera<br />
radissonica Harper et Méthot, décrit du nord du Québec, est reconnu comme un synonyme subjectif récent de Goera<br />
tungusensis Martynov d’abord trouvé en Sibérie. Une variante morphologique de Ceraclea nigronervosa (Retzius)<br />
est décrite. La répartition et le statut taxonomique de Mystacides interjectus (Banks) et de M. sepulchralis (Walker)<br />
sont révisés et clarifiés. Ce travail a permis d’établir un nouveau cadre de recherche taxonomique et conceptuel<br />
pour l’étude des trichoptères holarctiques.<br />
Table <strong>of</strong> Contents<br />
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 790<br />
Materials and Methods . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 791<br />
Annotated species list <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> Trichoptera . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 793<br />
Suborder Spicipalpia<br />
Family Glossosomatidae . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 793<br />
Family Hydroptilidae . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 793<br />
Family Rhyacophilidae . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 794
790 G.B. Wiggins and C.R. Parker<br />
Table <strong>of</strong> Contents (continued)<br />
Suborder Annulipalpia<br />
Family Hydropsychidae . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 796<br />
Family Philopotamidae . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 797<br />
Family Polycentropodidae. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 798<br />
Suborder Integripalpia<br />
Family Apataniidae . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 799<br />
Family Brachycentridae . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 799<br />
Family Goeridae . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 800<br />
Family Lepidostomatidae . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 800<br />
Family Leptoceridae . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 801<br />
Family Limnephilidae . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 803<br />
Family Molannidae . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 809<br />
Family Phryganeidae. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 809<br />
Family Uenoidae . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 811<br />
Taxonomic notes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 811<br />
Biogeographic analysis <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> and Beringian Trichoptera . . . . . . . . . . . . . . . . . . . . . . . . . 820<br />
Geological and climatic context . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 820<br />
<strong>Biological</strong> aspects . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 821<br />
Origin <strong>of</strong> <strong>the</strong> Beringian and Holarctic Trichoptera . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 824<br />
I. Nearctic species widespread in North America . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 825<br />
Greenland Trichoptera . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 828<br />
II. Holarctic species widespread in North America . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 830<br />
III. Palaearctic-East Beringian species . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 839<br />
IV. Beringian species . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 849<br />
V. Holarctic species not in Beringia . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 852<br />
Ecological considerations. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 854<br />
Acknowledgements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 861<br />
References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 862<br />
Introduction<br />
In this study we have undertaken to examine <strong>the</strong> Trichoptera <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> Territory <strong>of</strong><br />
nor<strong>the</strong>rn Canada from a dynamic viewpoint. Several lines <strong>of</strong> biological investigation<br />
coincide to make <strong>the</strong> <strong>Yukon</strong> especially appropriate for such an analysis.<br />
Repopulation <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> following <strong>the</strong> last recession <strong>of</strong> Pleistocene glaciers is <strong>of</strong><br />
unusual biogeographic interest because <strong>the</strong> western half <strong>of</strong> this area was part <strong>of</strong> <strong>the</strong><br />
unglaciated Beringian refugium that extended westward through central Alaska, over <strong>the</strong><br />
exposed continental shelf underlying Bering Strait and adjacent coastal areas, and incorporating<br />
a large part <strong>of</strong> nor<strong>the</strong>astern Asia. While nor<strong>the</strong>rn North America and extensive areas<br />
<strong>of</strong> Europe and Asia were covered by ice during <strong>the</strong> last glacial (Wisconsinan) advance, <strong>the</strong><br />
unglaciated Beringian refugium harboured cold-adapted species, enabling some <strong>of</strong> <strong>the</strong>m to<br />
move from one continent to <strong>the</strong> o<strong>the</strong>r across <strong>the</strong> Bering land bridge connecting North<br />
America and Asia. Evidence indicates that a number <strong>of</strong> <strong>the</strong> species in <strong>the</strong> present trichopteran<br />
fauna <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> entered North America in this way and passed <strong>the</strong> Pleistocene glacial<br />
periods in <strong>the</strong> Beringian refugium, while most Nearctic species were confined to <strong>the</strong> south<br />
<strong>of</strong> <strong>the</strong> advancing front <strong>of</strong> <strong>the</strong> Laurentide continental and Cordilleran montane glaciers.<br />
From an ecological viewpoint, an investigation <strong>of</strong> <strong>Yukon</strong> Trichoptera <strong>of</strong>fers an opportunity<br />
to contrast <strong>the</strong> ecological success <strong>of</strong> a diverse group <strong>of</strong> aquatic insects at high latitudes<br />
with <strong>the</strong> success <strong>of</strong> <strong>the</strong> same group in more temperate parts <strong>of</strong> North America. The species<br />
advancing from sou<strong>the</strong>rn refugia as <strong>the</strong> glacial ice receded had to contend with different<br />
habitat conditions at higher latitudes. For terrestrial insects, evidence on <strong>the</strong> ecological costs
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 791<br />
imposed by <strong>the</strong>se conditions is available from a number <strong>of</strong> groups; but for aquatic insects<br />
o<strong>the</strong>r than Chironomidae (e.g. Oliver 1968) <strong>the</strong>re is little evidence or syn<strong>the</strong>sis. To what<br />
extent did longer colder winters, shorter summers, and shorter photoperiods influence <strong>the</strong><br />
success <strong>of</strong> a group <strong>of</strong> insects with wholly aquatic larvae? Trichoptera are especially<br />
appropriate for seeking answers to questions <strong>of</strong> this kind because <strong>of</strong> <strong>the</strong>ir relatively high<br />
diversification; apart from Chironomidae, <strong>the</strong>re are more species <strong>of</strong> Trichoptera than <strong>of</strong> any<br />
o<strong>the</strong>r group <strong>of</strong> freshwater insects, and those species occupy an exceptionally wide range <strong>of</strong><br />
aquatic habitats and ecological niches (Wiggins and Mackay 1978).<br />
Combining both biogeographic and ecological viewpoints, investigation <strong>of</strong> <strong>the</strong> <strong>Yukon</strong><br />
Trichoptera reveals results in nature when related species <strong>of</strong> Nearctic and Palaearctic origins<br />
come toge<strong>the</strong>r to form aquatic communities. As an ecological testing ground for <strong>the</strong>se natural<br />
experiments, <strong>the</strong> <strong>Yukon</strong> is well suited because <strong>of</strong> its high diversity <strong>of</strong> aquatic habitats.<br />
Mountain ranges divide <strong>the</strong> land into several major drainage systems, giving rise to small<br />
rapid streams, which in turn unite to form river systems <strong>of</strong> increasing order and potential<br />
biological diversity. Marshes, ponds, and lakes abound, providing rich resources for aquatic<br />
insects adapted for life in lentic waters. The treeline, sou<strong>the</strong>rn margin <strong>of</strong> <strong>the</strong> arctic biome,<br />
passes through <strong>the</strong> nor<strong>the</strong>rn <strong>Yukon</strong> at about latitude 67°N. To <strong>the</strong> north, arctic tundra extends<br />
to <strong>the</strong> Arctic Ocean; on <strong>the</strong> slopes <strong>of</strong> <strong>the</strong> mountains, biotic zones range through coniferous<br />
forests to alpine tundra and ice-fields. Although freshwater habitats are highly diverse within<br />
<strong>the</strong> <strong>Yukon</strong>, <strong>the</strong> success <strong>of</strong> insects in forming aquatic communities under climatic conditions<br />
<strong>of</strong> high latitudes is not well understood. Therefore, examination <strong>of</strong> <strong>the</strong>se fundamental<br />
biological issues can add to our understanding <strong>of</strong> <strong>the</strong> biology <strong>of</strong> Trichoptera; <strong>the</strong> same issues<br />
underlie management <strong>of</strong> aquatic systems in <strong>the</strong> <strong>Yukon</strong> Territory.<br />
The analysis begins, necessarily, with a survey <strong>of</strong> <strong>the</strong> species <strong>of</strong> Trichoptera known to<br />
occur in <strong>the</strong> <strong>Yukon</strong>. Systematic interpretation <strong>of</strong> <strong>the</strong> species has been aided by <strong>the</strong> advanced<br />
state <strong>of</strong> knowledge on <strong>the</strong> Trichoptera <strong>of</strong> Russia (e.g. Lepneva 1964, 1966; Martynov 1924a),<br />
and by <strong>the</strong> recent syn<strong>the</strong>sis <strong>of</strong> aquatic insects <strong>of</strong> <strong>the</strong> Russian Far East by I.M. Levanidova<br />
(1982).<br />
Materials and Methods<br />
Most <strong>of</strong> <strong>the</strong> collections <strong>of</strong> Trichoptera studied were made by field parties from <strong>the</strong><br />
<strong>Department</strong> <strong>of</strong> Entomology, Royal Ontario Museum (ROME), over a 4-year period<br />
from 1979 to 1982, and are deposited <strong>the</strong>re. Collections from o<strong>the</strong>r institutions were also<br />
studied: Canadian National Collection <strong>of</strong> Insects, Ottawa (CNCI); Illinois Natural History<br />
Survey, Champaign, Illinois (INHS); Royal British Columbia Museum (BCPM);<br />
U.S. National Museum <strong>of</strong> Natural History (USNM); University <strong>of</strong> British Columbia Insect<br />
Collection, Vancouver (SMDV); Zoological Institute, Russian Academy <strong>of</strong> <strong>Sciences</strong>,<br />
St. Petersburg (ZMAS).<br />
All species known from <strong>the</strong> <strong>Yukon</strong> Territory are listed, including species recorded in<br />
<strong>the</strong> scientific literature but not represented in <strong>the</strong> material we examined—principally records<br />
compiled by Nimmo and Wickstrom 1984 (henceforth NW 1984). Although we have many<br />
larval collections, <strong>the</strong>se records are included only if larvae are <strong>the</strong> single source <strong>of</strong> evidence,<br />
as for Allomyia, or can be identified reliably to species as in some Hydropsyche. Distributional<br />
records are grouped by ecogeographic regions <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> (Fig. 1); <strong>the</strong> detailed list<br />
<strong>of</strong> our records is too long for inclusion here, but is deposited in <strong>the</strong> library <strong>of</strong> <strong>the</strong> Royal<br />
Ontario Museum. At this early stage in understanding <strong>the</strong> distribution <strong>of</strong> Trichoptera in <strong>the</strong><br />
<strong>Yukon</strong>, <strong>the</strong> records are highly correlated with <strong>the</strong> access roads; however, some general<br />
patterns seem to emerge. <strong>Biological</strong> and distributional characteristics <strong>of</strong> <strong>the</strong> higher taxa are
792 G.B. Wiggins and C.R. Parker<br />
FIG. 1. Ecogeographic regions <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> Territory (after Scudder 1997); collection records <strong>of</strong> <strong>Yukon</strong> Trichoptera<br />
are summarized in accordance with <strong>the</strong>se numbered regions. 1, Arctic Coastal Plain; 2, British Mountains; 3, Arctic<br />
Plateau; 4, Porcupine Plain (including Old Crow Plain, Old Crow Mts., N. Porcupine Plateau); 5, Richardson<br />
Mountains; 6, Eagle Plain (including S. Porcupine Plateau); 7, Peel Plateau (including Bonnet Plume Basin); 8,<br />
Ogilvie Mountains (including N. and S. Ogilvie Mts.); 9, Wernecke/Selwyn Mountains; 10, <strong>Yukon</strong>/Tintina<br />
(including Lewes Plateau, part <strong>of</strong> Klondike Plateau, and Tintina Trench); 11, Eastern Plateaus (including Stewart,<br />
Macmillan, and Pelly Plateaus); 12, Shakwak Trench (including Wellesley Basin); 13, Western Ranges (including<br />
Ruby, Nisling, and Dawson Ranges, part <strong>of</strong> Klondike Plateau); 14, Pelly Mountains; 15, Logan Mountains; 16,<br />
Saint Elias/Coast Mountains; 17, Sou<strong>the</strong>rn Lakes (including Aishihik Basin, Takhini Valley, Teslin Plateau, and<br />
Nisutlin Plateau); 18, Cassiar Mountains; 19, Liard Plain (including Dease Plateau); 20, Hyland/Liard Plateaus.
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 793<br />
outlined briefly to provide a broader context for <strong>the</strong> <strong>Yukon</strong> species; distributional information<br />
for North America is based on <strong>the</strong> manuscript for an Annotated Catalogue <strong>of</strong> <strong>the</strong><br />
Trichoptera <strong>of</strong> North America North <strong>of</strong> Mexico (Wiggins and Flint in prep.), and for Europe<br />
on BotojAneanu and Malicky (1978). Extended comment required on <strong>the</strong> status <strong>of</strong> certain<br />
species is included under Taxonomic Notes. Families, genera, and species are listed alphabetically<br />
under <strong>the</strong> 3 suborders <strong>of</strong> Trichoptera proposed by Wiggins and Wichard (1989)<br />
and Frania and Wiggins (1997). The classification <strong>of</strong> families and genera follows Wiggins<br />
(1996). Roman numerals following <strong>the</strong> names indicate <strong>the</strong> category to which <strong>the</strong> species is<br />
assigned for biogeographic analysis. Arabic numbers associated with <strong>the</strong> species names<br />
provide a cross-reference to distributional and o<strong>the</strong>r data in <strong>the</strong> annotated species list. Dates<br />
for collections <strong>of</strong> adults are based on material examined and on published records.<br />
Annotated Species List <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> Trichoptera<br />
This list records 145 species, mainly from specimens we examined, but also from<br />
literature records where no material was available. Additional species undoubtedly have yet<br />
to be found. Distributional records for <strong>the</strong> <strong>Yukon</strong> are grouped by ecogeographic regions as<br />
identified in Fig. 1.<br />
Suborder Spicipalpia<br />
These are <strong>the</strong> cocoon-making caddisflies, characterized by pupal cocoons <strong>of</strong> stout silk<br />
lacking openings <strong>of</strong> any kind for circulation <strong>of</strong> water over <strong>the</strong> pupa. They are for <strong>the</strong> most<br />
part inhabitants <strong>of</strong> cool running waters, although larvae <strong>of</strong> some genera <strong>of</strong> <strong>the</strong> Hydroptilidae<br />
are adapted to warmer lentic sites.<br />
Family Glossosomatidae<br />
Larvae occur on rocks in flowing waters where <strong>the</strong>y graze on diatoms, o<strong>the</strong>r algae, and<br />
deposits <strong>of</strong> fine organic particles. The family is represented in most faunal regions <strong>of</strong> <strong>the</strong><br />
world; 6 Nearctic genera are recognized, comprising approximately 75 species.<br />
Genus Glossosoma. Species occur through <strong>the</strong> Nearctic, Palaearctic, and Oriental faunal<br />
regions; <strong>of</strong> 22 North American species now known, all but 3 are confined to <strong>the</strong> western<br />
mountains. Three species are recorded from <strong>the</strong> <strong>Yukon</strong>, representing 2 <strong>of</strong> <strong>the</strong> 3 Nearctic<br />
subgenera: G. (Ripaeglossa) alascense; G. (Synafophora = Eomystra) intermedium and verdona.<br />
1. Glossosoma alascense Banks (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong>, Alaska, south to Utah.<br />
<strong>Yukon</strong> records: 8, 10, 12 (ROME); 16, 19 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 23 June – 9 August.<br />
2. Glossosoma intermedium (Klapalek) (II) Holarctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to Montana, Quebec, Tennessee; central Europe to Finland and <strong>the</strong> nor<strong>the</strong>rn part<br />
<strong>of</strong> European Russia, eastward through Siberia to Chukotka and Kamchatka (Levanidova 1975, 1982).<br />
<strong>Yukon</strong> records: 5, 10, 12, 19 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 23 May – 9 August.<br />
3. Glossosoma verdona Ross (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong>, Alaska, south to California and Utah.<br />
<strong>Yukon</strong> records: 8, 17 (SMDV); 10 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 31 May – 28 June.<br />
Family Hydroptilidae<br />
Hydroptilidae are widely distributed throughout <strong>the</strong> world with genera characteristic <strong>of</strong><br />
all types <strong>of</strong> fresh waters from cold springs to lakes. Larvae feed principally on algae,
794 G.B. Wiggins and C.R. Parker<br />
especially filamentous forms. At least 5 <strong>of</strong> <strong>the</strong> 16 North American genera are represented in<br />
<strong>the</strong> <strong>Yukon</strong>, each by a single species.<br />
Genus Agraylea. This is an Holarctic genus with 4 North American species; larvae live in<br />
standing waters <strong>of</strong> lakes, and areas <strong>of</strong> reduced current in streams.<br />
4. Agraylea cognatella McLachlan (III) Palaearctic-East Beringian<br />
Distribution: <strong>Yukon</strong>; nor<strong>the</strong>rn Europe (BotojAneanu and Malicky 1978); Russian Far East (Levanidova<br />
1975; BotojAneanu and Levanidova 1988).<br />
<strong>Yukon</strong> records: 4, 5, 11 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 5 – 23 July.<br />
Taxonomic notes: North American specimens recorded as A. multipunctata Curtis (e.g. NW 1984, YT:<br />
16, 17) will have to be re-examined because that species appears to be restricted to Europe and Asia<br />
(Vineyard and Wiggins in prep.).<br />
Genus Hydroptila. Representatives <strong>of</strong> this genus occur through much <strong>of</strong> <strong>the</strong> world, including<br />
approximately 90 species in North America alone; larvae occur in lakes and in flowing<br />
waters.<br />
5. Hydroptila rono Ross (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to California, Quebec, and Pennsylvania.<br />
<strong>Yukon</strong> records: 17 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 11– 26 July.<br />
Genus Ochrotrichia. This genus is confined to <strong>the</strong> New World, where approximately 50<br />
species are known in North America. Larvae occur in running-water habitats. An unidentified<br />
species was recorded from <strong>the</strong> <strong>Yukon</strong>.<br />
6. Ochrotrichia sp.<br />
Distribution: Unknown.<br />
<strong>Yukon</strong> records: 16 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 26 July.<br />
Genus Oxyethira. Occurring widely throughout <strong>the</strong> world, this genus includes approximately<br />
40 North American species; larvae live in beds <strong>of</strong> aquatic plants in lakes and slow<br />
rivers.<br />
7. Oxyethira araya Ross (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Minnesota to Maine; continued absence <strong>of</strong> records between <strong>Yukon</strong> and Minnesota<br />
raises <strong>the</strong> possibility that <strong>the</strong>se populations are disjunct, perhaps reflecting <strong>the</strong>ir separation during<br />
glaciation.<br />
<strong>Yukon</strong> records: 4, 10, 12 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 20 June –12 July.<br />
Genus Stactobiella. This is a small Holarctic genus with 6 North American species; larvae<br />
live in small, rapid streams.<br />
8. Stactobiella delira (Ross) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to California, Maine, and Tennessee, most <strong>of</strong> North America, but not recorded<br />
from central or eastern Canada.<br />
<strong>Yukon</strong> records: 10 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 22 – 25 July.<br />
Family Rhyacophilidae<br />
Larvae are confined to cool, running waters, and for <strong>the</strong> most part are predacious on<br />
o<strong>the</strong>r insects. The family occurs in all continents <strong>of</strong> <strong>the</strong> nor<strong>the</strong>rn hemisphere except Africa.<br />
Only 2 genera are recognized, and both occur in North America.
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 795<br />
Genus Rhyacophila. This is a genus <strong>of</strong> at least 500 species, <strong>the</strong> largest in <strong>the</strong> Trichoptera.<br />
More than 100 species occur in North America, chiefly in <strong>the</strong> western mountains; 14 species<br />
have been recorded in <strong>the</strong> <strong>Yukon</strong>. Evidence shows that life cycles <strong>of</strong> Rhyacophila species<br />
at high latitudes tend to be longer than <strong>the</strong> single year in temperate latitudes (Irons 1988).<br />
9. Rhyacophila alberta Banks (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong>, Alaska to New Mexico.<br />
<strong>Yukon</strong> records: 17 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 7 August.<br />
10. Rhyacophila angelita Banks (I) Nearctic, disjunct<br />
Distribution: <strong>Yukon</strong> to California, New Mexico; Minnesota, Quebec, New Hampshire. Distributional<br />
records indicate that <strong>the</strong> eastern and western populations <strong>of</strong> this species may be disjunct.<br />
<strong>Yukon</strong> records: Nimmo (1971).<br />
<strong>Biological</strong> information: None available.<br />
11. Rhyacophila bifila Banks (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong> to California.<br />
<strong>Yukon</strong> records: 17 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 9 August.<br />
12. Rhyacophila brunnea Banks (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to nor<strong>the</strong>astern North America, south to New Mexico and California.<br />
<strong>Yukon</strong> records: 10, 16 (ROME); 12, 17, 19 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 30 June – 26 August.<br />
Taxonomic notes: The present concept for this species (Smith and Manuel 1984) subsumes<br />
R. acropedes Banks, R. ignorata Schmid, and R. acuminata Fields.<br />
13. Rhyacophila hyalinata Banks (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong>, Alaska to California and New Mexico.<br />
<strong>Yukon</strong> records: 12, 17, 19 (NW 1984); 16 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 23 June – 8 August.<br />
14. Rhyacophila mongolica Schmid, Arefina and Levanidova (III) Palaearctic-East Beringian<br />
Distribution: Previously known only from Mongolia and <strong>the</strong> Russian Far East, this species is recorded<br />
from North America for <strong>the</strong> first time.<br />
<strong>Yukon</strong> records: 4 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 16 July.<br />
Taxonomic notes: See Taxonomic Note 1.<br />
15. Rhyacophila narvae Navas (II) Holarctic, western montane<br />
Distribution: Subsumes <strong>the</strong> western Nearctic R. vepulsa Milne (Schmid 1970), extending its distribution<br />
from <strong>the</strong> Russian Far East to California.<br />
<strong>Yukon</strong> records: 17 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 25 June.<br />
16. Rhyacophila pellisa Ross (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong> to California and Utah.<br />
<strong>Yukon</strong> records: 10, 16 (ROME); 19 (SMDV); 12 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 24 March – 28 August.<br />
17. Rhyacophila tucula Ross (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong>, Alaska to Utah and Colorado.<br />
<strong>Yukon</strong> records: 10 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 10 –12 August.<br />
18. Rhyacophila vao Milne (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong>, Alaska to Utah and Montana.<br />
<strong>Yukon</strong> records: 17, 19 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 23 July – 8 August.
796 G.B. Wiggins and C.R. Parker<br />
19. Rhyacophila verrula Milne (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong>, Alaska to California and New Mexico.<br />
<strong>Yukon</strong> records: 11, 17 (SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 23 July – 3 August.<br />
20. Rhyacophila vobara Milne (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong>, Alaska to Oregon and Montana.<br />
<strong>Yukon</strong> records: 12, 17 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 23 June – 27 July.<br />
21. Rhyacophila vocala Milne (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong> to Montana and California.<br />
<strong>Yukon</strong> records: 10 (CNCI); 16 (ROME); 17 (SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 9 – 27 July.<br />
22. Rhyacophila v<strong>of</strong>ixa Milne (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong>, Alaska to Utah<br />
<strong>Yukon</strong> records: 8 (ROME); 12, 16, 17, 19 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 23 July –1 August.<br />
Suborder Annulipalpia<br />
These are <strong>the</strong> retreat-making or net-spinning caddisflies whose larvae are concealed in<br />
fixed tubular retreats or nets on rocks, logs, and plants. Most <strong>of</strong> <strong>the</strong> larvae live in running<br />
waters; some construct fine-meshed nets <strong>of</strong> silk to strain suspended food materials from <strong>the</strong><br />
current, o<strong>the</strong>rs graze fine organic particles or prey on insects. Pupation occurs in open<br />
perforate cells, with water circulating through <strong>the</strong> cell around <strong>the</strong> pupa.<br />
Family Hydropsychidae<br />
Hydropsychids are <strong>the</strong> dominant caddisflies <strong>of</strong> running waters over much <strong>of</strong> North<br />
America, both in species diversity and in biomass, but <strong>the</strong> family is markedly reduced in <strong>the</strong><br />
<strong>Yukon</strong>. The family is widespread throughout <strong>the</strong> world; 11 genera with approximately 150<br />
species are represented in North America, but only 4 genera are known in <strong>the</strong> <strong>Yukon</strong>. Larvae<br />
construct nets <strong>of</strong> silken meshes which filter suspended particles and insects from <strong>the</strong> current,<br />
<strong>the</strong> size <strong>of</strong> <strong>the</strong> mesh differing in most genera.<br />
Genus Arctopsyche. Species <strong>of</strong> Arctopsyche occur through much <strong>of</strong> <strong>the</strong> Holarctic and<br />
Oriental regions; 4 are known in North America. Larvae construct coarse-meshed filter nets<br />
and are primarily insectivorous.<br />
23. Arctopsyche grandis (Banks) (I) Nearctic, disjunct<br />
Distribution: <strong>Yukon</strong> to California, with a disjunct occurrence in Quebec.<br />
<strong>Yukon</strong> records: 10, 11, 19 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 23 July – 3 August.<br />
24. Arctopsyche ladogensis (Kolenati) (II) Holarctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Newfoundland and New Hampshire; a record from Utah (Baumann<br />
and Unzicker 1981) leaves a large gap to <strong>the</strong> <strong>Yukon</strong> in <strong>the</strong> recorded distribution <strong>of</strong> this species in<br />
western North America; nor<strong>the</strong>rn Europe and Asia through Siberia to Mongolia and Kamchatka<br />
(Lepneva 1964), but not recorded from Chukotka (Levanidova 1982).<br />
<strong>Yukon</strong> records: 4, 12, 17 (ROME); 10 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 26 June –14 August.<br />
Genus Cheumatopsyche. Some 40 species <strong>of</strong> this genus are known in North America, and<br />
<strong>the</strong> group is widespread through most o<strong>the</strong>r faunal regions.
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 797<br />
25. Cheumatopsyche campyla Ross (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to California, Newfoundland, Alabama.<br />
<strong>Yukon</strong> records: 19 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 15 – 21 July.<br />
26. Cheumatopsyche sp. female (not C. campyla)<br />
Distribution: Unknown.<br />
<strong>Yukon</strong> records: 10 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 23 July.<br />
Genus Hydropsyche. This is <strong>the</strong> dominant North American genus <strong>of</strong> <strong>the</strong> family with more<br />
than 70 Nearctic species, but only 5 are represented in <strong>the</strong> <strong>Yukon</strong>.<br />
27. Hydropsyche alhedra Ross (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to Massachusetts and North Carolina.<br />
<strong>Yukon</strong> records: 10 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 14 June.<br />
28. Hydropsyche alternans (Walker) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska and British Columbia to Newfoundland and Massachusetts.<br />
<strong>Yukon</strong> records: 4, 10 (NW 1984); 16, 17 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 1– 20 July.<br />
29. Hydropsyche amblis Ross (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong> to Oregon.<br />
<strong>Yukon</strong> records: 17, 19 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 23 May –17 June.<br />
30. Hydropsyche cockerelli Banks (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong> to California.<br />
<strong>Yukon</strong> records: 17 (SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 2 – 25 July.<br />
31. Hydropsyche oslari Banks (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong> to California.<br />
<strong>Yukon</strong> records: 10 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 25 July –15 August.<br />
Genus Parapsyche. Species <strong>of</strong> Parapsyche are widely distributed through <strong>the</strong> nor<strong>the</strong>rn<br />
hemisphere; 7 species are known in North America, mainly in <strong>the</strong> western mountains. Larvae<br />
live in small, cold streams and, like Arctopsyche, construct filter nets <strong>of</strong> coarse meshes.<br />
32. Parapsyche elsis Milne (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong>, Alaska to California.<br />
<strong>Yukon</strong> records: 10, 12 (NW 1984); 16, 19 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 25 July – 8 August.<br />
Family Philopotamidae<br />
Larvae live in amorphous tubular silken nets <strong>of</strong> very small mesh which strain out fine<br />
particulate organic matter carried by <strong>the</strong> current. Wormaldia is <strong>the</strong> only one <strong>of</strong> <strong>the</strong> 3 North<br />
American genera recorded from <strong>the</strong> <strong>Yukon</strong>, although Dolophilodes is known from Alaska<br />
(Nimmo 1986).<br />
Genus Wormaldia. This genus is widely distributed in both <strong>the</strong> nor<strong>the</strong>rn and sou<strong>the</strong>rn<br />
hemispheres. We have Wormaldia larvae from <strong>the</strong> <strong>Yukon</strong> (10; ROME) and from <strong>the</strong><br />
Northwest Territories.
798 G.B. Wiggins and C.R. Parker<br />
33. Wormaldia gabriella (Banks) (I) Nearctic, disjunct<br />
Distribution: <strong>Yukon</strong> to California; Quebec. Records from <strong>the</strong> Hudson Bay drainage <strong>of</strong> nor<strong>the</strong>rn<br />
Quebec (Roy and Harper 1979) indicate a major disjunction from <strong>the</strong> general western range <strong>of</strong> this<br />
species.<br />
<strong>Yukon</strong> records: A record for this species from <strong>the</strong> nor<strong>the</strong>rn <strong>Yukon</strong> (NW 1984) was attributed to Schmid<br />
(1982); <strong>Yukon</strong> was not included among 9 provinces and states listed by Schmid but a <strong>Yukon</strong> record<br />
does appear on a distribution map. This species was recorded by Winchester (1984) from <strong>the</strong> area <strong>of</strong><br />
Inuvik, Northwest Territories (68°31.2′N 135°54.2′W), close to <strong>the</strong> Arctic coast and adjacent to <strong>the</strong> <strong>Yukon</strong>.<br />
Family Polycentropodidae<br />
This is an important cosmopolitan family <strong>of</strong> retreat-making caddisflies with 6 genera<br />
and more than 70 species in North America; 2 genera occur in <strong>the</strong> <strong>Yukon</strong>. Most larvae are<br />
predacious and construct a variety <strong>of</strong> silken retreats and capture-nets.<br />
Genus Neureclipsis. This is a small Holarctic genus with 6 North American species. Larvae<br />
live in slow currents, concealed in voluminous sack-like silken nets that filter suspended<br />
particles.<br />
34. Neureclipsis bimaculata (Linnaeus) (II) Holarctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Newfoundland, Illinois; Europe through Siberia to Kamchatka.<br />
<strong>Yukon</strong> records: 12 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 26 July.<br />
Genus Polycentropus. This is <strong>the</strong> largest genus in <strong>the</strong> family, widely distributed through <strong>the</strong><br />
world, and with more than 40 species in North America. Larvae <strong>of</strong> different species live in<br />
lotic and lentic habitats and also in bog ponds and temporary pools, confirming a broad<br />
ecological tolerance for <strong>the</strong> genus.<br />
35. Polycentropus aureolus (Banks) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to Newfoundland and Ohio.<br />
<strong>Yukon</strong> records: 4, 16 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 25 – 27 July.<br />
36. Polycentropus flavus (Banks) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Newfoundland, Illinois and California.<br />
<strong>Yukon</strong> records: 4, 5, 10, 12, 17 (ROME, SMDV); 11, 16 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 19 June – 24 July.<br />
37. Polycentropus remotus Banks (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Newfoundland and Kentucky.<br />
<strong>Yukon</strong> records: 4, 10, 12 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 29 June – 23 July.<br />
38. Polycentropus smithae Denning (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, British Columbia, Quebec, New Hampshire.<br />
<strong>Yukon</strong> records: 4, 10, 12 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 24 June – 27 July.<br />
39. Polycentropus weedi Blickle and Morse (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to Newfoundland, New Hampshire.<br />
<strong>Yukon</strong> records: 8 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 29 June – 6 August.<br />
Suborder Integripalpia<br />
These are <strong>the</strong> case-making caddisflies whose larvae construct portable tubular cases <strong>of</strong><br />
plant or mineral materials fastened toge<strong>the</strong>r with silk. In contrast to <strong>the</strong> Annulipalpia with<br />
fixed retreats, <strong>the</strong>se larvae move with <strong>the</strong>ir cases in search <strong>of</strong> food. Larvae in most families
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 799<br />
are detritivores, although some are algal grazers or predators. For pupation, <strong>the</strong> larval case<br />
is sealed with perforate silk at each end, allowing water to circulate directly over <strong>the</strong> pupa.<br />
Family Apataniidae<br />
Five North American genera are assigned to this family and 2 <strong>of</strong> <strong>the</strong>m occur in <strong>the</strong><br />
<strong>Yukon</strong>.<br />
Genus Allomyia. Most <strong>of</strong> <strong>the</strong> species known in this genus are confined to cold mountain<br />
streams <strong>of</strong> western North America; a few are known also in <strong>the</strong> Far East <strong>of</strong> Russia. Larvae<br />
graze diatoms and fine organic particles from rocks.<br />
40. Allomyia sp.<br />
This record is based on one larval collection from <strong>the</strong> <strong>Yukon</strong> (10 ROME) which cannot be<br />
identified to species.<br />
Genus Apatania. Seventeen species <strong>of</strong> Apatania are known in North America, and many<br />
o<strong>the</strong>rs occur in <strong>the</strong> Palaearctic and Oriental regions. Larvae live in cool waters, usually<br />
streams but also lakes at higher latitudes, where <strong>the</strong>y scrape diatoms and o<strong>the</strong>r algae from<br />
rocks (e.g. Irons 1988).<br />
41. Apatania crymophila McLachlan (II) Holarctic, northwestern and central<br />
Distribution: <strong>Yukon</strong>, Alaska, Manitoba; nor<strong>the</strong>rn Europe and Asia.<br />
<strong>Yukon</strong> records: 4, 8, 10, 12, 16 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 26 May –12 August.<br />
42. Apatania stigmatella (Zetterstedt) (II) Holarctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Newfoundland; nor<strong>the</strong>rn Europe through Siberia to Chukotka, Kamchatka<br />
and <strong>the</strong> Amur basin (Levanidova 1982).<br />
<strong>Yukon</strong> records: 8, 10, 11, 12, 16, 17 (ROME, SMDV, CNCI).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 15 July – 28 August.<br />
43. Apatania zonella (Zetterstedt) (II) Holarctic, transcontinental<br />
Distribution: Ellesmere Is. (Northwest Territories), <strong>Yukon</strong>, Alaska, Quebec, Minnesota; Greenland;<br />
through nor<strong>the</strong>rn Europe and Asia to <strong>the</strong> Amur basin (I.M. Levanidova, pers. comm.).<br />
<strong>Yukon</strong> records: 4, 12, 16 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 15 June – 6 August.<br />
Family Brachycentridae<br />
This is a small family <strong>of</strong> <strong>the</strong> nor<strong>the</strong>rn hemisphere with 5 genera and about 30 species<br />
in North America; larvae live mainly in flowing water.<br />
Genus Brachycentrus. Larvae <strong>of</strong> Brachycentrus species live in larger and, on <strong>the</strong> whole,<br />
warmer rivers and streams than do those <strong>of</strong> o<strong>the</strong>r genera in <strong>the</strong> family. Larvae feed on<br />
suspended particles from <strong>the</strong> current and graze periphytic algae.<br />
44. Brachycentrus americanus (Banks) (II) Holarctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to California, Quebec, Massachusetts; Siberia, Mongolia, Japan.<br />
<strong>Yukon</strong> records: 4, 10, 12 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 22 June – 9 August.<br />
Genus Micrasema. Larvae are confined to small cold streams, where <strong>the</strong>y graze algae and<br />
moss from rocks.<br />
45. Micrasema gelidum McLachlan (II) Holarctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Wisconsin and Quebec; nor<strong>the</strong>rn Europe and Asia.<br />
<strong>Yukon</strong> records: 1 (SMDV); 4, 8, 10 (ROME); 12 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 30 June – 29 July.<br />
Taxonomic notes: M. kluane Ross and Morse is a junior synonym (BotojAneanu 1988).
800 G.B. Wiggins and C.R. Parker<br />
46. Micrasema bactro Ross (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong> to Utah.<br />
<strong>Yukon</strong> records: 10, 12 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 23 June – 23 July.<br />
Family Goeridae<br />
This is a small family widely distributed in <strong>the</strong> nor<strong>the</strong>rn hemisphere, but extended to<br />
tropical latitudes in Asia and even to sou<strong>the</strong>rn Africa. There are 4 North American genera<br />
comprising about a dozen species. Larvae <strong>of</strong> most species live in cool running water, and<br />
some in <strong>the</strong> water-saturated muck <strong>of</strong> spring seepage.<br />
Genus Goera. This is <strong>the</strong> largest genus in <strong>the</strong> family with 6 species in North America. Larvae<br />
that are known live in streams and graze diatoms and organic particles from rocks.<br />
47. Goera tungusensis Martynov (II) Holarctic<br />
Distribution: Northwest Territories, Quebec; Siberia.<br />
<strong>Yukon</strong> records: This species is included provisionally in <strong>the</strong> <strong>Yukon</strong> fauna on <strong>the</strong> basis <strong>of</strong> a single<br />
female collected in <strong>the</strong> Northwest Territories, very close to <strong>the</strong> <strong>Yukon</strong> border (Midway L., 67°14′N<br />
135°26′W, 8 July85, SMDV). This female is similar to, but not entirely identical with, <strong>the</strong> female <strong>of</strong><br />
G. tungusensis from Siberia.<br />
Taxonomic notes: See Taxonomic Note 2.<br />
Family Lepidostomatidae<br />
This family is widely distributed in <strong>the</strong> nor<strong>the</strong>rn hemisphere, with some 70 Nearctic<br />
species. Two genera are recognized in North America, Theliopsyche and Lepidostoma, with<br />
most species assigned to <strong>the</strong> latter; 4 subgenera have been proposed for species in Lepidostoma,<br />
3 <strong>of</strong> <strong>the</strong>m represented in <strong>the</strong> <strong>Yukon</strong> fauna. No Holarctic species are known. Larvae <strong>of</strong><br />
most species live in cool, running water, where <strong>the</strong>y are important detritivores; some<br />
Lepidostoma larvae live in <strong>the</strong> littoral zone <strong>of</strong> lakes.<br />
Genus Lepidostoma. This genus is widely represented in North America and Eurasia.<br />
48. Lepidostoma cascadense (Milne) (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong>, Alaska to California.<br />
<strong>Yukon</strong> records: 17 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 28 June – 24 July.<br />
49. Lepidostoma cinereum Banks (I) Nearctic, transcontinental,<br />
western montane<br />
Distribution: <strong>Yukon</strong>, Alaska to California and Utah, to Newfoundland and Maine.<br />
<strong>Yukon</strong> records: 10, 14 (ROME); 11 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 5 July –12 August.<br />
Taxonomic notes: Lepidostoma strophe Ross is a junior synonym.<br />
50. Lepidostoma pluviale (Milne) (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong> to California.<br />
<strong>Yukon</strong> records: 10, 16, 19 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 27 July – 9 August.<br />
Taxonomic notes: Lepidostoma veleda Denning is a junior synonym.<br />
51. Lepidostoma roafi (Milne) (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong>, Alaska to California.<br />
<strong>Yukon</strong> records: 17 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 28 June – 24 July.<br />
52. Lepidostoma stigma Banks (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong> to Utah.<br />
<strong>Yukon</strong> records: 17 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 24 July.
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 801<br />
53. Lepidostoma unicolor (Banks) (I) Nearctic, transcontinental,<br />
western montane<br />
Distribution: <strong>Yukon</strong> to Quebec, California, and Arizona.<br />
<strong>Yukon</strong> records: 10 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 4 – 9 August.<br />
Family Leptoceridae<br />
The Leptoceridae are a large family represented on all continents. In North America<br />
<strong>the</strong>re are approximately 100 species assigned to 8 genera, 5 <strong>of</strong> which are represented in <strong>the</strong><br />
<strong>Yukon</strong> fauna. Larvae live in lakes, marshes, and slow rivers, feeding on organic particles<br />
and aquatic plants, or on insects.<br />
Genus Ceraclea. Of some 36 North American species in this genus, 4 are known in <strong>the</strong><br />
<strong>Yukon</strong>. Larvae live in large rivers and <strong>the</strong> littoral zone <strong>of</strong> lakes where some species feed on<br />
colonies <strong>of</strong> freshwater sponges (Resh et al. 1976).<br />
54. Ceraclea annulicornis (Stephens) (II) Holarctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to California, Newfoundland and Kentucky; nor<strong>the</strong>rn Europe and Asiatic<br />
Russia to <strong>the</strong> Amur region and Japan (Lepneva 1966).<br />
<strong>Yukon</strong> records: 4 (SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 3 July.<br />
55. Ceraclea cancellata (Betten) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Newfoundland, south to Florida and Arizona.<br />
<strong>Yukon</strong> records: 10, 12 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 20 July – 9 August.<br />
56. Ceraclea nigronervosa (Retzius) (II) Holarctic<br />
Distribution: <strong>Yukon</strong>, Alaska, British Columbia and Wyoming; nor<strong>the</strong>rn Europe and Asia.<br />
<strong>Yukon</strong> records: 4, 10, 12, 17 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 5 June –11 July.<br />
Taxonomic notes: See Taxonomic Note 3.<br />
57. Ceraclea resurgens (Walker) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Oregon to Maine and Louisiana.<br />
<strong>Yukon</strong> records: 10, 12, 14, 15 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 17 – 28 July.<br />
Genus Mystacides. This is an Holarctic and Oriental genus with 3 widely distributed North<br />
American species; larvae live in standing or slowly moving water, where <strong>the</strong>y are mainly<br />
predacious.<br />
58. Mystacides alafimbriata Hill-Griffin (I) Nearctic, western<br />
Distribution: <strong>Yukon</strong>, Alaska to California and Mexico.<br />
<strong>Yukon</strong> records: 10, 17 (ROME); 12 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 2 July – 5 August.<br />
Taxonomic notes: See Taxonomic Note 4.<br />
59. Mystacides interjectus (Banks) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Quebec, Massachusetts and Ohio.<br />
<strong>Yukon</strong> records: 4, 10, 12, 17, 19 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 24 June – 5 August.<br />
Taxonomic notes: See Taxonomic Note 5.<br />
60. Mystacides sepulchralis (Walker) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to California, Newfoundland and Georgia.<br />
<strong>Yukon</strong> records: 4, 5, 8, 10, 12, 14, 16, 17, 19 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 26 June –19 August.<br />
Taxonomic notes: See Taxonomic Note 4.
802 G.B. Wiggins and C.R. Parker<br />
Genus Oecetis. This is a genus <strong>of</strong> worldwide distribution, with approximately 20 Nearctic<br />
species; larvae are predacious and live in both lentic and lotic waters.<br />
61. Oecetis immobilis (Hagen) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Montana to Maine, Ohio.<br />
<strong>Yukon</strong> records: 4 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 14 – 23 July.<br />
62. Oecetis inconspicua (Walker) (I) Nearctic, transcontinental;<br />
Neotropical<br />
Distribution: <strong>Yukon</strong>, Alaska to California, Newfoundland, Florida, Texas; Mexico, Bahamas, Cuba,<br />
Venezuela.<br />
<strong>Yukon</strong> records: 4, 10, 12, 14, 15, 17, 19 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 17 July – 2 August.<br />
63. Oecetis ochracea (Curtis) (II) Holarctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to California, Tennessee; central and nor<strong>the</strong>rn Europe (Malicky 1988),<br />
through Siberia to Chukotka and Kamchatka, and Mongolia (Levanidova 1982).<br />
<strong>Yukon</strong> records: 4, 10, 12, 16 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 26 June – 28 July.<br />
Genus Triaenodes. This is a widely distributed genus with about 25 Nearctic species; larvae<br />
occur in lentic and lotic habitats, where <strong>the</strong>y feed on plant materials.<br />
64. Triaenodes baris (Ross) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to Wisconsin, Maine.<br />
<strong>Yukon</strong> records: 10 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 1 July.<br />
65. Triaenodes tardus Milne (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Arizona, Maine, Tennessee.<br />
<strong>Yukon</strong> records: 10 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 20 – 23 July.<br />
Genus Ylodes. Some 15 species are now assigned to this Holarctic genus, most <strong>of</strong> <strong>the</strong>m from<br />
nor<strong>the</strong>rn Asia. All 4 species known in North America occur in <strong>the</strong> <strong>Yukon</strong>. Larvae live in<br />
lentic waters.<br />
66. Ylodes frontalis (Banks) (I) Nearctic, western and central<br />
Distribution: <strong>Yukon</strong>, Alaska to California, Saskatchewan, South Dakota.<br />
<strong>Yukon</strong> records: 10, 12 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 26 June – 8 August.<br />
67. Ylodes kaszabi (Schmid) (III) Palaearctic-East Beringian<br />
Distribution: <strong>Yukon</strong>, Alaska, Northwest Territories; Mongolia (Schmid 1965b).<br />
<strong>Yukon</strong> records: 12 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 27 July.<br />
68. Ylodes reuteri (McLachlan) (II) Holarctic<br />
Distribution: <strong>Yukon</strong> to Colorado and Manitoba; through much <strong>of</strong> Europe (BotojAneanu and Malicky<br />
1978), Caucasus (Martynov 1909), Egypt and Saudi Arabia (L. BotojAneanu, pers. comm.), Siberia<br />
(I.M. Levanidova, pers. comm.) to Mongolia (Mey and Dulmaa 1985).<br />
<strong>Yukon</strong> records: 17 (SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 27 July.<br />
Taxonomic notes: Triaenodes griseus Banks is a junior synonym (Manuel and Nimmo 1984).<br />
69. Ylodes schmidi Manuel and Nimmo (IV) East Beringian<br />
Distribution: Known only from <strong>the</strong> <strong>Yukon</strong>.<br />
<strong>Yukon</strong> records: 17 (CNCI) (Manuel and Nimmo 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 24 July.
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 803<br />
Family Limnephilidae<br />
This is <strong>the</strong> largest family <strong>of</strong> Trichoptera in North America with some 230 species in 39<br />
genera. Limnephilidae are <strong>the</strong> dominant and most diverse group in nor<strong>the</strong>rn latitudes, and<br />
constitute more than half <strong>of</strong> <strong>the</strong> species <strong>of</strong> Trichoptera in <strong>the</strong> <strong>Yukon</strong>. Larvae occur in all<br />
types <strong>of</strong> aquatic habitats, and are largely detritivorous.<br />
Genus Anabolia. This is an Holarctic genus with 5 North American species widely<br />
distributed over <strong>the</strong> nor<strong>the</strong>rn part <strong>of</strong> <strong>the</strong> continent. Larvae are detritivores in slow streams,<br />
marshes, and temporary pools.<br />
70. Anabolia bimaculata (Walker) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to Utah, Newfoundland, New Hampshire.<br />
<strong>Yukon</strong> records: 6, 10, 11, 12, 15, 16, 17 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 12 July – 29 August.<br />
Genus Arctopora. This is a small, Holarctic genus <strong>of</strong> 3 species (Fig. 19). Larvae <strong>of</strong> at least<br />
A. pulchella live in temporary pools, and probably marshy sites generally.<br />
71. Arctopora pulchella (Banks) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Newfoundland and New Hampshire.<br />
<strong>Yukon</strong> records: 4, 8, 11, 12, 14, 16, 17 (ROME, SMDV); 10 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 23 June –12 August.<br />
72. Arctopora trimaculata (Zetterstedt) (III) Palaearctic-East Beringian<br />
Distribution: <strong>Yukon</strong>, Alaska; nor<strong>the</strong>rn Europe (BotojAneanu and Malicky 1978) and Asia from<br />
Scandinavia through Siberia to <strong>the</strong> Amur region and Sakhalin (Schmid 1952).<br />
<strong>Yukon</strong> records: 4, 5 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 8 – 23 July.<br />
Genus Asynarchus. Species <strong>of</strong> Asynarchus occur over <strong>the</strong> nor<strong>the</strong>rn half <strong>of</strong> North America,<br />
and several <strong>of</strong> <strong>the</strong>m also occur in Eurasia. Larvae live in streams, ponds, and temporary<br />
pools, and are probably detritivores.<br />
73. Asynarchus aldinus (Ross) (I) Nearctic, western<br />
Distribution: <strong>Yukon</strong> to Utah.<br />
<strong>Yukon</strong> records: 10, 16, 17 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 23 July – 21 August.<br />
74. Asynarchus iteratus McLachlan (II) Holarctic, northwestern and central<br />
Distribution: <strong>Yukon</strong>, Alaska to Manitoba (Churchill: Lehmkuhl and Kerst 1979); nor<strong>the</strong>rn Asia to<br />
Kamchatka (Lepneva 1966).<br />
<strong>Yukon</strong> records: 4 (CNCI); 5, 10 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 25 July – 6 August.<br />
75. Asynarchus lapponicus (Zetterstedt) (II) Holarctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to Newfoundland and Maine; nor<strong>the</strong>rn and central Europe (Malicky 1988, fig. 11)<br />
through Siberia to Chukotka (Levanidova 1982).<br />
<strong>Yukon</strong> records: 1 (SMDV); 2 (CNCI); 8 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 21 July –1 August.<br />
76. Asynarchus montanus Banks (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Newfoundland and Utah.<br />
<strong>Yukon</strong> records: 4, 6, 8, 10, 11, 12, 14, 16, 17, 19 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 30 June – 29 August.<br />
77. Asynarchus mutatus (Hagen) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to Newfoundland.<br />
<strong>Yukon</strong> records: 16 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 29 July – 6 August.
804 G.B. Wiggins and C.R. Parker<br />
Genus Chyranda. The single species <strong>of</strong> Chyranda is widely distributed in small, cold<br />
streams in nor<strong>the</strong>rn and western montane regions <strong>of</strong> North America. Larvae are detritivorous<br />
(e.g. Irons 1988).<br />
78. Chyranda centralis (Banks) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to California and Quebec.<br />
<strong>Yukon</strong> records: 8, 11, 14, 16, 17 (ROME, SMDV); 12 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 27 July –12 August.<br />
Genus Clistoronia. Clistoronia are confined to western North America, where 4 species are<br />
known. Larvae live in small lakes and ponds, where <strong>the</strong>y are detritivorous.<br />
79. Clistoronia magnifica (Banks) (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong>, Alaska to California.<br />
<strong>Yukon</strong> records: 11, 12, 16, 17 (CNCI, ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 17 June –17 August.<br />
Genus Dicosmoecus. Four species <strong>of</strong> Dicosmoecus inhabit running waters in western<br />
montane North America, and 2 o<strong>the</strong>rs occur in eastern Asia (Wiggins and Richardson 1982).<br />
The 2 <strong>Yukon</strong> species feed mainly on vascular plant materials and insects.<br />
80. Dicosmoecus atripes (Hagen) (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong>, Alaska to California, New Mexico.<br />
<strong>Yukon</strong> records: 8, 10, 12, 14, 16 (ROME); 17, 19 (CNCI).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 23 June –18 August. Dippers, or<br />
water ouzels, representing a small family <strong>of</strong> passeriform birds (Cinclidae) frequenting streams, feed<br />
on larvae <strong>of</strong> this species in Alaska (Ellis 1978b).<br />
81. Dicosmoecus obscuripennis Banks (III) Palaearctic-East Beringian<br />
Distribution: <strong>Yukon</strong>, Alaska; Russian Far East (Nagayasu and Ito 1993).<br />
<strong>Yukon</strong> records: 4, 8, 10, 12 (ROME); 17 (CNCI).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 26 July – 7 August.<br />
Genus Ecclisomyia. This is a small Holarctic genus confined to montane areas <strong>of</strong> western<br />
North America and eastern Asia; larvae live in small streams or rocky lake shores, and feed<br />
on diatoms and plant detritus (Irons 1988).<br />
82. Ecclisomyia conspersa Banks (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong>, Alaska to California.<br />
<strong>Yukon</strong> records: 8, 10, 12, 14, 16, 17 (CNCI, ROME, SMDV); 19 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 26 May –17 August.<br />
Genus Glyphopsyche. This is a Nearctic genus <strong>of</strong> 2 species; larvae <strong>of</strong> G. irrorata live in<br />
accumulations <strong>of</strong> plant materials in slowly flowing waters and in marshes, where <strong>the</strong>y are<br />
probably detritivorous.<br />
83. Glyphopsyche irrorata (Fabricius) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to California and Newfoundland.<br />
<strong>Yukon</strong> records: 12 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 26 May.<br />
Genus Grammotaulius. This is a widespread Holarctic genus <strong>of</strong> nor<strong>the</strong>rn and montane<br />
ponds and slow streams. Larvae are probably detritivorous.<br />
84. Grammotaulius alascensis Schmid (IV) East Beringian<br />
Distribution: <strong>Yukon</strong>, Alaska, Northwest Territories.<br />
<strong>Yukon</strong> records: 2 (NW 1984); 4, 8, 10 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 23 – 31 July.<br />
Taxonomic notes: Grammotaulius subborealis Schmid (1964) is a new synonym. See Taxonomic Note 6.
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 805<br />
85. Grammotaulius interrogationis (Zetterstedt) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Newfoundland; Greenland.<br />
<strong>Yukon</strong> records: 8, 10, 11, 12, 14, 16, 17 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 24 June – 25 August.<br />
86. Grammotaulius signatipennis McLachlan (III) Palaearctic-East Beringian<br />
Distribution: <strong>Yukon</strong>; nor<strong>the</strong>rn Eurasia from Sweden and Poland to Kamchatka (Schmid 1950a;<br />
Levanidova 1982).<br />
<strong>Yukon</strong> records: 11 (CNCI).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 4 September.<br />
Taxonomic notes: See Taxonomic Note 6.<br />
Genus Grensia. A single circumpolar species is assigned to this genus; larvae live mainly<br />
in tundra lakes and ponds.<br />
87. Grensia praeterita (Walker) (III) Holarctic, far nor<strong>the</strong>rn<br />
Distribution: <strong>Yukon</strong>, Alaska, Northwest Territories; Greenland; far nor<strong>the</strong>rn Eurasia.<br />
<strong>Yukon</strong> records: 8 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 18 June.<br />
Genus Hesperophylax. Seven species are recognized in <strong>the</strong> Nearctic genus Hesperophylax,<br />
and <strong>the</strong>y inhabit an unusually wide range <strong>of</strong> habitats from springs to rivers and lakes (Parker<br />
and Wiggins 1985). Larvae feed mainly on detritus.<br />
88. Hesperophylax designatus (Walker) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to California, Newfoundland, and Illinois.<br />
<strong>Yukon</strong> records: 10, 11, 12, 17 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 9 June – 6 August.<br />
Genus Lenarchus. This is a nor<strong>the</strong>rn Holarctic genus in which 5 <strong>of</strong> <strong>the</strong> 9 Nearctic species<br />
have been recorded from <strong>the</strong> <strong>Yukon</strong>. Larvae live in lentic sites—small lakes, marshes, and<br />
temporary pools, especially at higher elevations and latitudes, and feed on organic debris.<br />
89. Lenarchus crassus (Banks) (I) Nearctic, transcontinental, nor<strong>the</strong>rn<br />
Distribution: <strong>Yukon</strong> to Newfoundland.<br />
<strong>Yukon</strong> records: 4, 8, 14 (ROME); 12 (CNCI).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 8 July –12 August.<br />
90. Lenarchus expansus Martynov (IV) Palaearctic-East Beringian<br />
Distribution: <strong>Yukon</strong>, Alaska; Siberia and <strong>the</strong> Russian Far East (I.M. Levanidova, pers. comm).<br />
<strong>Yukon</strong> records: 1, 8, 10 (CNCI); 4 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 29 June – 27 July.<br />
91. Lenarchus fautini (Denning) (I) Nearctic, western<br />
Distribution: <strong>Yukon</strong> to Utah.<br />
<strong>Yukon</strong> records: 8, 12 (ROME); 14, 15 (SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 6 – 31 July.<br />
92. Lenarchus keratus Ross (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to Michigan, Quebec.<br />
<strong>Yukon</strong> records: 10 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 24 July.<br />
93. Lenarchus vastus (Hagen) (I) Nearctic, western<br />
Distribution: <strong>Yukon</strong> to California.<br />
<strong>Yukon</strong> records: 8, 10, 11, 12, 14, 16, 17 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 11 June – 2 September.<br />
Genus Limnephilus. This is <strong>the</strong> dominant trichopteran genus <strong>of</strong> nor<strong>the</strong>rn latitudes <strong>of</strong> <strong>the</strong><br />
globe, and includes approximately 85 Nearctic species (Ruiter 1995); about 27 species are
806 G.B. Wiggins and C.R. Parker<br />
known in <strong>the</strong> <strong>Yukon</strong>, constituting 20 per cent <strong>of</strong> <strong>the</strong> Trichoptera. Larvae range widely in<br />
habitat, although most live in standing waters where <strong>the</strong>y are major detritivores.<br />
94. Limnephilus argenteus Banks (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Newfoundland.<br />
<strong>Yukon</strong> records: 4, 6, 8, 10, 17 (ROME); 11, 16 (SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 3 June –18 July.<br />
95. Limnephilus canadensis Banks (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to Maine, Oregon.<br />
<strong>Yukon</strong> records: 10 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 6 August.<br />
96. Limnephilus diphyes McLachlan (III) Palaearctic-East Beringian<br />
Distribution: Previously known from Scandinavia through northwestern Siberia (BotojAneanu and<br />
Malicky 1978) to <strong>the</strong> Amur district and Kamchatka (I.M. Levanidova, pers. comm), this species is<br />
recorded from North America for <strong>the</strong> first time.<br />
<strong>Yukon</strong> records: 5, 8, 10 (ROME); 11, 17 (SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 10 June – 26 July.<br />
Taxonomic notes: See Taxonomic Note 7.<br />
97. Limnephilus dispar McLachlan (II) Holarctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Colorado, Newfoundland; nor<strong>the</strong>rn and central Europe (Malicky 1988,<br />
fig. 15), Siberia (Martynov 1914, 1924a), <strong>the</strong> Amur basin and Kamchatka (Levanidova 1982).<br />
<strong>Yukon</strong> records: 4, 8, 11, 16, 17 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 31 May –18 July.<br />
Taxonomic notes: L. minusculus (Banks) is a junior synonym (Malicky 1979).<br />
98. Limnephilus externus Hagen (II) Holarctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to California, Newfoundland; nor<strong>the</strong>rn Europe, Siberia, China (Fischer<br />
1968).<br />
<strong>Yukon</strong> records: 4, 8, 10, 11, 12, 14 (ROME); 16, 17 (SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 21 July – 2 September.<br />
99. Limnephilus extractus Walker (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to Utah, Quebec.<br />
<strong>Yukon</strong> records: 4, 10, 16, 17 (ROME); 12 (SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 14 June – 29 July.<br />
100. Limnephilus femoralis Kirby (II) Holarctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to Washington and Maine; Greenland (Mosely 1929); nor<strong>the</strong>rn Europe, Kamchatka<br />
(Ulmer 1927) but not Siberia.<br />
<strong>Yukon</strong> records: 4 (SMDV); 17 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 5 July – 6 August.<br />
101. Limnephilus fenestratus (Zetterstedt) (III) Palaearctic-East Beringian<br />
Distribution: <strong>Yukon</strong>, Alaska, Northwest Territories; nor<strong>the</strong>rn Europe, Iceland (Gislason 1981),<br />
Mongolia (Mey and Dulmaa 1985), Bering Island (I.M. Levanidova, pers. comm.) (Fig. 21).<br />
<strong>Yukon</strong> records: 4, 8 (ROME); 12 (CNCI, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 21 July – 2 September.<br />
102. Limnephilus fumosus Banks (IV) East Beringian<br />
Distribution: <strong>Yukon</strong>, Alaska, Northwest Territories.<br />
<strong>Yukon</strong> records: 11 (SMDV); 12 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 12 June – 22 July.<br />
Taxonomic notes: This species has been confused with L. santanus Ross; Limnephilus isobela Nimmo<br />
(1991) is a new synonym. See Taxonomic Note 8.<br />
103. Limnephilus hageni Banks (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, British Columbia to Quebec.<br />
<strong>Yukon</strong> records: 4, 10, 11, 12, 14, 15, 19 (ROME); 16 (NW 1984).
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 807<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 18 July –12 August.<br />
104. Limnephilus hyalinus Hagen (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Maine, Colorado.<br />
<strong>Yukon</strong> records: 10 (ROME, CNCI); 16 (NW 1984); 17 (SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 23 July – 28 August.<br />
105. Limnephilus infernalis (Banks) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Maine.<br />
<strong>Yukon</strong> records: 4, 10, 11, 17 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 28 July –17 August.<br />
106. Limnephilus janus Ross (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to Colorado, Maine.<br />
<strong>Yukon</strong> records: 10 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 21– 27 July.<br />
107. Limnephilus kennicotti Banks (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Oregon to Newfoundland; Greenland (Fig. 21).<br />
<strong>Yukon</strong> records: 2 (ROME); 10 (CNCI); 12 (NW 1984); 16, 19 (SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 28 July – 24 August.<br />
Taxonomic notes: See under L. fenestratus (category III).<br />
108. Limnephilus nigriceps (Zetterstedt) (II) Holarctic, nor<strong>the</strong>rn<br />
Distribution: <strong>Yukon</strong>, Alaska to Manitoba; Europe and Siberia to Kamchatka (Lepneva 1966).<br />
<strong>Yukon</strong> records: 8 (SMDV); 10 (CNCI); 12, 16 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 27 July – 21 August.<br />
109. Limnephilus pallens Banks (IV) Nearctic<br />
Distribution: <strong>Yukon</strong>, Alaska, Northwest Territories, Michigan (Ruiter 1995).<br />
<strong>Yukon</strong> records: 1 (SMDV); 2 (CNCI); 16 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 14 July –18 August.<br />
110. Limnephilus partitus Walker (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Newfoundland.<br />
<strong>Yukon</strong> records: 4 (SMDV); 10, 12, 16 (ROME); 17 (CNCI).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 14 July – 9 August.<br />
111. Limnephilus parvulus (Banks) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to Newfoundland, Maine.<br />
<strong>Yukon</strong> records: 11 (SMDV); 17 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 31 May – 25 June.<br />
112. Limnephilus perpusillus Walker (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to Newfoundland, south to Colorado.<br />
<strong>Yukon</strong> records: 10 (SMDV); 12 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 16 – 27 July.<br />
113. Limnephilus picturatus McLachlan (II) Holarctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Colorado, east to Quebec; Greenland; nor<strong>the</strong>rn Europe, Iceland<br />
(Gislason 1981) through Siberia to Kamchatka, <strong>the</strong> Amur region, Sakhalin, and Chukotka (Levanidova<br />
1982).<br />
<strong>Yukon</strong> records: 1, 16 (NW 1984); 2 (CNCI); 5, 6, 12 (ROME); 4, 8, 14, 19 (ROME, SMDV); 17<br />
(SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 9 July – 20 August.<br />
114. Limnephilus rhombicus (Linnaeus) (II) Holarctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Colorado, east to Newfoundland, West Virginia, Ohio; Greenland;<br />
Europe (Malicky 1988, fig. 18), through Turkestan (Schmid 1955), Siberia, Mongolia to Kamchatka<br />
(Lepneva 1966; Levanidova 1982).<br />
<strong>Yukon</strong> records: 4, 10, 12 (ROME); 17 (INHS).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 16 June – 30 July.
808 G.B. Wiggins and C.R. Parker<br />
115. Limnephilus sansoni Banks (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong>, Alaska to Colorado.<br />
<strong>Yukon</strong> records: 6, 8, 10, 11, 12 (ROME); 11, 16 (ROME, SMDV); 17 (CNCI).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 14 July – 29 August.<br />
116. Limnephilus secludens Banks (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to California, Quebec.<br />
<strong>Yukon</strong> records: 10, 12, 17 (CNCI, ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 21 July – 2 September.<br />
117. Limnephilus sericeus (Say) (II) Holarctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Colorado, Newfoundland to Ohio; Europe (Malicky 1988, fig. 16),<br />
Siberia to Kamchatka, Japan (Lepneva 1966).<br />
<strong>Yukon</strong> records: 10, 14, 16 (ROME); 12, 17 (SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 2 – 31 August.<br />
118. Limnephilus stigma Curtis (III) Palaearctic-East Beringian<br />
Distribution: <strong>Yukon</strong>, Alaska (Flint 1964); Europe (BotojAneanu and Malicky 1978), through Siberia<br />
to Kamchatka and <strong>the</strong> eastern extremity <strong>of</strong> Russia (Lepneva 1966; Levanidova 1975).<br />
<strong>Yukon</strong> records: 4, 12, 17 (SMDV); 8, 10, 11 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 15 July – 31 August.<br />
Taxonomic notes: L. indivisus (Figs. 26, 27), <strong>the</strong> similar sister species, has not been confirmed for <strong>the</strong><br />
<strong>Yukon</strong>; see under L. stigma (III).<br />
119. Limnephilus sublunatus Provancher (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to Newfoundland, Colorado.<br />
<strong>Yukon</strong> records: 10, 11 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 3 –10 August.<br />
120. Limnephilus tarsalis (Banks) (I) Nearctic, western<br />
Distribution: <strong>Yukon</strong>, Alaska, Northwest Territories, Alberta, British Columbia, Wyoming, Colorado.<br />
<strong>Yukon</strong> records: 10 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 27 July.<br />
Taxonomic notes: L. alvatus Denning is a junior synonym (Ruiter 1995).<br />
Genus Nemotaulius. This is a small Holarctic genus with a single species in North America<br />
distributed across <strong>the</strong> nor<strong>the</strong>rn part <strong>of</strong> <strong>the</strong> continent. Larvae live in lentic waters.<br />
121. Nemotaulius hostilis (Hagen) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Newfoundland, West Virginia, Colorado.<br />
<strong>Yukon</strong> records: 4, 10, 12 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 27 June – 27 July.<br />
Genus Onocosmoecus. Onocosmoecus is a small Holarctic genus <strong>of</strong> 2 species (Wiggins and<br />
Richardson 1987); larvae live in cool waters <strong>of</strong> slow streams and lake margins where <strong>the</strong>y<br />
are detritivores.<br />
122. Onocosmoecus unicolor (Banks) (II) Holarctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to California, Colorado, Michigan, Newfoundland; eastern Siberia and<br />
Kamchatka to <strong>the</strong> Kurile Islands.<br />
<strong>Yukon</strong> records: 6, 8, 10, 11, 12, 14, 16, 17, 19 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 24 July – 20 August.<br />
Genus Philarctus. Several species <strong>of</strong> Philarctus occur in Asia but only one in North<br />
America, from Manitoba westward; larvae live in slow streams and small ponds where <strong>the</strong>y<br />
are probably detritivores.<br />
123. Philarctus quaeris (Milne) (I) Nearctic, western and north-central<br />
Distribution: <strong>Yukon</strong>, British Columbia to Manitoba, Colorado.<br />
<strong>Yukon</strong> records: 17 (ROME, SMDV).
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 809<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 26 June – 3 August.<br />
Genus Psychoglypha. This is an important Nearctic genus wholly confined to western<br />
montane areas except for one nor<strong>the</strong>rn and transcontinental species, P. subborealis. Larvae<br />
are confined to cool running waters and are detritivorous.<br />
124. Psychoglypha alascensis (Banks) (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong>, Alaska to California.<br />
<strong>Yukon</strong> records: 10 (SMDV); 14 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 15 June – 22 August.<br />
125. Psychoglypha subborealis (Banks) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to California, New Hampshire, Newfoundland.<br />
<strong>Yukon</strong> records: 12, 17 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 26 April – 24 July.<br />
Genus Sphagnophylax. Sphagnophylax is a relict genus <strong>of</strong> arctic tundra in <strong>the</strong> <strong>Yukon</strong> and<br />
adjacent Northwest Territories (Wiggins and Winchester 1984). Larvae <strong>of</strong> <strong>the</strong> single species<br />
live in transient tundra pools and feed on plant materials.<br />
126. Sphagnophylax meiops Wiggins and Winchester (IV) East Beringian<br />
Distribution: <strong>Yukon</strong>, Northwest Territories.<br />
<strong>Yukon</strong> records: 4 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 23 July.<br />
Family Molannidae<br />
The Molannidae are a small family mainly confined to <strong>the</strong> nor<strong>the</strong>rn hemisphere; larvae<br />
are omnivorous, living in lentic or slowly flowing waters.<br />
Genus Molanna. This is an Holarctic and Oriental genus with 6 North American species.<br />
Only one, M. flavicornis, is transcontinental; <strong>the</strong> o<strong>the</strong>rs are eastern.<br />
127. Molanna flavicornis Banks (II) Holarctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to Nova Scotia, New York, disjunct populations in Colorado; Eurasia.<br />
<strong>Yukon</strong> records: 4, 10 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 2 June – 26 July.<br />
Taxonomic notes: Designation <strong>of</strong> this species as Holarctic hinges on <strong>the</strong> proposal by Fuller (1987) that<br />
it is conspecific with <strong>the</strong> Eurasian M. albicans Zett.<br />
Genus Molannodes. Molannodes is an Holarctic genus with one species widely distributed<br />
across nor<strong>the</strong>rn Europe and Asia, and highly localized in nor<strong>the</strong>rn North America; a second<br />
species occurs in Japan.<br />
128. Molannodes tinctus Zetterstedt (II) Holarctic<br />
Distribution: <strong>Yukon</strong>, Alaska, Northwest Territories, Saskatchewan, Ontario; nor<strong>the</strong>rn Europe, Asia.<br />
<strong>Yukon</strong> records: 4, 12 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 23 June – 25 July.<br />
Family Phryganeidae<br />
The Phryganeidae are an Holarctic and Oriental family <strong>of</strong> 74 species, well represented<br />
throughout nor<strong>the</strong>rn North America (Wiggins in press). Larvae live in lakes and marshes or<br />
in slow rivers and streams; <strong>the</strong>y are omnivorous to largely predacious in feeding. The largest<br />
caddisflies belong to this family.<br />
Genus Agrypnia. This is an Holarctic genus with 10 North American species, mainly<br />
transcontinental and nor<strong>the</strong>rn; all but one <strong>of</strong> <strong>the</strong> 10 are known in <strong>the</strong> <strong>Yukon</strong>. Larvae occur<br />
in marshes, lakes and slow rivers, and are mainly predacious.
810 G.B. Wiggins and C.R. Parker<br />
129. Agrypnia colorata Hagen (II) Holarctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Utah to Newfoundland; Siberia to Mongolia, Finland.<br />
<strong>Yukon</strong> records: 4, 6, 12 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 25 June – 27 July.<br />
130. Agrypnia deflata (Milne) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Colorado, Newfoundland.<br />
<strong>Yukon</strong> records: 4, 12 (ROME, SMDV); 6, 10, 16 (ROME); 17 (CNCI).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 29 June – 8 August.<br />
131. Agrypnia glacialis Hagen (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to California, North Dakota, Newfoundland; Greenland.<br />
<strong>Yukon</strong> records: 4, 11, 12, 17 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 6 June –15 July.<br />
132. Agrypnia improba (Hagen) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Oregon to Newfoundland, North Carolina.<br />
<strong>Yukon</strong> records: 10, 12, 15, 17 (ROME, SMDV); 19 (CNCI).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 16 June – 30 July.<br />
133. Agrypnia macdunnoughi (Milne) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to Newfoundland.<br />
<strong>Yukon</strong> records: 10, 17 (ROME, SMDV); 19 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 17 June – 20 July.<br />
134. Agrypnia obsoleta (Hagen) (III) Palaearctic-East Beringian<br />
Distribution: <strong>Yukon</strong> and British Columbia; Europe and Asia.<br />
<strong>Yukon</strong> records: 2 (SMDV); 4 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 25 June – 23 July.<br />
135. Agrypnia pagetana Curtis (II) Holarctic<br />
Distribution: <strong>Yukon</strong>, Alaska, British Columbia through <strong>the</strong> Northwest Territories to Manitoba;<br />
nor<strong>the</strong>rn and central Europe to nor<strong>the</strong>rn Italy, Bulgaria, Caucasus (BotojAneanu and Malicky 1978).<br />
<strong>Yukon</strong> records: 2, 4, 10, 11, 12, 19 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 6 June – 23 July.<br />
136. Agrypnia sahlbergi (McLachlan) (III) Palaearctic-East Beringian<br />
Distribution: <strong>Yukon</strong>, Alaska, and British Columbia; nor<strong>the</strong>rn Scandinavia, Asia.<br />
<strong>Yukon</strong> records: 5, 8, 12 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 5 June – 30 July.<br />
137. Agrypnia straminea Hagen (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to Newfoundland and Colorado, Illinois, North Dakota.<br />
<strong>Yukon</strong> records: 8, 10, 11, 12, 15, 16, 17, 19 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 10 June – 5 August.<br />
Genus Banksiola. This is a Nearctic genus <strong>of</strong> 4 eastern species, and one common and<br />
widespread transcontinental species (Wiggins 1956). Larvae live in lentic waters.<br />
138. Banksiola crotchi Banks (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to California, Newfoundland, Ohio.<br />
<strong>Yukon</strong> records: 4, 10 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 27 June – 27 July.<br />
Genus Oligotricha. This is a small Eurasian genus with a single species extending into<br />
northwestern North America (Wiggins and Kuwayama 1971). Larvae live in standing waters.<br />
139. Oligotricha lapponica (Hagen) (III) Palaearctic-East Beringian<br />
Distribution: <strong>Yukon</strong>, Alaska; nor<strong>the</strong>rn Europe, Asia.<br />
<strong>Yukon</strong> records: 4, 12 (ROME, SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 6 – 25 July.
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 811<br />
Genus Phryganea. This is an Holarctic genus with 2 North American species living in lentic<br />
waters. One <strong>of</strong> <strong>the</strong>m, P. cinerea, is common throughout nor<strong>the</strong>rn and montane areas.<br />
140. Phryganea cinerea Walker (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong>, Alaska to Newfoundland and California, North Dakota, Ohio.<br />
<strong>Yukon</strong> records: 4, 10, 12, 17 (ROME); 17 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 15 June – 27 July.<br />
Genus Ptilostomis. This Nearctic genus <strong>of</strong> 4 species includes 2 with transcontinental<br />
distributions; in addition to P. semifasciata, P. ocellifera (Walker) has been recorded from<br />
Alaska and British Columbia (Liard R. Hot Springs Prov. Pk., 59°26′N 126°04′W, 8.vi.80,<br />
ROME), and probably occurs in <strong>the</strong> <strong>Yukon</strong>. Larvae are predacious for <strong>the</strong> most part, and<br />
live in all types <strong>of</strong> aquatic habitats, including temporary pools.<br />
141. Ptilostomis semifasciata (Say) (I) Nearctic, transcontinental<br />
Distribution: <strong>Yukon</strong> to Newfoundland, Virginia.<br />
<strong>Yukon</strong> records: 6, 12, 17 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 22 June –13 July.<br />
Family Uenoidae<br />
This is a family <strong>of</strong> <strong>the</strong> nor<strong>the</strong>rn hemisphere with 5 North American genera, all inhabiting<br />
rapid streams (Vineyard and Wiggins 1988). Two genera are recorded from <strong>the</strong> <strong>Yukon</strong>, and<br />
one o<strong>the</strong>r, Neophylax, is represented in Alaska. Larvae graze algae and fine organic particles<br />
on rocks.<br />
Genus Neothremma. This genus is restricted to western montane North America where 7<br />
species are known (Wiggins and Wisseman 1992). Larvae occur in rapid streams, and we<br />
have collected <strong>the</strong>m at several <strong>Yukon</strong> sites.<br />
142. Neothremma didactyla Ross (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong> to Oregon.<br />
<strong>Yukon</strong> records: 15, 17 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 23 July.<br />
Genus Oligophlebodes. This is a Nearctic genus <strong>of</strong> 7 western species; larvae are confined<br />
to turbulent mountain streams.<br />
143. Oligophlebodes ruthae Ross (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong> to Utah.<br />
<strong>Yukon</strong> records: 17 (SMDV).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 23 July.<br />
144. Oligophlebodes sierra Ross (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong>, Alaska to California.<br />
<strong>Yukon</strong> records: 16 (ROME).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 29 July.<br />
145. Oligophlebodes zelti Nimmo (I) Nearctic, western montane<br />
Distribution: <strong>Yukon</strong> to Montana.<br />
<strong>Yukon</strong> records: 17, 19 (NW 1984).<br />
<strong>Biological</strong> information: Adults have been collected in <strong>the</strong> <strong>Yukon</strong> 23 – 24 July.<br />
Taxonomic Notes<br />
Note 1. Rhyacophila mongolica Schmid, Arefina and Levanidova (14)<br />
Among a series <strong>of</strong> 5 pharate adults collected by a Royal Ontario Museum field party in<br />
<strong>the</strong> <strong>Yukon</strong> (30 mi W Old Crow, Sunaghun Cr., 16.vii. 1981, ROME #810565), one well<br />
developed male (Fig. 2) is considered conspecific with specimens <strong>of</strong> R. mongolica (Schmid
812 G.B. Wiggins and C.R. Parker<br />
FIGS. 2 – 3. 2, Rhyacophila mongolica Schmid, Arefina and Levanidova (14) (Rhyacophilidae), male genitalia: a,<br />
lateral; b, segment X, caudal (YT: ROME 810565); 3, Rhyacophila sibirica McLachlan, segment X, caudal (Russia,<br />
Siberia: ROME).<br />
et al. 1993); our material has been compared with specimens provided by <strong>the</strong>se authors from<br />
Russia. This is <strong>the</strong> first North American record for this species, o<strong>the</strong>rwise widely distributed<br />
in <strong>the</strong> Russian Far East and Mongolia. The pharate male differs from its sister species R.<br />
sibirica McLachlan in details <strong>of</strong> segment X (cf. Figs. 2b, 3).<br />
Note 2. Goera tungusensis Martynov (47)<br />
Goera radissonica Harper and Méthot 1975, new synonymy<br />
During this study, we found that Goera radissonica Harper and Méthot (1975) from<br />
nor<strong>the</strong>rn Quebec is a junior subjective synonym <strong>of</strong> Goera tungusensis Martynov (1909) from<br />
Siberia, confirmed through comparison by W.K. Gall <strong>of</strong> <strong>the</strong> holotype male <strong>of</strong> G. radissonica<br />
with specimens <strong>of</strong> G. tungusensis obtained from <strong>the</strong> Zoological Institute, St. Petersburg.<br />
Note 3. Ceraclea nigronervosa (Retzius) (56)<br />
We found 2 forms <strong>of</strong> adults <strong>of</strong> this species in <strong>the</strong> <strong>Yukon</strong> and Beringian collections<br />
studied. In both forms <strong>the</strong> dark veins <strong>of</strong> <strong>the</strong> forewing contrast strongly against <strong>the</strong> membrane,<br />
but <strong>the</strong> wing membrane is reddish brown in one and gray to colourless in <strong>the</strong> o<strong>the</strong>r. The<br />
gray-winged form is inferred to be typical for <strong>the</strong> species, in accordance with McLachlan’s<br />
(1877) description which applies to specimens from nor<strong>the</strong>rn and central Europe; we have<br />
similar material from Finland. However, <strong>the</strong> brown-winged form shows several distinctive<br />
characters in <strong>the</strong> male genitalia as described below.<br />
Male genitalia (brown-winged form, Fig. 4). Segment IX in ventral view approximately<br />
twice as wide as long (width less than twice length in gray-winged form, Fig. 6); basal<br />
segment <strong>of</strong> inferior appendages extended into a prominent posteroventral process (little<br />
extended in gray-winged form). Segment X with superior appendages long and tapered<br />
apically (shorter and not tapered in gray-winged form); segment X with each ventrolateral<br />
lobe bearing short stout setae basally (stout setae lacking in gray-winged form). Phallus with<br />
apical membranous endo<strong>the</strong>ca spherical in caudal view (less expanded in gray-winged form).<br />
Female genitalia. The female genitalic structure is variable and no characters concordant<br />
with <strong>the</strong> males were found; characters illustrated for each form (Figs. 5, 7) can occur in both
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 813<br />
FIGS. 4 – 7. Ceraclea nigronervosa (Retzius) (56) (Leptoceridae). 4, Brown-winged form (YT: 810525d ROME),<br />
male genitalia: a, lateral; b, dorsal; c, caudal; d, ventral; e; phallus, lateral; 5, Brown-winged form (YT: 810525d<br />
ROME), female genitalia: a, lateral; b, dorsal; 6, Gray-winged form (YT: 810522b ROME), male genitalia: a, lateral;<br />
b, dorsal; c, caudal; d, ventral; e, phallus, lateral; 7, Gray-winged form (Russia: Tunguska, Yenisei R., ROME),<br />
female genitalia: a, lateral; b, dorsal.
814 G.B. Wiggins and C.R. Parker<br />
brown- and gray-winged specimens. Thus females can be identified only by <strong>the</strong> brown<br />
membrane <strong>of</strong> <strong>the</strong> forewings and by association with males.<br />
In North America material <strong>of</strong> <strong>the</strong> brown-winged form was examined from <strong>the</strong> <strong>Yukon</strong><br />
(10, 12, 17; ROME, SMDV), Alaska, and British Columbia; <strong>the</strong> gray-winged form was<br />
collected only in <strong>the</strong> <strong>Yukon</strong> (4, 10, 12; ROME). Adult specimens were collected along lakes<br />
and streams and on <strong>the</strong> banks <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> River. However, we received a series <strong>of</strong> <strong>the</strong><br />
brown-winged form collected near St. Petersburg, Russia, by V.D. Ivanov; genitalic structure<br />
<strong>of</strong> males is entirely consistent with our Beringian variant (Fig. 4). Consequently, we infer<br />
that two morphs may occur sympatrically over much <strong>of</strong> <strong>the</strong> range attributed to C. nigronervosa,<br />
and perhaps <strong>the</strong> two differ in preferred habitat or life cycle. The issue has to be resolved<br />
by a worker with access to populations in Europe and Asia.<br />
Note 4. Mystacides sepulchralis (Walker) (60)<br />
Although this species is common and widely distributed over much <strong>of</strong> North America<br />
including <strong>the</strong> <strong>Yukon</strong> and Alaska (Yamamoto and Wiggins 1964), its status beyond North<br />
America requires clarification. Holarctic distribution was attributed to M. sepulchralis by<br />
Yamamoto and Ross (1966), even while recognizing as valid <strong>the</strong> Siberian species M. bifidus<br />
Martynov (1924b); supporting evidence was not given, and we know <strong>of</strong> none apart from a<br />
record from Siberia attributed initially to M. sepulchralis (Martynov 1910) but later assigned<br />
to M. bifidus (Martynov 1935).<br />
There is considerable variation in genitalic characters <strong>of</strong> M. sepulchralis. Through <strong>the</strong><br />
assistance <strong>of</strong> V.D. Ivanov, we have obtained information on genitalic characters <strong>of</strong> <strong>the</strong><br />
holotype <strong>of</strong> M. bifidus in <strong>the</strong> Zoological Institute, St. Petersburg; and we have examined<br />
o<strong>the</strong>r specimens, indicating that <strong>the</strong>re is at least some variation in this species, too. The two<br />
forms are very close, and clearly are sister taxa; but Martynov affirmed his view (Betten and<br />
Mosely 1940) that <strong>the</strong> Siberian M. bifidus Martynov and <strong>the</strong> North American M. sepulchralis<br />
(Walker) are separate species. We follow that interpretation here because small morphological<br />
differences in o<strong>the</strong>r species are used here as <strong>the</strong> basis for inferring separation during<br />
glacial periods. Detailed analysis <strong>of</strong> <strong>the</strong> variation in populations within each continent might<br />
reveal evidence <strong>of</strong> a Beringian interchange; whe<strong>the</strong>r such differences would confirm status<br />
as species or as intraspecific variants would emerge from <strong>the</strong> analysis. For <strong>the</strong> present, we<br />
interpret M. sepulchralis as a strictly North American species.<br />
Larvae <strong>of</strong> M. bifidus, for which we have no information, could be relevant to this<br />
question. In recent years, distinctive larvae in some populations <strong>of</strong> M. sepulchralis from<br />
eastern North America have been found with head markings <strong>of</strong> discrete spots similar to those<br />
in M. alafimbriata, and quite unlike <strong>the</strong> largely black heads characteristic <strong>of</strong> most sepulchralis<br />
populations in North America (cf. figs. 3 and 4, Yamamoto and Wiggins 1964).<br />
Association <strong>of</strong> <strong>the</strong>se larvae with adults confirms that <strong>the</strong>y are M. sepulchralis. This larval<br />
dimorphism could have been established in populations isolated during <strong>the</strong> glacial period to<br />
<strong>the</strong> sou<strong>the</strong>ast <strong>of</strong> <strong>the</strong> continental ice mass, although it does not appear to be reflected in<br />
characters <strong>of</strong> <strong>the</strong> adults.<br />
The sister species <strong>of</strong> M. sepulchralis/bifidus is M. alafimbriata, confined to western<br />
North America including <strong>the</strong> <strong>Yukon</strong> and Alaska (Yamamoto and Wiggins 1964). Mystacides<br />
alafimbriata and sepulchralis are only slightly different morphologically; and although <strong>the</strong>re<br />
appears to be little overlap in <strong>the</strong>ir ranges, <strong>the</strong> two have been taken in <strong>the</strong> same collections<br />
in a few localities, all in <strong>the</strong> <strong>Yukon</strong> and Alaska. Possibly M. alafimbriata was derived from<br />
populations isolated to <strong>the</strong> south <strong>of</strong> <strong>the</strong> Cordilleran glacier in <strong>the</strong> mountains <strong>of</strong> western North<br />
America. Outlying montane glaciers along <strong>the</strong> Rocky and Cascade Mountain ranges served
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 815<br />
FIGS. 8 – 9. 8, Mystacides sibiricus Martynov (Leptoceridae) (Russia, Lake Baikal: ROME), male genitalia, caudal<br />
with detail at apex <strong>of</strong> phallus, lateral; 9, Mystacides interjectus (Banks) (59) (YT: ROME 795058), male genitalia,<br />
caudal with detail <strong>of</strong> apex <strong>of</strong> phallus, lateral.<br />
to isolate refugia in <strong>the</strong> coniferous forest zone south <strong>of</strong> <strong>the</strong> continental ice sheet and in <strong>the</strong><br />
sou<strong>the</strong>rn coastal refugium (Kavanaugh 1988, fig. 37).<br />
Note 5. Mystacides interjectus (Banks) (59)<br />
This species is widely distributed across <strong>the</strong> nor<strong>the</strong>rn half <strong>of</strong> North America (Yamamoto<br />
and Wiggins 1964, as longicornis), including <strong>the</strong> <strong>Yukon</strong> and Alaska. In distinguishing<br />
between <strong>the</strong> North American M. interjectus (Banks) and <strong>the</strong> Eurasian M. longicornis<br />
(Linnaeus), Yamamoto and Ross (1966) treated M. sibiricus Martynov (1935) as a junior<br />
synonym <strong>of</strong> M. interjectus (Banks 1914), <strong>the</strong>reby apparently extending <strong>the</strong> range <strong>of</strong> <strong>the</strong> North<br />
American form into Asia. This interpretation was followed by Mey and Dulmaa (1985) in<br />
identifying specimens from Mongolia as M. interjectus. However, illustrations <strong>of</strong> <strong>the</strong> male<br />
genitalia <strong>of</strong> M. sibiricus by Martynov (1935, figs. 28 – 30) show that this species belongs not<br />
to <strong>the</strong> interjectus lineage, but to <strong>the</strong> longicornis complex. Our interpretation is consistent<br />
with a male from Lake Baikal identified as M. sibiricus (Fig. 8) in <strong>the</strong> Zoological Institute,<br />
St. Petersburg; it is confirmed from examination <strong>of</strong> <strong>the</strong> syntype series <strong>of</strong> approximately 100<br />
specimens in <strong>the</strong> Zoological Institute by V.D. Ivanov, who has designated a lectotype male<br />
<strong>of</strong> Mystacides sibiricus Martynov (Ivan Lake, 45 km NW <strong>of</strong> Chita City, 15.vii.1925,<br />
Vinogradov leg.). Whe<strong>the</strong>r Mystacides sibiricus Martynov and longicornis Linnaeus are<br />
distinct species will have to be determined by examination <strong>of</strong> specimens over a wide<br />
geographic range in Eurasia; currently, we know <strong>of</strong> no evidence to support <strong>the</strong> occurrence<br />
<strong>of</strong> M. interjectus in <strong>the</strong> Palaearctic region.<br />
Distinction between M. interjectus and longicornis was based by Yamamoto and Ross<br />
(1966) on <strong>the</strong> structure <strong>of</strong> segment X; but we believe that <strong>the</strong> two are best distinguished by<br />
<strong>the</strong> structure <strong>of</strong> <strong>the</strong> male inferior appendages in caudal aspect. In M. interjectus (Fig. 9) <strong>the</strong><br />
concave ventral face <strong>of</strong> <strong>the</strong> inferior appendage is conspicuously narrowed in caudal aspect;<br />
in M. longicornis and M. sibiricus, <strong>the</strong> concave ventral face is wide, and ra<strong>the</strong>r uniformly<br />
so throughout. Moreover, <strong>the</strong> phallo<strong>the</strong>ca in M. longicornis and M. sibiricus terminates<br />
apically in a pair <strong>of</strong> stout sclerotized hooks; <strong>the</strong>se hooks are scarcely developed in M. interjectus.<br />
Over <strong>the</strong> whole <strong>of</strong> its range in North America, M. interjectus is somewhat variable<br />
in genitalic morphology, but we find no indication <strong>of</strong> regional differences in <strong>the</strong> <strong>Yukon</strong>,<br />
Alaska, or elsewhere to indicate that populations were separated during glaciation.
816 G.B. Wiggins and C.R. Parker<br />
The longicornis species group comprising M. nigra, longicornis, and interjectus<br />
(Yamamoto and Ross 1966) is apparently <strong>of</strong> Palaearctic origin. Separation between M. longicornis<br />
and interjectus was probably by vicariant speciation <strong>of</strong> a continuous circumboreal<br />
ancestral range brought about by separation <strong>of</strong> Asia and North America.<br />
Note 6. Grammotaulius alascensis Schmid (84)<br />
Grammotaulius subborealis Schmid 1964, new synonymy<br />
These 2 taxa, toge<strong>the</strong>r with G. signatipennis McLachlan, constitute a complex in which<br />
identification is rendered all <strong>the</strong> more difficult by <strong>the</strong> fact that no explicit diagnosis has been<br />
proposed to distinguish one from ano<strong>the</strong>r. Because a series <strong>of</strong> <strong>Yukon</strong> specimens (Dempster<br />
Hwy. km 105, nr. Blackstone R., 30.vii.1979, ROME #791183, 19 males) shows characters<br />
intermediate between those illustrated for males <strong>of</strong> G. alascensis and G. subborealis (Schmid<br />
1964, figs. 6 –13), <strong>the</strong> latter name is placed here as a junior subjective synonym under<br />
G. alascensis which has page priority. In <strong>the</strong> characters illustrated by Schmid for <strong>the</strong> females,<br />
<strong>the</strong> pair <strong>of</strong> slender membranous lobes arising from <strong>the</strong> posterodorsal margin <strong>of</strong> segment<br />
IX + X was interpreted inconsistently (Schmid 1964, figs. 4, 5): in G. alascensis <strong>the</strong> slender<br />
lobe is shown as an appendage <strong>of</strong> a sclerotized rounded lobe at <strong>the</strong> base <strong>of</strong> <strong>the</strong> anal tube; in<br />
G. subborealis <strong>the</strong> slender lobe is shown correctly as a median structure separate from <strong>the</strong><br />
sclerotized lobe. These paired membranous lobes are actually eversible, and appear in<br />
genitalic preparations <strong>of</strong> Grammotaulius and many o<strong>the</strong>r Limnephilidae in variable conditions<br />
<strong>of</strong> eversion; in Schmid’s figures <strong>of</strong> G. signatipennis (1950a, figs. 52 – 54) <strong>the</strong>y do not<br />
appear at all, as is <strong>of</strong>ten <strong>the</strong> case, although <strong>the</strong> lobes are extended in o<strong>the</strong>r cleared specimens<br />
<strong>of</strong> this species.<br />
In this emended sense, G. alascensis appears to be <strong>the</strong> North American sister species <strong>of</strong><br />
G. signatipennis. Our material <strong>of</strong> G. signatipennis is not extensive, but <strong>the</strong> principal<br />
distinguishing character <strong>of</strong> <strong>the</strong> males is evidently <strong>the</strong> posteroventral edge <strong>of</strong> <strong>the</strong> superior<br />
appendages, which is very heavily sclerotized, darkened, and ra<strong>the</strong>r linear in G. alascensis,<br />
but more rounded and less sclerotized in G. signatipennis. In some specimens <strong>of</strong> <strong>the</strong> latter<br />
species, <strong>the</strong> posteroventral margin <strong>of</strong> <strong>the</strong> superior appendage is somewhat more heavily<br />
sclerotized than illustrated (Schmid 1950a, fig. 50). The intermediate appendages in our<br />
North American material show considerable variation, as was illustrated by Schmid. We<br />
have seen a specimen <strong>of</strong> G. alascensis from <strong>the</strong> Hudson Bay coastline <strong>of</strong> <strong>the</strong> Northwest<br />
Territories (Eskimo Point, 30.ix.1939, CNCI). From all <strong>of</strong> this, G. alascensis can be regarded<br />
as a highly variable North American species. We do not have sufficient material to assess<br />
variation for a consistent character separating females <strong>of</strong> <strong>the</strong>se 2 species.<br />
We are advised by O.S. Flint that <strong>the</strong> Alaskan specimens attributed to G. signatipennis<br />
McLachlan (Flint 1964: Nunivak Is., USNM) are in fact G. alascensis Schmid. However,<br />
G. signatipennis must remain as part <strong>of</strong> <strong>the</strong> North American fauna because we have found<br />
a single male from <strong>the</strong> <strong>Yukon</strong> (Dawson, Moose Cr., CNCI) in which <strong>the</strong> genitalic structure<br />
is typical for this species. The forewings show <strong>the</strong> typical markings only faintly, which we<br />
attribute to <strong>the</strong> ra<strong>the</strong>r teneral condition <strong>of</strong> <strong>the</strong> specimen.<br />
Note 7. Limnephilus diphyes McLachlan (96)<br />
This species is recorded here for <strong>the</strong> first time from North America; diagnostic<br />
characters in genitalic morphology are illustrated (Figs. 10, 11; and also by Malicky 1983,<br />
pp. 191 and 200). The original description by McLachlan (1880) provides a good account<br />
<strong>of</strong> <strong>the</strong> general morphology. Limnephilus diphyes appears to belong to <strong>the</strong> sitchensis group<br />
(Schmid 1955), and can be distinguished from <strong>the</strong> o<strong>the</strong>r species by <strong>the</strong> prominent notch in<br />
<strong>the</strong> superior appendages <strong>of</strong> <strong>the</strong> male.
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 817<br />
FIGS. 10 –11. Limnephilus diphyes McLachlan (96) (Limnephilidae) (YT: ROME 800105a). 10, Male genitalia: a,<br />
lateral; b, ventral; c, phallus, lateral; 11, Female genitalia: a, lateral; b, ventral.<br />
Note 8. Limnephilus fumosus Banks (102)<br />
Limnephilus isobela Nimmo 1991, new synonymy<br />
Information available in <strong>the</strong> taxonomic literature is inadequate for <strong>the</strong> distinction <strong>of</strong><br />
L. fumosus Banks from its very similar sister species L. santanus Ross. Banks (1900) based<br />
L. fumosus on a series <strong>of</strong> 3 male and 2 female specimens from Alaska; diagnostic characters<br />
for this species were not clearly described, and Banks’ assertion that L. fumosus also occurred<br />
in Washington probably stemmed from failure to distinguish between L. fumosus and <strong>the</strong><br />
more sou<strong>the</strong>rly sister taxon later recognized as L. santanus. In <strong>the</strong> original description <strong>of</strong><br />
L. santanus from Oregon, Ross (1949) gave an explicit diagnosis only for <strong>the</strong> female. To<br />
establish <strong>the</strong> identity <strong>of</strong> <strong>the</strong>se 2 species, we studied <strong>the</strong> type material <strong>of</strong> L. fumosus in <strong>the</strong><br />
U.S. National Museum <strong>of</strong> Natural History, and <strong>of</strong> L. santanus in <strong>the</strong> Illinois Natural History<br />
Survey; genitalic morphology <strong>of</strong> both sexes is illustrated here. We also designate Limnephi-
818 G.B. Wiggins and C.R. Parker<br />
FIGS. 12 –13. Limnephilus fumosus Banks (102) (Limnephilidae) (AK: USNM). 12, Lectotype male, genitalia: a,<br />
lateral; b, dorsal; c, caudal; d, phallus, lateral; e, phallus, dorsal; 13, Paratype female, genitalia: a, lateral; b, ventral.<br />
lus isobela Nimmo (1991) as a junior subjective synonym <strong>of</strong> L. fumosus Banks; <strong>the</strong> type<br />
locality given for L. isobela Nimmo is Isobel Pass (= Isabel or Isabell Pass) mi. 206,<br />
Richardson Highway, <strong>Yukon</strong>, but <strong>the</strong> Richardson Highway and Isabel Pass are within<br />
Alaska.<br />
Males <strong>of</strong> <strong>the</strong> 2 species can be separated by <strong>the</strong> parameres, which are bifid apically in<br />
L. fumosus (Fig. 12) but undivided in L. santanus (Fig. 14). The females are distinguished<br />
by <strong>the</strong> structure <strong>of</strong> segment X; in L. fumosus segment X is produced as a pair <strong>of</strong> heavily<br />
sclerotized blade-like projections (Fig. 13), broad apically in ventral aspect with <strong>the</strong> posterior<br />
margin broadly U-shaped. In females <strong>of</strong> L. santanus (Fig. 15) segment X terminates in a pair<br />
<strong>of</strong> elongate slender processes, and in ventral aspect is narrowed apically. In addition to<br />
genitalic differences, L. fumosus and L. santanus differ in <strong>the</strong> colour <strong>of</strong> <strong>the</strong> forewings:<br />
Limnephilus fumosus is uniformly brown with conspicuous light areas, much as illustrated<br />
by Banks (1900, fig. 10), whereas L. santanus is brown with light speckling widely<br />
distributed over <strong>the</strong> wing, in addition to <strong>the</strong> large light areas along <strong>the</strong> chord and at <strong>the</strong> apex,<br />
as described by Ross (1949).
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 819<br />
FIGS. 14 –15. Limnephilus santanus Ross (Limnephilidae) (OR: INHS). 14, Holotype male, genitalia: a, lateral; b,<br />
dorsal; c, caudal; d, phallus, lateral; e, phallus, dorsal; 15, Allotype female, genitalia: a, lateral; b, ventral.<br />
Ross (1949) stated that <strong>the</strong> type <strong>of</strong> L. fumosus is a female; however, a type has not been<br />
designated. Therefore, we select <strong>the</strong> following male from <strong>the</strong> type series as <strong>the</strong> lectotype <strong>of</strong><br />
Limnephilus fumosus (Banks, 1900): LECTOTYPE male: Alaska, Berg Bay, June 10 ’99;<br />
Harriman Expedition ’99, T. Kincaid, collector; #192, USNM Paratype (sensu cotype)<br />
No. 5262.<br />
Records confirmed for Limnephilus fumosus indicate that this species is known from<br />
Alaska, <strong>Yukon</strong>, and <strong>the</strong> Northwest Territories (NT: Midway L., 67°14′N 135°26′W,<br />
8.vii.1985, SMDV). A series in <strong>the</strong> ROME identified as L. santanus was cited by Nimmo<br />
and Wickstrom (1984: YT. Mirror Creek, Alaska Hwy. mile 1209, 28.vi.1958, ROME);<br />
<strong>the</strong>se specimens have been re-examined in light <strong>of</strong> <strong>the</strong> information now available for <strong>the</strong>se<br />
2 species, and <strong>the</strong>y are L. fumosus. Nimmo’s list <strong>of</strong> Alaskan Trichoptera (1986) includes<br />
L. fumosus based on earlier records from <strong>the</strong> literature, and also L. santanus based on material
820 G.B. Wiggins and C.R. Parker<br />
deposited in <strong>the</strong> CNCI; we have been unable to locate this recent material in <strong>the</strong> CNCI, but<br />
we suspect that it belongs to L. fumosus. Consequently, occurrence <strong>of</strong> L. santanus in Alaska<br />
remains subject to confirmation. Nimmo and Scudder (1983) record L. santanus from British<br />
Columbia; we have been unable to locate <strong>the</strong>ir material in <strong>the</strong> CNCI, but <strong>the</strong> record should<br />
be confirmed in light <strong>of</strong> this new information.<br />
The morphological distinction between <strong>the</strong>se 2 species is ra<strong>the</strong>r slight but seems<br />
consistent; as in a number <strong>of</strong> close congeneric species in <strong>the</strong> Limnephilidae, <strong>the</strong> structural<br />
distinction between <strong>the</strong>m appears to have arisen in <strong>the</strong> parameres <strong>of</strong> <strong>the</strong> males and in small,<br />
presumably reciprocal, changes in <strong>the</strong> genitalic structure <strong>of</strong> <strong>the</strong> females.<br />
Biogeographic Analysis <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> and Holarctic Trichoptera<br />
Here we seek some understanding <strong>of</strong> <strong>the</strong> sources from which Trichoptera have come to<br />
occupy <strong>the</strong> <strong>Yukon</strong>. The number <strong>of</strong> species <strong>of</strong> Trichoptera known in <strong>the</strong> <strong>Yukon</strong> Territory<br />
stands now at 145, constituting 11 per cent <strong>of</strong> <strong>the</strong> Nearctic Trichoptera known north <strong>of</strong><br />
Mexico, and doubtless more have yet to be recorded. The history <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> trichopteran<br />
fauna is analyzed under 4 categories <strong>of</strong> species; several o<strong>the</strong>r species from adjacent Alaska<br />
or <strong>the</strong> Northwest Territories, although not recorded from <strong>the</strong> <strong>Yukon</strong>, are relevant to this<br />
analysis and are included (designated by †). The remaining Holarctic species that have not<br />
been recorded in Beringia are brought toge<strong>the</strong>r for comparison in a fifth category; and<br />
consequently all North American species <strong>of</strong> Trichoptera that also occur in Europe or Asia<br />
are treated here in some manner.<br />
Category I. Nearctic species widespread in North America<br />
II. Holarctic species widespread in North America<br />
III. Palaearctic-East Beringian species<br />
IV. Beringian species<br />
V. Holarctic species not in Beringia<br />
Relevant evidence is outlined for <strong>the</strong> species discussed, and although it is incomplete<br />
and <strong>of</strong>ten speculative in interpretation, a taxonomic and conceptual context is established<br />
from which more focussed questions can be identified. Phylogenetic analysis <strong>of</strong> <strong>the</strong> genera<br />
for <strong>the</strong> species concerned, although an important asset, is well beyond <strong>the</strong> scope <strong>of</strong> this study.<br />
In addition to <strong>the</strong> investigation <strong>of</strong> <strong>Yukon</strong> Trichoptera, our objective in this study is an initial<br />
outline <strong>of</strong> issues relating to <strong>the</strong> origin <strong>of</strong> Holarctic Trichoptera in North America. Particular<br />
attention has been given to intraspecific morphological variation in <strong>the</strong> Holarctic and<br />
amphiberingian species, in an attempt to detect differences in populations that could be<br />
indicative <strong>of</strong> geographic disjunction during glacial periods. The broad scope <strong>of</strong> this study<br />
has precluded special efforts to enlarge series <strong>of</strong> <strong>the</strong> variable species to statistically significant<br />
levels; our observations, based on <strong>the</strong> material available, are to be regarded as an<br />
indication <strong>of</strong> <strong>the</strong> potential for more exacting analysis <strong>of</strong> intraspecific variation in certain<br />
species. In <strong>the</strong> absence <strong>of</strong> evidence to <strong>the</strong> contrary, we have assumed that <strong>the</strong>se differences<br />
have a genetic basis, but <strong>the</strong> possibility cannot be excluded that ecological factors could be<br />
involved (e.g. Danks 1981: 360).<br />
Geological and Climatic Context. For much <strong>of</strong> <strong>the</strong> past 65 million years <strong>of</strong> <strong>the</strong> Cenozoic<br />
era, overland connections between <strong>the</strong> major continents at <strong>the</strong> nor<strong>the</strong>rn end <strong>of</strong> <strong>the</strong> globe<br />
permitted interchange <strong>of</strong> animals and plants (Mat<strong>the</strong>ws 1979a, fig. 2.6), establishing <strong>the</strong><br />
ancestry <strong>of</strong> <strong>the</strong> present biota. Continental crust connecting North America with Asia stood
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 821<br />
above <strong>the</strong> sea for <strong>the</strong> first 40 million years <strong>of</strong> <strong>the</strong> Cenozoic era during <strong>the</strong> Palaeocene (65 – 58<br />
million years B.P.), Eocene (58 – 37 ma B.P.), and Oligocene (37 – 24 ma B.P.) epochs,<br />
permitting open biotic interchange between <strong>the</strong> 2 continents (Stanley 1986). The effective<br />
barrier to biotic interchange through early Cenozoic epochs was <strong>the</strong> Turgai Strait and perhaps<br />
<strong>the</strong> Cherskiy Seaway subdividing Siberia on a north-south axis (Mat<strong>the</strong>ws 1979a, fig. 2.2).<br />
The Bering land bridge connecting North America and Asia was inundated by <strong>the</strong> sea at<br />
intervals during <strong>the</strong> last 25 million years <strong>of</strong> <strong>the</strong> Cenozoic, through <strong>the</strong> Miocene (24 – 5 ma<br />
B.P.) and Pliocene (5 –1.8 ma B.P.) epochs (Stanley 1986); flooding during <strong>the</strong> mid-Pliocene<br />
some 3 ma B.P. closed <strong>the</strong> Bering land bridge until lowered sea level again exposed it during<br />
<strong>the</strong> major Pleistocene glacial advances.<br />
Combined with <strong>the</strong>se intercontinental connections, warm moist climatic conditions<br />
prevailed at arctic latitudes through <strong>the</strong> Palaeocene to <strong>the</strong> Eocene. Cooler and drier<br />
climates followed in late Eocene, probably caused by changes <strong>of</strong> geography, ocean currents,<br />
volcanism, and variations in Earth’s solar orbit. This cooling trend continued through <strong>the</strong><br />
Oligocene, Miocene, and Pliocene until marked decline in temperature led to establishment<br />
<strong>of</strong> an arctic climate in <strong>the</strong> north, with glaciers forming about 2 ma B.P. (Stanley 1986).<br />
Exposure <strong>of</strong> <strong>the</strong> Bering land bridge (Fig. 16) resulted from declining sea levels as large<br />
volumes <strong>of</strong> water were bound up in <strong>the</strong> glacial ice that covered much <strong>of</strong> nor<strong>the</strong>rn North<br />
America, Asia, and Europe. A number <strong>of</strong> major glacial advances followed in <strong>the</strong> Pleistocene,<br />
each advance receding to some extent during a subsequent interglacial period. The last major<br />
Pleistocene glacial advance in North America, <strong>the</strong> Wisconsinan, began approximately<br />
100 000 years ago and reached its maximum about 18 000 years ago, before beginning <strong>the</strong><br />
present period <strong>of</strong> glacial recession.<br />
Land connections between nor<strong>the</strong>astern North America and Europe had disappeared by<br />
mid-Miocene time, some 15 million years ago. Prior to that, in early Cenozoic epochs before<br />
sea-floor spreading between North America and Europe had separated <strong>the</strong> land masses, <strong>the</strong>re<br />
were at least 2 principal overland connections (Mat<strong>the</strong>ws 1979a, fig. 2.3): <strong>the</strong> De Geer bridge<br />
interconnecting through nor<strong>the</strong>rn Greenland; and <strong>the</strong> Greenland – Faeroes bridge through<br />
Iceland.<br />
<strong>Biological</strong> Aspects. As a result <strong>of</strong> this geological and climatic history, <strong>the</strong> present fauna <strong>of</strong><br />
North America has been derived over millions <strong>of</strong> years when intercontinental movement<br />
across nor<strong>the</strong>rn land connections was favoured by warm to mild climates. When ocean<br />
barriers arose between <strong>the</strong> continents, and nor<strong>the</strong>rn climates cooled, <strong>the</strong> North American<br />
biota was isolated from that <strong>of</strong> Europe, followed intermittently by separation from Asia. The<br />
Pleistocene epoch <strong>of</strong> <strong>the</strong> past 1.8 million years provided an opportunity once again for biotic<br />
interchange between North America and Asia. This time, however, harsh climates prevailed,<br />
and <strong>the</strong> largely unglaciated Bering land bridge connecting Nearctic (East) and Palaearctic<br />
(West) Beringia permitted only cold-adapted species to pass from one continent to <strong>the</strong> o<strong>the</strong>r.<br />
Formation <strong>of</strong> <strong>the</strong> glaciers was governed by <strong>the</strong> configuration <strong>of</strong> land, sea, and mountains—relationships<br />
that differ between Asia and North America. Over much <strong>of</strong> nor<strong>the</strong>astern<br />
Asia glaciers were confined to higher mountains (Fig. 16), leaving most <strong>of</strong> that area<br />
unglaciated throughout <strong>the</strong> Pleistocene epoch. In North America, by contrast, <strong>the</strong> Laurentide<br />
continental glacier and <strong>the</strong> Cordilleran glacier <strong>of</strong> <strong>the</strong> western mountain ranges flowed<br />
toge<strong>the</strong>r to <strong>the</strong> east and south <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> (Fig. 16); and with each glacial advance <strong>the</strong><br />
unglaciated Nearctic Beringian refugium was closed <strong>of</strong>f as <strong>the</strong> advancing ice eliminated<br />
animals and plants in its path, reducing <strong>the</strong> levels <strong>of</strong> species-packing in Beringian communities.<br />
During periods <strong>of</strong> interglacial recession, contact may have been possible between <strong>the</strong>
822 G.B. Wiggins and C.R. Parker<br />
FIG. 16. Approximate boundaries <strong>of</strong> unglaciated Beringia during Wisconsinan glacial maximum; based on Ager<br />
(1982), Lafontaine and Wood (1988). Separation between Laurentide and Cordilleran glaciers indicates location<br />
<strong>of</strong> postulated intermittent ice-free corridor.<br />
Beringian refugium, and perhaps o<strong>the</strong>r glacial refugia, with <strong>the</strong> main body <strong>of</strong> <strong>the</strong> North<br />
American biota to <strong>the</strong> south <strong>of</strong> <strong>the</strong> glaciated areas. An ice-free corridor was opened between<br />
<strong>the</strong> Laurentide and Cordilleran glaciers during periods <strong>of</strong> interglacial recession, connecting<br />
Beringia with unglaciated areas to <strong>the</strong> south for extensive periods (e.g. Reeves 1973; Pielou<br />
1991). This corridor would have held major freshwater drainage systems arising from glacial<br />
melting, but whe<strong>the</strong>r <strong>the</strong> climate sustained suitable habitats for aquatic insects such as<br />
Trichoptera is an open question. To provide food for aquatic insects, habitats must support<br />
plant materials <strong>of</strong> both allochthonous and autochthonous origin. However, at least one<br />
species <strong>of</strong> Trichoptera now exists in <strong>the</strong> rigorous climate <strong>of</strong> Ellesmere Island, indicating that<br />
<strong>the</strong> ice-free corridor may have supported some species during <strong>the</strong> Pleistocene. Should that<br />
have happened, Beringian species including those <strong>of</strong> Palaearctic origin could have passed<br />
to <strong>the</strong> south <strong>of</strong> <strong>the</strong> Laurentide and Cordilleran glaciers during Pleistocene interglacial<br />
periods. A Nahanni glacial refuge for freshwater organisms in <strong>the</strong> sou<strong>the</strong>astern <strong>Yukon</strong> is<br />
supported by genetic analysis <strong>of</strong> lake whitefish stocks (Foote et al. 1992), adding support to<br />
<strong>the</strong> possibility <strong>of</strong> survival <strong>of</strong> aquatic insects in <strong>the</strong> corridor area during glaciation. But if <strong>the</strong><br />
ice-free corridor did function as an interglacial passage for Trichoptera, it must have been a<br />
two-way corridor; and <strong>the</strong> ecological resistance <strong>of</strong> expanding Nearctic communities in <strong>the</strong><br />
south to dispersing Palaearctic species could have been a constraint to <strong>the</strong> southbound<br />
movement <strong>of</strong> species from Beringia (e.g. Vermeij 1991).<br />
Exposure <strong>of</strong> <strong>the</strong> Bering land bridge during Pleistocene glaciations connected quite<br />
different refugial areas in Nearctic and Palaearctic Beringia (Fig. 16). Palaearctic Beringia
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 823<br />
was much larger than Nearctic Beringia and also extended to lower latitudes; <strong>the</strong>refore, it<br />
would have supported more species <strong>of</strong> plants and animals, including Trichoptera (see under<br />
Ecological Considerations). By contrast, glaciation in North America isolated <strong>the</strong> Alaska –<br />
<strong>Yukon</strong> peninsula, largely if not entirely closing <strong>of</strong>f exchange <strong>of</strong> species with lower latitudes<br />
(above). Consequently, <strong>the</strong> 2 species pools must have been imbalanced when <strong>the</strong> Bering land<br />
bridge was exposed. We infer that whatever dispersal followed <strong>the</strong> opening <strong>of</strong> <strong>the</strong> Bering<br />
land bridge would necessarily have been weighted in favour <strong>of</strong> a Palaearctic-to-Nearctic<br />
movement; in a group-to-group comparison, not only would <strong>the</strong>re have been more species<br />
in Palaearctic Beringia, but also <strong>the</strong>re probably would have been more vacant ecological<br />
niches in Nearctic Beringia because <strong>of</strong> extinctions caused by glaciation. For mammalian<br />
species, predominantly eastward dispersal across <strong>the</strong> Bering land bridge has been inferred<br />
from evidence for <strong>the</strong> Pleistocene, but movement at about <strong>the</strong> same level in each direction<br />
during <strong>the</strong> Pliocene (Vermeij 1991). These conclusions are consistent with <strong>the</strong> concept that<br />
dissimilar patterns <strong>of</strong> glaciation in North America and Asia are correlated with asymmetric<br />
interchange <strong>of</strong> species. Moreover, global analysis <strong>of</strong> past biotic interchanges leads to <strong>the</strong><br />
general conclusion that <strong>the</strong> resistance <strong>of</strong> a biota to invasion is reduced by previous extinction<br />
<strong>of</strong> species in that biota (Vermeij 1991).<br />
Superimposed upon <strong>the</strong>se general factors influencing <strong>the</strong> interchange <strong>of</strong> animals and<br />
plants between North America and Asia are <strong>the</strong> special requirements <strong>of</strong> aquatic insects. Only<br />
<strong>the</strong> Odonata have <strong>the</strong> capacity for active dispersal over longer distances between <strong>the</strong> aquatic<br />
habitats <strong>of</strong> larvae. Adult Trichoptera are subject at best to passive dispersal by strong winds;<br />
even <strong>the</strong>n, ovipositing females would have to be relocated in <strong>the</strong> vicinity <strong>of</strong> appropriate<br />
aquatic habitats. Dispersal by wind over <strong>the</strong> narrow sea barrier <strong>of</strong> <strong>the</strong> present-day Bering<br />
Strait seems at least as likely as dispersal across long distances overland; but <strong>the</strong>re is little<br />
supporting evidence for successful dispersal across Bering Strait, nor is <strong>the</strong>re evidence for<br />
post-Pleistocene intercontinental interchange <strong>of</strong> Trichoptera by way <strong>of</strong> <strong>the</strong> Aleutian Island<br />
chain (e.g. Karlstrom and Ball 1969). Even so, several species <strong>of</strong> Trichoptera evidently did<br />
reach Greenland by dispersal at high latitudes over sea barriers from distant glacial refugia<br />
(see category I, Greenland Trichoptera). Beringia during much <strong>of</strong> <strong>the</strong> Pleistocene is depicted<br />
as arid and steppe-like with <strong>the</strong> muskeg and wet tundra, now so typical, greatly diminished<br />
in extent (Schweger et al. 1982). Thus, Nearctic Beringia, <strong>the</strong> corridor between Asia and<br />
North America, would seem to have provided a different mix <strong>of</strong> habitats and probably more<br />
restricted habitats for aquatic insects than are now available. Moreover, <strong>the</strong> glacier-fed water<br />
courses were probably similar to glacial streams <strong>of</strong> today that support few aquatic insects<br />
because <strong>the</strong>y carry heavy loads <strong>of</strong> suspended glacial debris which are deposited to form<br />
unstable and largely unproductive substrates, shifted by braided channels as <strong>the</strong> volume <strong>of</strong><br />
meltwater fluctuates. Unglaciated upland parts <strong>of</strong> Beringia, however, probably had streams<br />
that were similar to present-day upland streams in tundra regions. These streams would have<br />
joined <strong>the</strong> lowland river systems that traversed <strong>the</strong> exposed coastal shelf (Fig. 16), forming<br />
a network <strong>of</strong> freshwater systems that is believed to have facilitated an exchange <strong>of</strong> fish<br />
species between Asia and North America (Lindsey and McPhail 1986). Because <strong>of</strong> <strong>the</strong> high<br />
biological diversity <strong>of</strong> Trichoptera, however, <strong>the</strong> availability <strong>of</strong> freshwater habitats across<br />
Beringia is only one aspect <strong>of</strong> <strong>the</strong> issue; ano<strong>the</strong>r aspect is <strong>the</strong> extent to which <strong>the</strong>se freshwater<br />
habitats coincided with <strong>the</strong> requirements <strong>of</strong> <strong>the</strong> species that had access to <strong>the</strong>m. These issues<br />
are explored under Ecological Considerations.<br />
We infer that <strong>the</strong> Bering land bridge <strong>of</strong> Pleistocene time would have been a difficult<br />
passage for many aquatic insects because <strong>the</strong> cold dry climate was not well suited to dispersal<br />
<strong>of</strong> <strong>the</strong> adult insects, and because freshwater habitats would have been localized, unsuitable,
824 G.B. Wiggins and C.R. Parker<br />
or different in o<strong>the</strong>r ways from those <strong>of</strong> <strong>the</strong> present. More favourable conditions for <strong>the</strong><br />
movement <strong>of</strong> aquatic insects between Asia and North America would seem to have been<br />
available during <strong>the</strong> millions <strong>of</strong> years that <strong>the</strong> 2 continents were connected in Pliocene time<br />
and earlier, before <strong>the</strong> land connection between <strong>the</strong> 2 continents was inundated in <strong>the</strong><br />
mid-Pliocene, some 3 million years ago.<br />
Origin <strong>of</strong> <strong>the</strong> Beringian and Holarctic Trichoptera. The origin <strong>of</strong> Beringian and Holarctic<br />
species can be interpreted in several ways. Some species could have had an Holarctic<br />
distribution pre-dating <strong>the</strong> separation <strong>of</strong> North America from Asia in Pliocene time some<br />
3 ma B.P. Thus, some Holarctic species (category II) could have been confined to <strong>the</strong> south<br />
<strong>of</strong> <strong>the</strong> advancing ice in unglaciated parts <strong>of</strong> <strong>the</strong> continent, giving rise to intraspecific variants<br />
or even sibling species which now can be detected; disjunct populations <strong>of</strong> some <strong>of</strong> <strong>the</strong>se<br />
species may have been isolated in unglaciated Nearctic Beringia by <strong>the</strong> converging Laurentide<br />
and Cordilleran ice masses. Palaearctic-East Beringian species (category III) may never<br />
have occurred much beyond <strong>the</strong>ir present range in North America, or, if <strong>the</strong>y did, were<br />
overtaken by <strong>the</strong> advancing ice and confined as glacial relicts to <strong>the</strong> Beringian refugium<br />
where <strong>the</strong>y remain. Some o<strong>the</strong>rs could be Nearctic species that dispersed from Beringia to<br />
Asia during <strong>the</strong> past 1.8 million years <strong>of</strong> <strong>the</strong> Pleistocene. For most <strong>of</strong> <strong>the</strong> Beringian Holarctic<br />
species, <strong>the</strong> evidence now available is not sufficient to interpret clearly which <strong>of</strong> <strong>the</strong><br />
possibilities seems most likely. However, a pattern <strong>of</strong> vicariant distribution <strong>of</strong> sister species<br />
in North America and Eurasia <strong>of</strong>ten emerges, dating perhaps from <strong>the</strong> Pliocene or earlier,<br />
and augmented in some cases by apparent later dispersal <strong>of</strong> <strong>the</strong> Palaearctic form to North<br />
America, presumably by way <strong>of</strong> <strong>the</strong> Bering land bridge during <strong>the</strong> Pleistocene.<br />
The fundamental issues in piecing toge<strong>the</strong>r <strong>the</strong> origin <strong>of</strong> <strong>the</strong> Beringian Trichoptera are<br />
<strong>the</strong> age <strong>of</strong> <strong>the</strong> species and <strong>the</strong>ir rate <strong>of</strong> structural change over time. This information is<br />
unknown, but clearly we are dealing with species that differ from <strong>the</strong>ir closest living relative<br />
(sister species) by a wide range <strong>of</strong> distinguishing characters, and thus could and probably<br />
do represent species <strong>of</strong> different ages. Some Beringian species pairs are virtually sibling<br />
species, e.g. Limnephilus fenestratus (101) and L. kennicotti (107). The small, but consistent,<br />
morphological distinctions between some pairs <strong>of</strong> sister species could have arisen in disjunct<br />
populations along <strong>the</strong> sou<strong>the</strong>rn margin <strong>of</strong> <strong>the</strong> North American glaciers. O<strong>the</strong>rs are well<br />
differentiated although clearly sister species, e.g. Agrypnia deflata (130) and A. obsoleta<br />
(134), which probably had a vicariant origin arising from an earlier intercontinental subdivision<br />
<strong>of</strong> <strong>the</strong> range <strong>of</strong> a common circumboreal ancestor. Flooding <strong>of</strong> <strong>the</strong> land connection<br />
between Asia and North America in <strong>the</strong> mid-Pliocene, some 3 million years ago, or earlier,<br />
<strong>of</strong>fers possible origins for dichotomies <strong>of</strong> this sort, although disjunctions between some<br />
conspecific North American and Asian populations could have been imposed by cold climate<br />
even before <strong>the</strong> land connection was overrun by <strong>the</strong> sea. At <strong>the</strong> far end <strong>of</strong> <strong>the</strong> scale for<br />
<strong>the</strong> age <strong>of</strong> trichopteran taxa inferred from morphological divergence is Sphagnophylax<br />
meiops (126) (Frontispiece, Fig. 28), known only from unglaciated Nearctic Beringia or<br />
marginally beyond, and which appears to have lost <strong>the</strong> power <strong>of</strong> flight. The monotypic genus<br />
Sphagnophylax is <strong>the</strong> only genus-level taxon known in Trichoptera that is confined to<br />
Beringia, and is clearly both a phylogenetic and a geographic relict (Wiggins and Winchester<br />
1984).<br />
Placing <strong>the</strong>se speciation events in geological time with any assurance is not yet possible<br />
for Trichoptera. Inferring <strong>the</strong> relative times <strong>of</strong> origin <strong>of</strong> species from levels <strong>of</strong> morphological<br />
divergence achieves some consistency in interpreting <strong>the</strong> history <strong>of</strong> a number <strong>of</strong> species <strong>of</strong><br />
disparate age, and is employed in this study because few o<strong>the</strong>r clues are available; but we
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 825<br />
recognize that if correct for some species, this morphological guideline may be illusory for<br />
o<strong>the</strong>rs. One indication that <strong>the</strong> species did not diverge at uniform rates is provided by<br />
members <strong>of</strong> category V; several <strong>of</strong> <strong>the</strong>se are widespread Holarctic species that must have<br />
passed between Asia and North America at some time before <strong>the</strong> onset <strong>of</strong> harsh Pleistocene<br />
climates because <strong>the</strong>re is little evidence that <strong>the</strong>y are adapted now to far nor<strong>the</strong>rn latitudes.<br />
Secondly, fossil evidence <strong>of</strong> known age is rarely available for species <strong>of</strong> Trichoptera, but in<br />
Coleoptera where it is available, extant species <strong>of</strong> <strong>the</strong> north were found to be unexpectedly<br />
old and clearly in existence well before Pleistocene time, with some identifiable in late<br />
Miocene 5.7 ma B.P. (e.g. Downes and Kavanaugh 1988). A very slow rate <strong>of</strong> evolutionary<br />
change in some Trichoptera is demonstrated by <strong>the</strong> small structural differences between a<br />
Baltic amber species <strong>of</strong> Lype (Psychomyiidae) and a Recent European species (Ross 1958,<br />
fig. 1); <strong>the</strong> age <strong>of</strong> <strong>the</strong> amber species is placed at upper Eocene—some 50 ma B.P. Perhaps<br />
this is an isolated and atypical example, but for insect species generally an age <strong>of</strong> 25 million<br />
years has been estimated (e.g. Briggs 1966). Comprehensive analysis <strong>of</strong> <strong>the</strong> fossil record<br />
reveals that insects show very low rates <strong>of</strong> extinction; among vertebrates, by contrast, few<br />
living species are more than a million years old (Labandeira and Sepkoski 1993). The age<br />
<strong>of</strong> species and <strong>the</strong> time required for <strong>the</strong>ir origin are different concepts; but inferences<br />
reflecting <strong>the</strong> rate <strong>of</strong> evolution for insect species must be tempered by <strong>the</strong> evidence that at<br />
least 760 species <strong>of</strong> Drosophilidae have evolved on <strong>the</strong> Hawaiian Islands since <strong>the</strong>ir origins<br />
6 million to 500 000 years ago (Kaneshiro 1993).<br />
Finally, <strong>the</strong>re is <strong>the</strong> larger question <strong>of</strong> <strong>the</strong> age <strong>of</strong> <strong>the</strong> cold, far nor<strong>the</strong>rn biomes<br />
<strong>the</strong>mselves. Tundra develops in regions that are too cold for <strong>the</strong> growth <strong>of</strong> trees, and arctic<br />
tundra is believed to have arisen in <strong>the</strong> north as disjunct areas <strong>of</strong> ecological regression in late<br />
Miocene forests (Mat<strong>the</strong>ws 1979b; Danks 1981). The Seward Peninsula <strong>of</strong> Alaska, just<br />
below <strong>the</strong> Arctic Circle (67°N lat.), was forested up to about 5.7 million years ago in late<br />
Miocene time (Hopkins et al. 1971). Palaeobotanical evidence indicates that <strong>the</strong> forest-tundra<br />
ecotone probably lay just to <strong>the</strong> north <strong>of</strong> <strong>the</strong> Seward Peninsula at that time, suggesting that<br />
tundra probably covered most <strong>of</strong> <strong>the</strong> North American Arctic Archipelago by 6 ma B.P.<br />
(Mat<strong>the</strong>ws 1979b). Thus, tundra may be a relatively young biome in <strong>the</strong> north, and adaptation<br />
<strong>of</strong> tundra species a ra<strong>the</strong>r recent phenomenon. Some arctic tundra Trichoptera may have been<br />
derived from alpine tundra habitats, which in Beringia would have dated from <strong>the</strong> time that<br />
<strong>the</strong> high mountains <strong>of</strong> <strong>the</strong> western Cordillera and <strong>of</strong> nor<strong>the</strong>astern Asia reached elevations<br />
which precluded <strong>the</strong> growth <strong>of</strong> trees. Evidence from fossil insects suggests, however, that<br />
species may have adapted to arctic tundra conditions from lineages occurring in <strong>the</strong> boreal<br />
forest (Mat<strong>the</strong>ws 1979a; Danks 1981). Because <strong>of</strong> <strong>the</strong>ir diversity and broad ecological<br />
penetration, Trichoptera could be an appropriate group for testing <strong>the</strong>se hypo<strong>the</strong>ses. Phylogenetic<br />
analysis in <strong>the</strong> highly diverse boreomontane genus Limnephilus would be a promising<br />
avenue <strong>of</strong> investigation to reveal <strong>the</strong> habitats <strong>of</strong> <strong>the</strong> phyletic ancestors <strong>of</strong> <strong>the</strong> present<br />
inhabitants <strong>of</strong> arctic and alpine tundra. However, a good deal more basic information about<br />
<strong>the</strong> biology, distribution, taxonomy, and phylogenetic relationships <strong>of</strong> <strong>the</strong> species <strong>of</strong> Limnephilus<br />
will be required. Trichoptera established in pre-Pleistocene arctic tundra might have<br />
found glacial refuge in alpine tundra <strong>of</strong> accessible mountain ranges, or in disjunct lowland<br />
tundra refugia along <strong>the</strong> sou<strong>the</strong>rn margin <strong>of</strong> <strong>the</strong> continental glacier.<br />
I. Nearctic Species Widespread in North America<br />
This is <strong>the</strong> largest category <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> Trichoptera—98 species comprising 68 per<br />
cent <strong>of</strong> <strong>the</strong> total fauna. None <strong>of</strong> <strong>the</strong>se species is known from Europe or Asia, and <strong>the</strong>ir<br />
generally widespread occurrence now at more sou<strong>the</strong>rly latitudes in North America suggests
826 G.B. Wiggins and C.R. Parker<br />
that <strong>the</strong>y passed <strong>the</strong> last glaciation south <strong>of</strong> <strong>the</strong> ice or in o<strong>the</strong>r refugia under climatic<br />
conditions less severe than those in Beringia, and returned to nor<strong>the</strong>rn latitudes as <strong>the</strong> glaciers<br />
receded. The possibility cannot be excluded that some <strong>of</strong> <strong>the</strong>se species could have passed<br />
<strong>the</strong> last glacial period in East Beringia, failed to cross <strong>the</strong> Bering land bridge to Asia, and<br />
dispersed southward after recession <strong>of</strong> <strong>the</strong> glaciers. Most <strong>of</strong> <strong>the</strong>se strictly Nearctic caddisfly<br />
species which now occur in <strong>the</strong> <strong>Yukon</strong> probably originated before <strong>the</strong> glaciation <strong>of</strong> <strong>the</strong> past<br />
2 million years, although some may be <strong>the</strong> product <strong>of</strong> isolation in separate refugial areas to<br />
<strong>the</strong> south <strong>of</strong> <strong>the</strong> glaciers, e.g. <strong>the</strong> dichotomy between Mystacides alafimbriata and M. sepulchralis.<br />
Numbers in paren<strong>the</strong>ses following <strong>the</strong> species names provide a cross-reference to<br />
distributional and o<strong>the</strong>r data in <strong>the</strong> Annotated List <strong>of</strong> <strong>Yukon</strong> Trichoptera.<br />
Spicipalpia<br />
Glossosomatidae<br />
Glossosoma alascense Banks (1)<br />
Glossosoma verdona Ross (3)<br />
Hydroptilidae<br />
Hydroptila rono Ross (5)<br />
Ochrotrichia sp. (6)<br />
Oxyethira araya Ross (7)<br />
Stactobiella delira (Ross) (8)<br />
Rhyacophilidae<br />
Rhyacophila alberta Banks (9)<br />
Rhyacophila angelita Banks (10)<br />
Rhyacophila bifila Banks (11)<br />
Rhyacophila brunnea Banks (12)<br />
Rhyacophila hyalinata Banks (13)<br />
Rhyacophila pellisa Ross (16)<br />
Rhyacophila tucula Ross (17)<br />
Rhyacophila vao Milne (18)<br />
Rhyacophila verrula Milne (19)<br />
Rhyacophila vobara Milne (20)<br />
Rhyacophila vocala Milne (21)<br />
Rhyacophila v<strong>of</strong>ixa Milne (22)<br />
Annulipalpia<br />
Hydropsychidae<br />
Arctopsyche grandis (Banks) (23)<br />
Cheumatopsyche campyla Ross (25)<br />
Cheumatopsyche sp. (26)<br />
Hydropsyche alhedra Ross (27)<br />
Hydropsyche alternans (Walker) (28)<br />
Hydropsyche amblis Ross (29)<br />
Hydropsyche cockerelli Banks (30)<br />
Hydropsyche oslari Banks (31)<br />
Parapsyche elsis Milne (32)<br />
Philopotamidae<br />
Wormaldia gabriella (Banks) (33)<br />
Polycentropodidae<br />
Polycentropus aureolus (Banks) (35)<br />
Polycentropus flavus (Banks) (36)<br />
Polycentropus remotus Banks (37)<br />
Polycentropus smithae Denning (38)<br />
Polycentropus weedi Blickle and Morse (39)<br />
Integripalpia<br />
Apataniidae<br />
Allomyia sp. (40)<br />
Brachycentridae<br />
Micrasema bactro Ross (46)<br />
Lepidostomatidae<br />
Lepidostoma cascadense (Milne) (48)<br />
Lepidostoma cinereum Banks (49)<br />
Lepidostoma pluviale (Milne) (50)<br />
Lepidostoma roafi (Milne) (51)<br />
Lepidostoma stigma Banks (52)<br />
Lepidostoma unicolor (Banks) (53)<br />
Leptoceridae<br />
Ceraclea cancellata (Betten) (55)<br />
Ceraclea resurgens (Walker) (57)<br />
Mystacides alafimbriata Hill-Griffin (58)<br />
Mystacides interjectus (Banks) (59)<br />
Mystacides sepulchralis (Walker) (60)<br />
Oecetis immobilis (Hagen) (61)<br />
Oecetis inconspicua (Walker) (62)<br />
Triaenodes baris (Ross) (64)<br />
Triaenodes tardus Milne (65)<br />
Ylodes frontalis (Banks) (66)<br />
Limnephilidae<br />
Anabolia bimaculata (Walker) (70)<br />
Arctopora pulchella (Banks) (71)<br />
Asynarchus aldinus (Ross) (73)<br />
Asynarchus montanus Banks (76)<br />
Asynarchus mutatus (Hagen) (77)<br />
Chyranda centralis (Banks) (78)<br />
Clistoronia magnifica (Banks) (79)<br />
Dicosmoecus atripes (Hagen) (80)<br />
Ecclisomyia conspersa Banks (82)<br />
Glyphopsyche irrorata (Fabricius) (83)<br />
Grammotaulius interrogationis<br />
(Zetterstedt) (85)<br />
Hesperophylax designatus (Walker) (88)<br />
Lenarchus crassus (Banks) (89)<br />
Lenarchus fautini (Denning) (91)<br />
Lenarchus keratus Ross (92)<br />
Lenarchus vastus (Hagen) (93)<br />
Limnephilus argenteus Banks (94)<br />
Limnephilus canadensis Banks (95)
Limnephilus extractus Walker (99)<br />
Limnephilus hageni Banks (103)<br />
Limnephilus hyalinus Hagen (104)<br />
Limnephilus infernalis (Banks) (105)<br />
Limnephilus janus Ross (106)<br />
Limnephilus kennicotti (Banks) (107)<br />
Limnephilus partitus Walker (110)<br />
Limnephilus parvulus (Banks) (111)<br />
Limnephilus perpusillus Walker (112)<br />
Limnephilus sansoni Banks (115)<br />
Limnephilus secludens Banks (116)<br />
Limnephilus sublunatus Provancher (119)<br />
Limnephilus tarsalis (Banks) (120)<br />
Nemotaulius hostilis (Hagen) (121)<br />
Philarctus quaeris (Milne) (123)<br />
Psychoglypha alascensis (Banks) (124)<br />
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 827<br />
Psychoglypha subborealis (Banks) (125)<br />
Phryganeidae<br />
Agrypnia deflata (Milne) (130)<br />
Agrypnia glacialis Hagen (131)<br />
Agrypnia improba (Hagen) (132)<br />
Agrypnia macdunnoughi (Milne) (133)<br />
Agrypnia straminea Hagen (137)<br />
Banksiola crotchi Banks (138)<br />
Phryganea cinerea Walker (140)<br />
Ptilostomis semifasciata (Say) (141)<br />
Uenoidae<br />
Neothremma didactyla Ross (142)<br />
Oligophlebodes ruthae Ross (143)<br />
Oligophlebodes sierra Ross (144)<br />
Oligophlebodes zelti Nimmo (145)<br />
These Nearctic Trichoptera from glacial refuges to <strong>the</strong> south are <strong>of</strong> 2 broad groups—<br />
western montane species and transcontinental species. The western montane species are<br />
mainly members <strong>of</strong> <strong>the</strong> Spicipalpia and Annulipalpia whose larvae live in lotic habitats<br />
characteristic <strong>of</strong> mountainous terrain. This group is particularly diverse southward through<br />
Oregon to California and Colorado, but markedly less successful to <strong>the</strong> north (Table 1<br />
below): Glossosomatidae, Rhyacophilidae, Hydropsychidae, Philopotamidae, Polycentropodidae,<br />
Brachycentridae (Micrasema), Lepidostomatidae, Limnephilidae (Chyranda,<br />
Discosmoecus, Ecclisomyia, Psychoglypha), and Uenoidae. Figures for declining species<br />
diversity at higher latitudes (Table 1) indicate that <strong>the</strong> lotic species <strong>of</strong> <strong>the</strong> western montane<br />
Trichoptera have populated <strong>the</strong> glaciated terrain <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> with limited success. Lentic<br />
species <strong>of</strong> western montane origin in a few genera <strong>of</strong> <strong>the</strong> Limnephilidae (Asynarchus,<br />
Clistoronia, Lenarchus, Limnephilus) and <strong>the</strong> Leptoceridae (Mystacides, Ylodes) have<br />
extended far<strong>the</strong>r north in <strong>the</strong> <strong>Yukon</strong> than have <strong>the</strong> lotic species <strong>of</strong> montane origin.<br />
Transcontinental species are inferred to have passed glaciation along a much wider ice<br />
front because overall <strong>the</strong>y are now widely distributed in North America. They are, for <strong>the</strong><br />
most part, species <strong>of</strong> lentic waters and slowly moving streams. These species are primarily<br />
case-makers <strong>of</strong> <strong>the</strong> Integripalpia; <strong>the</strong>ir diversity also declines with increasing latitude<br />
(Table 1), but not as much as in <strong>the</strong> Spicipalpia and Annulipalpia where most species live<br />
in lotic waters. Transcontinental Trichoptera occur to a large extent across <strong>the</strong> boreal forest<br />
biome but <strong>the</strong>ir lake and marsh habitats also extend through deciduous forests and grasslands.<br />
Larvae <strong>of</strong> <strong>the</strong>se species, and <strong>of</strong> Trichoptera generally, have little or no specific relationship<br />
with vascular plants, and feed for <strong>the</strong> most part on <strong>the</strong> fungi colonizing plant detritus and on<br />
algae, or on o<strong>the</strong>r insects. In general, <strong>the</strong> effect <strong>of</strong> low temperature on aquatic habitats seems<br />
to be <strong>the</strong> major constraint to repopulation <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> by species in both groups <strong>of</strong> Category<br />
I Trichoptera; this topic is explored fur<strong>the</strong>r under Ecological Considerations.<br />
There is an interesting recurrence in nor<strong>the</strong>rn Quebec <strong>of</strong> disjunct populations <strong>of</strong> several<br />
species which o<strong>the</strong>rwise appear to be confined to western or northwestern North America<br />
(Roy and Harper 1979; Harper 1989): Arctopsyche grandis (23) (Hydropsychidae), Wormaldia<br />
gabriella (33) (Philopotamidae), and Goera tungusensis (47) (Goeridae). The first<br />
2 are Nearctic species (category I) which probably passed <strong>the</strong> Wisconsinan glaciation south<br />
<strong>of</strong> <strong>the</strong> ice, and now range broadly in western montane areas from <strong>the</strong> <strong>Yukon</strong> southward.<br />
Goera tungusensis is Holarctic (category II) and may also have passed <strong>the</strong> last glacial period
828 G.B. Wiggins and C.R. Parker<br />
along <strong>the</strong> sou<strong>the</strong>rn margin <strong>of</strong> <strong>the</strong> ice, or it may have entered North America for <strong>the</strong> first time<br />
during <strong>the</strong> Pleistocene. This shared pattern <strong>of</strong> postglacial distribution might indicate that<br />
each <strong>of</strong> <strong>the</strong>se species passed <strong>the</strong> Wisconsinan glaciation as disjunct eastern populations;<br />
larvae <strong>of</strong> each <strong>of</strong> <strong>the</strong>m usually occur in cool lotic habitats.<br />
Greenland Trichoptera. Species <strong>of</strong> Trichoptera occurring in both North America and<br />
Greenland pose relevant distributional questions arising from glaciation because most <strong>of</strong><br />
<strong>the</strong>m occur in <strong>the</strong> <strong>Yukon</strong> as well. Some are Holarctic species, and some are strictly Nearctic,<br />
but <strong>the</strong> fact that all <strong>of</strong> <strong>the</strong>m occur in Greenland raises <strong>the</strong> possibility that <strong>the</strong>y illustrate some<br />
general principles <strong>of</strong> high-latitude distribution. Since <strong>the</strong> insect fauna <strong>of</strong> Greenland includes<br />
few if any endemic species, and was derived to a large extent by postglacial dispersal from<br />
North America (Downes 1966), we infer that <strong>the</strong>se Nearctic species <strong>of</strong> Trichoptera reached<br />
Greenland after recession <strong>of</strong> <strong>the</strong> glaciers. Occurrence <strong>of</strong> a Palaearctic species, Limnephilus<br />
griseus (Linnaeus), in Greenland (Fristrup 1942) raises a fur<strong>the</strong>r possibility that some <strong>of</strong><br />
<strong>the</strong> Holarctic species might have reached Greenland from Europe; records for a second<br />
Palaearctic limnephilid from Greenland, Halesus radiatus (Curt.), are believed to be in error<br />
(N.P. Kristensen, pers. comm.). Ice rafting from western Norway has been postulated as a<br />
sweepstakes route for <strong>the</strong> repopulation <strong>of</strong> Iceland and o<strong>the</strong>r North Atlantic islands by insects<br />
about 10 000 years ago (Buckland et al. 1986). Although adult Trichoptera are generally<br />
short-lived, an adult diapause in species <strong>of</strong> Limnephilus adapted to transient waters could<br />
prolong <strong>the</strong> life span, perhaps enabling <strong>the</strong>se species to endure such a passage; <strong>the</strong>ir egg<br />
masses embedded in gelatinous matrix are resistant to desiccation (Wiggins 1973). All <strong>of</strong><br />
<strong>the</strong> Greenland Trichoptera are case-making species <strong>of</strong> <strong>the</strong> Integripalpia whose larvae live in<br />
lentic waters or in slow currents <strong>of</strong> streams.<br />
Common patterns in <strong>the</strong> far nor<strong>the</strong>rn distribution <strong>of</strong> Lepidoptera and <strong>of</strong> several o<strong>the</strong>r<br />
groups <strong>of</strong> insects reveal 2 elements in <strong>the</strong> fauna <strong>of</strong> Greenland (Downes 1966). Species <strong>of</strong><br />
<strong>the</strong> high arctic occurring on adjacent Ellesmere Island are very largely shared with nor<strong>the</strong>rn<br />
Greenland. In <strong>the</strong> Trichoptera, only 2 species <strong>of</strong> <strong>the</strong> Greenland fauna can be considered<br />
high-arctic forms—Apatania zonella (43) and Grensia praeterita (87). The first occurs on<br />
Ellesmere Island (Corbet 1966) where it is <strong>the</strong> most nor<strong>the</strong>rly species <strong>of</strong> Trichoptera known<br />
in North America, although it is an Holarctic species and is recorded also from <strong>Yukon</strong> to<br />
Quebec; Grensia praeterita is a circumpolar species recorded mainly above treeline although<br />
not from Ellesmere Island. The high-arctic species are held to have reached Greenland by<br />
way <strong>of</strong> a far nor<strong>the</strong>rn route across <strong>the</strong> Arctic Archipelago (Downes 1966), raising <strong>the</strong><br />
possibility that <strong>the</strong>y could have passed <strong>the</strong> Wisconsinan glaciation in a Peary Land refugium<br />
along <strong>the</strong> nor<strong>the</strong>rn edge <strong>of</strong> Greenland (e.g. Danks 1981, fig. 5). The relatively small number<br />
<strong>of</strong> high-arctic Trichoptera indicates that <strong>the</strong>se insects are not well adapted to <strong>the</strong> rigours <strong>of</strong><br />
existence at high latitudes.<br />
The second element in <strong>the</strong> Greenland insect fauna comprises species <strong>of</strong> more sou<strong>the</strong>rly<br />
distribution that are <strong>of</strong> low-arctic or boreal origin (Downes 1966). Most <strong>of</strong> <strong>the</strong> Greenland<br />
Trichoptera appear to be <strong>of</strong> this group because <strong>the</strong>y range widely across <strong>the</strong> boreal forest<br />
and <strong>of</strong>ten far<strong>the</strong>r to <strong>the</strong> south. These species are likely to be postglacial dispersants to<br />
Greenland, although probably by way <strong>of</strong> <strong>the</strong> Arctic Archipelago and Baffin Island. It appears<br />
that most <strong>of</strong> <strong>the</strong>m moved into deglaciated North America from refuges in <strong>the</strong> central part <strong>of</strong><br />
<strong>the</strong> continent, finally reaching Greenland.<br />
Evidence relevant to category I species in Greenland is outlined below, and for o<strong>the</strong>r<br />
species under <strong>the</strong> category indicated; 2 <strong>of</strong> <strong>the</strong> Nearctic species known from Greenland have<br />
not been recorded from <strong>the</strong> <strong>Yukon</strong> (†).
Apataniidae<br />
Apatania zonella (Zetterstedt) (II, 43)<br />
Limnephilidae<br />
Grammotaulius interrogationis (Zetterstedt) (I, 85)<br />
The type locality <strong>of</strong> this transcontinental Nearctic species is in Greenland (Mosely 1929;<br />
Schmid 1950a). Genitalic structures <strong>of</strong> males showed marked variation both within and<br />
between populations, but we found no congruent pattern. Schmid (1950a) considered<br />
G. interrogationis to be <strong>the</strong> sister species <strong>of</strong> 3 o<strong>the</strong>rs (2 Palaearctic, 1 Nearctic).<br />
Grensia praeterita (Walker) (III, 87)<br />
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 829<br />
Limnephilus femoralis Kirby (II, 100); L. picturatus McLachlan (II, 113); L. rhombicus<br />
(Linnaeus) (II, 114)<br />
These 3 widely distributed Holarctic species could have reached Greenland from North<br />
America, or from Europe as did <strong>the</strong> Palaearctic species L. griseus. In <strong>Yukon</strong> and o<strong>the</strong>r<br />
Nearctic material <strong>of</strong> L. rhombicus <strong>the</strong>re is substantial variation in both male and female<br />
genitalic structure, but we found no geographic correlation to indicate separation <strong>of</strong> populations<br />
by glacial barriers. Congruent variation found in L. picturatus was analyzed (II).<br />
Limnephilius kennicotti Banks (I, 107)<br />
This Nearctic species is discussed under L. fenestratus (III), its very similar sister<br />
species.<br />
Limnephilus moestus Banks (†)<br />
The range <strong>of</strong> this Nearctic species extends from <strong>the</strong> Northwest Territories to Colorado,<br />
east to Newfoundland and West Virginia, and to Greenland (Forsslund 1932). It has not been<br />
recorded from <strong>the</strong> <strong>Yukon</strong> or Alaska. The species was assigned to <strong>the</strong> sitchensis group <strong>of</strong> 6<br />
Nearctic species (Schmid 1955). Few species <strong>of</strong> Nearctic Trichoptera have <strong>the</strong> broad<br />
latitudinal range from West Virginia to Greenland shared by L. moestus and <strong>the</strong> following<br />
species L. ornatus.<br />
Limnephilus ornatus Banks (†)<br />
This species is known from Alaska [Naknek (CNCI); Parks Hwy. (ROME 821118);<br />
Kodiak Is. (ROME); Pop<strong>of</strong> Is. (Banks 1900)], and from Alberta and Montana to Newfoundland,<br />
south to West Virginia. Although recorded from Alaska, L. ornatus is not known from<br />
<strong>the</strong> <strong>Yukon</strong> or from British Columbia (Nimmo and Scudder 1978, 1983), nor is it known<br />
elsewhere in <strong>the</strong> western montane states; this is a large gap for a conspicuous species and<br />
suggests a disjunct population in Alaska. The species has been recorded from Greenland<br />
(Mosely 1932), but not from nor<strong>the</strong>rn Asia; a record from Japan (Ulmer 1907), never<br />
confirmed, was rejected by Schmid (1965a). If L. ornatus passed <strong>the</strong> last glacial period in<br />
<strong>the</strong> Beringian refugium, <strong>the</strong>re is no evidence that it spread to Asia. No close relatives <strong>of</strong><br />
L. ornatus were indicated in <strong>the</strong> classification by Schmid (1955).<br />
Phryganeidae<br />
Agrypnia glacialis Hagen (I, 131)<br />
This transcontinental Nearctic species is <strong>the</strong> sister <strong>of</strong> <strong>the</strong> Palaearctic A. picta Kolenati,<br />
widely distributed through nor<strong>the</strong>rn Europe and Asia to Kamchatka. There is no evidence<br />
that ei<strong>the</strong>r species has moved across <strong>the</strong> Bering land bridge, in contrast to <strong>the</strong> congeneric<br />
species pair A. deflata and obsoleta (see category III). However, <strong>the</strong>re are records for<br />
A. glacialis from Greenland, and <strong>the</strong> Eurasian sister species A. picta occurs as far west as
830 G.B. Wiggins and C.R. Parker<br />
Britain and Iceland. We infer that this evidence indicates vicariant speciation from a<br />
circumboreal ancestor. Outlying records <strong>of</strong> A. glacialis in Idaho, Utah and California suggest<br />
relict montane populations from <strong>the</strong> time when <strong>the</strong> species occurred south <strong>of</strong> <strong>the</strong> Pleistocene<br />
glacial ice. In <strong>the</strong>se populations, and also in <strong>the</strong> Greenland material, variations in male<br />
genitalic structure are more common than in <strong>the</strong> populations <strong>of</strong> nor<strong>the</strong>rn North America<br />
(Wiggins in press).<br />
II. Holarctic Species Widespread in North America<br />
Each <strong>of</strong> <strong>the</strong>se species is now widely distributed in both North America and Eurasia; 28<br />
<strong>of</strong> <strong>the</strong>m are known in <strong>the</strong> <strong>Yukon</strong>, constituting about 18 per cent <strong>of</strong> <strong>the</strong> Trichoptera. Many<br />
are now transcontinental, and <strong>the</strong>se broad Nearctic ranges seem more likely to follow from<br />
glacial refuge along <strong>the</strong> sou<strong>the</strong>rn margin <strong>of</strong> <strong>the</strong> ice. Thus, a number <strong>of</strong> <strong>the</strong>se Holarctic species<br />
probably occurred in North America before <strong>the</strong> Pleistocene, and probably have been on this<br />
continent for at least <strong>the</strong> past 3 million years, before <strong>the</strong> connection between Asia and North<br />
America was overrun by <strong>the</strong> sea in mid-Pliocene time. However, southward movement <strong>of</strong><br />
any <strong>of</strong> <strong>the</strong>se species from Beringia through <strong>the</strong> ice-free corridor between <strong>the</strong> Laurentide and<br />
Cordilleran glaciers might have occurred during interglacial periods <strong>of</strong> <strong>the</strong> Pleistocene (see<br />
<strong>Biological</strong> Aspects), and is difficult to detect. For some category II species, sister relationships<br />
between Nearctic and Palaearctic species pairs suggest origin from common circumboreal<br />
ancestors, and perhaps arose from intercontinental vicariance in <strong>the</strong> Pliocene or<br />
earlier. Evidence indicates that for <strong>the</strong> most part species involved in recent dispersal moved<br />
from Asia to North America (see Biogeographic Analysis). A few species, e.g. Brachycentrus<br />
americanus and Onocosmoecus unicolor, appear to have originated in North America<br />
and dispersed to Asia across <strong>the</strong> Bering land bridge.<br />
Evidence from intraspecific morphological variation suggests that some Nearctic species<br />
were divided, with populations confined to <strong>the</strong> south <strong>of</strong> <strong>the</strong> advancing glacial front while<br />
disjunct conspecific populations were restricted to Beringia: e.g. Brachycentrus americanus<br />
(44), Micrasema gelidum (45), Oecetis ochracea (63), Ylodes reuteri (68), Limnephilus<br />
picturatus (113), Onocosmoecus unicolor (122), and Agrypnia colorata (129). For <strong>the</strong><br />
remaining species <strong>of</strong> category II, evidence available now is inadequate to support any<br />
inference about <strong>the</strong>ir origin and direction <strong>of</strong> dispersal.<br />
Although several <strong>of</strong> <strong>the</strong>se species now occur in arctic tundra habitats, most <strong>of</strong> <strong>the</strong>m are<br />
also widely distributed in forest and grassland biomes at more temperate latitudes. Consequently,<br />
<strong>the</strong>se species do not comply with <strong>the</strong> general pattern in some o<strong>the</strong>r groups that <strong>the</strong><br />
arctic and treeline species were derived from Beringia, while <strong>the</strong> boreal and subarctic fauna<br />
moved northward from refugia below <strong>the</strong> glacial front. The most nor<strong>the</strong>rly species <strong>of</strong><br />
category II are Asynarchus iteratus (74) and Apatania zonella (43).<br />
Two species (†) known from Alaska or <strong>the</strong> Northwest Territories are also considered<br />
under this category; although not yet recorded from <strong>the</strong> <strong>Yukon</strong>, <strong>the</strong>y probably occur <strong>the</strong>re.<br />
Spicipalpia<br />
Glossosomatidae<br />
Glossosoma intermedium (Klapalek)<br />
Hydroptilidae<br />
Oxyethira ecornuta Morton (†)<br />
Rhyacophilidae<br />
Rhyacophila narvae Navas<br />
Annulipalpia<br />
Hydropsychidae<br />
Arctopsyche ladogensis (Kolenati)<br />
Polycentropodidae<br />
Neureclipsis bimaculata McLachlan
Integripalpia<br />
Apataniidae<br />
Apatania crymophila McLachlan<br />
Apatania stigmatella (Zetterstedt)<br />
Apatania zonella (Zetterstedt)<br />
Brachycentridae<br />
Brachycentrus americanus (Banks)<br />
Micrasema gelidum McLachlan<br />
Goeridae<br />
Goera tungusensis Martynov<br />
Leptoceridae<br />
Ceraclea annulicornis (Stephens)<br />
Ceraclea excisa (Morton) (†)<br />
Ceraclea nigronervosa (Retzius)<br />
Oecetis ochracea (Curtis)<br />
Ylodes reuteri (McLachlan)<br />
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 831<br />
Limnephilidae<br />
Asynarchus iteratus McLachlan<br />
Asynarchus lapponicus (Zetterstedt)<br />
Limnephilus dispar McLachlan<br />
Limnephilus externus Hagen<br />
Limnephilus femoralis Kirby<br />
Limnephilus nigriceps (Zetterstedt)<br />
Limnephilus picturatus McLachlan<br />
Limnephilus rhombicus (Linnaeus)<br />
Limnephilus sericeus (Say)<br />
Onocosmoecus unicolor (Banks)<br />
Molannidae<br />
Molanna flavicornis Banks<br />
Molannodes tinctus Zetterstedt<br />
Phryganeidae<br />
Agrypnia colorata Hagen<br />
Agrypnia pagetana Curtis<br />
Glossosomatidae<br />
Glossosoma intermedium (Klapalek) (2)<br />
This is <strong>the</strong> only Holarctic species <strong>of</strong> Glossosoma, and <strong>the</strong> only transcontinental species<br />
in North America. According to Ross (1956, charts 31, 43), <strong>the</strong> sister species <strong>of</strong> G. intermedium<br />
is G. verdona Ross; he inferred that <strong>the</strong> two originated in North America and<br />
intermedium spread to Eurasia by way <strong>of</strong> <strong>the</strong> Bering land bridge. If this dispersal occurred<br />
during <strong>the</strong> Pleistocene, G. intermedium would be one <strong>of</strong> <strong>the</strong> few species that moved from<br />
North America to Asia. However, by this interpretation, some populations <strong>of</strong> G. intermedium<br />
probably would have been confined south <strong>of</strong> <strong>the</strong> ice, accounting for <strong>the</strong> present transcontinental<br />
distribution. This sister-group relationship opens a second possibility that G. intermedium<br />
is <strong>the</strong> Eurasian vicariant from a common Holarctic ancestor; this species might <strong>the</strong>n<br />
have reached North America by way <strong>of</strong> <strong>the</strong> Bering land bridge. Occurrence <strong>of</strong> G. intermedium<br />
in <strong>the</strong> nor<strong>the</strong>rn <strong>Yukon</strong> (Fig. 1, region 5) indicates its adaptation to far nor<strong>the</strong>rn<br />
conditions; and if G. intermedium reached North America during <strong>the</strong> Pleistocene glaciation,<br />
its present transcontinental distribution would have been achieved after glacial recession. In<br />
any event, occurrence <strong>of</strong> <strong>the</strong> 2 sister species in <strong>the</strong> <strong>Yukon</strong> invites ecological comparison (e.g.<br />
Irons 1988), leading perhaps to some understanding <strong>of</strong> factors underlying <strong>the</strong> wide distribution<br />
<strong>of</strong> G. intermedium. Ross considered <strong>the</strong> subgenus G. (Synafophora = Eomystra), to which<br />
G. intermedium belongs, to be <strong>the</strong> biological analogue <strong>of</strong> <strong>the</strong> sibirica group <strong>of</strong> Rhyacophila—species<br />
<strong>of</strong> both groups tolerant <strong>of</strong> low gradient streams and sharing <strong>the</strong> dispersal<br />
capability <strong>of</strong> ecological generalists which enabled some to reach eastern North America.<br />
Hydroptilidae<br />
Oxyethira ecornuta Morton (†)<br />
One <strong>of</strong> <strong>the</strong> few Holarctic species known in this family, O. ecornuta occurs through<br />
nor<strong>the</strong>rn Europe (BotojAneanu and Malicky 1978) to <strong>the</strong> Far East <strong>of</strong> Russia (BotojAneanu<br />
and Levanidova 1988); our collections include specimens from Alaska, and although not yet<br />
recorded from <strong>the</strong> <strong>Yukon</strong>, this species probably occurs <strong>the</strong>re. There is material from Ontario<br />
in <strong>the</strong> ROME collection, suggesting a disjunct distribution and <strong>the</strong> possibility that this<br />
species at least in part passed <strong>the</strong> last glaciation south <strong>of</strong> <strong>the</strong> continental glacier. The species<br />
was placed in <strong>the</strong> flavicornis group by Kelley (1984), and was believed to be <strong>the</strong> sister species<br />
<strong>of</strong> O. flavicornis (Pictet) from Europe; both are said to occur in lentic habitats.
832 G.B. Wiggins and C.R. Parker<br />
Rhyacophilidae<br />
Rhyacophila narvae Navas (15)<br />
This species is a member <strong>of</strong> <strong>the</strong> sibirica group, and was proposed as <strong>the</strong> sister taxon <strong>of</strong><br />
R. transquilla Tsuda from Japan (Ross 1956; Schmid 1970). Origin <strong>of</strong> Rhyacophila narvae<br />
in Asia was proposed by <strong>the</strong>se authors. If dispersal to North America occurred during <strong>the</strong><br />
Pleistocene, river systems <strong>of</strong> <strong>the</strong> Bering land bridge must have provided larval habitats <strong>of</strong><br />
cool, fast-flowing water required by <strong>the</strong> larvae (Lepneva 1964). If dispersal <strong>of</strong> R. narvae to<br />
North America occurred before <strong>the</strong> Pliocene separation <strong>of</strong> <strong>the</strong> 2 continents, this Holarctic<br />
species has resisted <strong>the</strong> vicariant subdivision postulated for some o<strong>the</strong>rs.<br />
Hydropsychidae<br />
Arctopsyche ladogensis (Kolenati) (24)<br />
This is <strong>the</strong> only Holarctic species in <strong>the</strong> genus and, <strong>of</strong> 4 species <strong>of</strong> Arctopsyche in North<br />
America, it is <strong>the</strong> only one with a transcontinental distribution. It is <strong>the</strong> sister species <strong>of</strong><br />
A. amurensis Martynov from <strong>the</strong> Amur region <strong>of</strong> Russia (Schmid 1968). If <strong>the</strong> 2 species<br />
originated in Asia, A. ladogensis could have reached North America during <strong>the</strong> Pleistocene<br />
or before by way <strong>of</strong> <strong>the</strong> Bering land bridge. However, vicariant speciation <strong>of</strong> a circumboreal<br />
ancestor following separation <strong>of</strong> North America and Asia, perhaps in <strong>the</strong> Pliocene, is also a<br />
possible explanation for <strong>the</strong> sister-group relationship. In this interpretation, A. ladogensis<br />
would be a Nearctic species confined at least in part to <strong>the</strong> Beringian refugium, and dispersing<br />
to Asia across <strong>the</strong> land bridge; a disjunct population in Utah (Baumann and Unzicker 1981)<br />
is consistent with Nearctic origin <strong>of</strong> A. ladogensis and its occurrence both north and south<br />
<strong>of</strong> <strong>the</strong> ice. We found no consistent morphological differences between Nearctic and<br />
Palaearctic representatives, but have not examined specimens from Utah. Larvae live in<br />
strong currents <strong>of</strong> rivers and larger streams (Lepneva 1964), and occurrence <strong>of</strong> this species<br />
in <strong>the</strong> far north <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> (Fig. 1, region 4) indicates that it is suited for trans-Beringian<br />
dispersal.<br />
Polycentropodidae<br />
Neureclipsis bimaculata (Linnaeus) (34)<br />
This is <strong>the</strong> only Holarctic species <strong>of</strong> Neureclipsis, and <strong>the</strong> only transcontinental species<br />
in North America. There are morphological differences between Nearctic and Palaearctic<br />
specimens which could reflect distinctions at <strong>the</strong> level <strong>of</strong> populations or even species, but<br />
<strong>the</strong> general background variation has yet to be assessed over broad geographic areas. These<br />
differences suggest <strong>the</strong> possibility <strong>of</strong> vicariant subdivision <strong>of</strong> a circumboreal ancestor when<br />
North America and Asia were separated during <strong>the</strong> Pliocene. Transcontinental distribution<br />
across central North America suggests that, at least in part, this species occurred to <strong>the</strong> south<br />
<strong>of</strong> <strong>the</strong> ice during glaciation.<br />
Apataniidae<br />
Apatania crymophila McLachlan (41); A. stigmatella (Zetterstedt) (42); A. zonella (Zetterstedt)<br />
(43)<br />
These are <strong>the</strong> only Holarctic species known in Apatania, and all 3 could have passed<br />
<strong>the</strong> last glaciation in Beringia. However, transcontinental penetration <strong>of</strong> North America<br />
seems likely to have been achieved through postglacial dispersal from refugial areas south<br />
<strong>of</strong> <strong>the</strong> ice mass, implying that at least A. stigmatella and zonella occurred in North America<br />
before <strong>the</strong> Pleistocene, and thus probably before <strong>the</strong> Pliocene separation between North<br />
America and Asia. Apatania crymophila was proposed as <strong>the</strong> sister species <strong>of</strong> <strong>the</strong> European<br />
A. wallengreni McLachlan, and A. stigmatella <strong>the</strong> sister species <strong>of</strong> A. shoshone Banks in
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 833<br />
western North America (Schmid 1954a). Apatania zonella shows ra<strong>the</strong>r distant relationship<br />
with o<strong>the</strong>r species (Schmid 1954a), and is par<strong>the</strong>nogenetic (Corbet 1966). Recorded<br />
from Ellesmere Island and Greenland, A. zonella is <strong>the</strong> most nor<strong>the</strong>rly <strong>of</strong> Nearctic<br />
Trichoptera.<br />
Brachycentridae<br />
Brachycentrus americanus Banks (44)<br />
This species occurs through nor<strong>the</strong>rn and western North America, and its range has been<br />
extended to include Siberia, Mongolia, and Japan with recognition <strong>of</strong> Oligoplectrodes<br />
potanini Martynov as a junior subjective synonym (Schmid 1983); it has been recorded also<br />
from Kamchatka, but not Chukotka (Levanidova 1982). This is <strong>the</strong> only Nearctic species<br />
assigned to <strong>the</strong> subgenus B. (Oligoplectrodes) (Flint 1984); 2 o<strong>the</strong>r species occur in<br />
Asia—B. kozlovi Martynov and B. punctatus Forsslund. From illustrations <strong>of</strong> <strong>the</strong> male<br />
genitalia <strong>of</strong> <strong>the</strong>se species, it appears that <strong>the</strong> 2 Asian taxa are sister species, toge<strong>the</strong>r<br />
constituting <strong>the</strong> sister lineage <strong>of</strong> B. americanus.<br />
We have examined specimens from much <strong>of</strong> <strong>the</strong> range in North America [159 #,<br />
220 !] and also material from Siberia, Mongolia, and Japan; a unique characteristic <strong>of</strong><br />
B. americanus is <strong>the</strong> high variability <strong>of</strong> tibial spurs in North American populations. The<br />
typical and plesiomorphic tibial spur complement for all Brachycentrus species is 2,3,3; but<br />
in populations <strong>of</strong> B. americanus in Asia and Alaska tibial spurs are largely consistent at 2,2,3<br />
(Fig. 17). Most <strong>Yukon</strong> specimens also have spur counts <strong>of</strong> 2,2,3; although approximately<br />
20 per cent <strong>of</strong> those examined have 2,2,2, and occasionally <strong>the</strong> full complement <strong>of</strong> 2,3,3,<br />
with some spurs reduced in size. Regional variation representing all 3 spur conditions <strong>of</strong><br />
B. americanus was found in varying proportions over much <strong>of</strong> North America to <strong>the</strong> south;<br />
in Manitoba, nor<strong>the</strong>rn Ontario, and in Utah and Colorado <strong>the</strong> plesiomorphic 2,3,3 condition<br />
occurred in most specimens examined. Assuming genetic control for this variation, <strong>the</strong><br />
parsimonious interpretation for <strong>the</strong> pattern <strong>of</strong> character distribution is that B. americanus<br />
arose in North America where <strong>the</strong> plesiomorphic spur count <strong>of</strong> 2,3,3 occurs. Uniformity in<br />
nor<strong>the</strong>astern Asia for <strong>the</strong> reduced spur condition <strong>of</strong> 2,2,3 indicates a pervasive founder effect<br />
from an apomorphic ancestor that probably came from North America. In Nearctic Beringian<br />
populations isolated by encroaching glaciers, tibial spurs could have stabilized at 2,2,3, with<br />
colonizers from <strong>the</strong>se populations entering Asia by way <strong>of</strong> rivers <strong>of</strong> <strong>the</strong> Bering land bridge.<br />
Occurrence <strong>of</strong> B. americanus in <strong>the</strong> nor<strong>the</strong>rn <strong>Yukon</strong> (Fig. 1, region 4: Porcupine Plain)<br />
indicates that it is adapted to conditions that would have been available in trans-Beringian<br />
dispersal during <strong>the</strong> Pleistocene. The main Nearctic body <strong>of</strong> B. americanus south <strong>of</strong> <strong>the</strong><br />
continental ice mass would have been characterized by plesiomorphic tibial spurs <strong>of</strong> 2,3,3<br />
(Fig. 17). Distribution for B. americanus in North America (Flint 1984, fig. 23) suggests<br />
that disjunct populations occur in <strong>the</strong> nor<strong>the</strong>ast, in Wisconsin, Minnesota and Michigan, and<br />
in <strong>the</strong> western mountains. These disjunctions may be maintained now by <strong>the</strong> Great Lakes<br />
and by extensive areas where <strong>the</strong> cool streams required by larvae <strong>of</strong> this species are lacking.<br />
The disjunctions could be a consequence <strong>of</strong> separate refugia during glacial advances,<br />
restricting gene flow and enabling differing numbers <strong>of</strong> tibial spurs to become established<br />
in different areas. Following retreat <strong>of</strong> <strong>the</strong> ice, renewed contact between <strong>the</strong> Nearctic<br />
populations could have led to <strong>the</strong> mixture <strong>of</strong> spur counts against a strong background <strong>of</strong> <strong>the</strong><br />
plesiomorphic 2,3,3 that now characterizes populations <strong>of</strong> B. americanus in North America<br />
(Fig. 17). The high incidence <strong>of</strong> <strong>the</strong> reduced 2,2,3 condition in western North America might<br />
also be a result <strong>of</strong> southward dispersal <strong>of</strong> Beringian populations. Based on life-history data<br />
from <strong>the</strong> interior <strong>of</strong> Alaska, a life cycle <strong>of</strong> 2 years was inferred (Irons 1988).
834 G.B. Wiggins and C.R. Parker<br />
FIG. 17. Approximate distribution <strong>of</strong> character states for tibial spurs in Brachycentrus americanus (Banks) (44)<br />
(Brachycentridae).
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 835<br />
Micrasema gelidum McLachlan (45)<br />
An intensive taxonomic analysis <strong>of</strong> this species complex was carried out by<br />
BotojAneanu (1988), who concluded from a study <strong>of</strong> male genitalic morphology that<br />
M. gelidum represents a polytypic superspecies <strong>of</strong> 6 prospecies, allopatrically distributed<br />
over a large part <strong>of</strong> nor<strong>the</strong>rn Eurasia and North America. Three <strong>of</strong> <strong>the</strong> prospecies are<br />
represented in <strong>the</strong> <strong>Yukon</strong> and Alaska and in <strong>the</strong> Far East <strong>of</strong> Russia: M. (gelidum) gentile<br />
McLachlan (junior synonym M. scissum McLachlan, Kimmins and Denning 1951);<br />
M. (gelidum) extremum BotojAneanu; and M. (gelidum) gelidum McLachlan which also<br />
extends across <strong>the</strong> whole <strong>of</strong> nor<strong>the</strong>rn Eurasia to Norway (junior synonyms: M. kluane Ross<br />
and Morse 1973; M. subscissum Martynov; M. sibiricum Martynov). Because M. gelidum<br />
is a member <strong>of</strong> a Nearctic group comprising M. bactro Ross, M. diteris Ross, M. onisca<br />
Ross, and M. sprulesi Ross, its origin was believed to have been in western North America<br />
(BotojAneanu 1988); and <strong>the</strong> mosaic <strong>of</strong> prospecies constituting M. gelidum was attributed<br />
to <strong>the</strong> isolation <strong>of</strong> populations during Pleistocene glacial periods and <strong>the</strong>ir recolonization<br />
following glacial retreat. Complexity within <strong>the</strong> superspecies M. gelidum was interpreted to<br />
indicate that glacial intervals preceding <strong>the</strong> last Wisconsinan period were effective in<br />
isolating populations for periods sufficient to effect morphological divergence. One form in<br />
<strong>the</strong> complex, M. (gelidum) canusa BotojAneanu (1988), occurs in central North America,<br />
and probably was isolated along <strong>the</strong> sou<strong>the</strong>rn margin <strong>of</strong> <strong>the</strong> glacial ice; but <strong>the</strong> more nor<strong>the</strong>rly<br />
Nearctic forms were inferred to have passed periods <strong>of</strong> glacial advance in <strong>the</strong> Beringian<br />
refugium. As with species <strong>of</strong> Apatania, larval habitats for members <strong>of</strong> <strong>the</strong> gelidum complex<br />
broaden with increasing latitude—those in <strong>the</strong> most sou<strong>the</strong>rly localities living in spring<br />
streams, but northward habitats include rivers and also lakes in <strong>the</strong> most nor<strong>the</strong>rly localities<br />
(BotojAneanu 1988). Consequently larval habitat probably would not have been a<br />
deterrent to dispersal <strong>of</strong> <strong>the</strong> nor<strong>the</strong>rn populations across <strong>the</strong> Bering land bridge during <strong>the</strong><br />
Pleistocene, an inference fur<strong>the</strong>r supported by occurrence <strong>of</strong> M. gelidum on <strong>the</strong> Arctic coastal<br />
plain (Fig. 1, region 1).<br />
Goeridae<br />
Goera tungusensis Martynov (47)<br />
The range <strong>of</strong> this species known originally from Siberia must now be extended to<br />
nor<strong>the</strong>rn Quebec, <strong>the</strong> Northwest Territories, and almost certainly <strong>the</strong> <strong>Yukon</strong> (see Taxonomic<br />
Note 2). We also have from Alaska a pharate male that may represent a morphological<br />
variant <strong>of</strong> this species, or perhaps an unnamed sister taxon. If Pleistocene dispersal <strong>of</strong><br />
G. tungusensis across <strong>the</strong> Bering land bridge to North America is indicated, <strong>the</strong> species<br />
could have extended eastward in North America after withdrawal <strong>of</strong> <strong>the</strong> glaciers. However,<br />
<strong>the</strong> widely scattered North American records suggest <strong>the</strong> possibility <strong>of</strong> repopulation<br />
<strong>of</strong> deglaciated areas from south <strong>of</strong> <strong>the</strong> ice where a pre-Pliocene circumboreal<br />
ancestor took refuge, and from which a disjunct Alaskan population has begun to<br />
diverge.<br />
Leptoceridae<br />
Ceraclea annulicornis (Stephens) (54)<br />
Phylogenetic analysis (Yang and Morse 1988) indicated that C. annulicornis is one <strong>of</strong><br />
an unresolved group <strong>of</strong> 4 species—2 in China, and C. ruthae (Flint) from eastern North<br />
America. Species that are now transcontinental in North America, such as C. annulicornis,<br />
probably passed <strong>the</strong> Pleistocene to <strong>the</strong> south <strong>of</strong> <strong>the</strong> ice mass and if so, would have occurred<br />
in North America before glaciation.
836 G.B. Wiggins and C.R. Parker<br />
Ceraclea excisa (Morton) (†)<br />
Although not known from <strong>the</strong> <strong>Yukon</strong>, this species probably occurs <strong>the</strong>re because it is<br />
recorded from Alaska (Milne 1934; Morse 1975), Wisconsin, and Michigan to Quebec and<br />
Massachusetts; and it extends through nor<strong>the</strong>rn Europe and Siberia to <strong>the</strong> Amur region<br />
(Lepneva 1966). It is considered to be <strong>the</strong> sister taxon <strong>of</strong> <strong>the</strong> group <strong>of</strong> 4 species that includes<br />
C. annulicornis (Yang and Morse 1988); its widespread occurrence in North America leads to<br />
<strong>the</strong> same interpretation as for <strong>the</strong> preceding species.<br />
Ceraclea nigronervosa (Retzius) (56)<br />
Although most species <strong>of</strong> <strong>the</strong> nigronervosa group are confined to North America (Morse<br />
1975), C. nigronervosa is <strong>the</strong> sole Holarctic species. Larvae live in large rivers, where <strong>the</strong>y<br />
feed on colonies <strong>of</strong> freshwater sponge (Resh 1976; Solem and Resh 1981). In addition to<br />
typical nigronervosa specimens that are consistent with most o<strong>the</strong>rs examined from Europe<br />
and Asia, our North American material reveals a brown-winged variant with distinctive male<br />
genitalic characters (Fig. 4) which is also represented in Europe (Taxonomic Note 3). Thus<br />
<strong>the</strong> 2 forms appear to be broadly sympatric and could have reached North America during<br />
<strong>the</strong> Pleistocene by way <strong>of</strong> unglaciated Beringia, and spread southward following glacial<br />
retreat. This interpretation would require that sponges, <strong>the</strong> food <strong>of</strong> <strong>the</strong> larvae, occurred in<br />
waters <strong>of</strong> <strong>the</strong> Bering land bridge; and this is feasible because C. nigronervosa now occurs<br />
in <strong>the</strong> far nor<strong>the</strong>rn <strong>Yukon</strong> (Fig. 1, region 4). However, this species might have been in North<br />
America before <strong>the</strong> Pliocene separation from Asia because <strong>the</strong> range <strong>of</strong> C. nigronervosa now<br />
extends to Wyoming.<br />
Oecetis ochracea (Curtis) (63)<br />
This is <strong>the</strong> only Holarctic species in Oecetis, and no phylogenetic analysis <strong>of</strong> <strong>the</strong> genus<br />
has been made. The subspecies O. o. carri Milne (1934) was segregated to discriminate<br />
between North American and European populations by genitalic characters. We found no<br />
evidence to support this interpretation; variation in genitalic characters was evident, but<br />
showed no geographic correlation. However, specimens from nor<strong>the</strong>rn Europe and <strong>the</strong><br />
<strong>Yukon</strong> are darker brown in colour overall than those from o<strong>the</strong>r parts <strong>of</strong> North America.<br />
Although slight, this indication <strong>of</strong> genetic continuity in O. ochracea through Nearctic<br />
Beringia and Europe suggests that <strong>the</strong> light-coloured Nearctic populations were derived from<br />
ancestors forced to <strong>the</strong> south <strong>of</strong> <strong>the</strong> continental glacier; if so, this species probably was<br />
present in North America before <strong>the</strong> Pleistocene glaciation.<br />
Ylodes reuteri (McLachlan) (68)<br />
In discussing <strong>the</strong> Nearctic range <strong>of</strong> this species, Ross (1965, as Triaenodes griseus)<br />
alluded to morphological variants in <strong>the</strong> Rocky Mountains, <strong>the</strong> western part <strong>of</strong> <strong>the</strong> Northwest<br />
Territories, and <strong>the</strong> Hudson Bay area. Highly variable populations in sou<strong>the</strong>rn Saskatchewan<br />
were interpreted as an intergrading blend from all 3 areas—an example <strong>of</strong> caddisfly species<br />
that passed <strong>the</strong> last glaciation in discrete areas in <strong>the</strong> Rocky Mountains, in eastern North<br />
America, and in Beringia. If so, Y. reuteri or its ancestor must have occupied North America<br />
early enough, before Asia and North America were separated in <strong>the</strong> Pliocene, to have become<br />
established to <strong>the</strong> south <strong>of</strong> <strong>the</strong> glaciers.<br />
Limnephilidae<br />
Asynarchus iteratus McLachlan (74)<br />
This species was assigned to <strong>the</strong> lapponicus group (Schmid 1954b), and was considered<br />
to be so close to A. aldinus (Ross) from Alberta that <strong>the</strong> two could be geographic variants<br />
<strong>of</strong> <strong>the</strong> same species. The close similarity suggests that separation between <strong>the</strong>m might have
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 837<br />
been brought about by segregation <strong>of</strong> a portion <strong>of</strong> <strong>the</strong> Nearctic population <strong>of</strong> A. iteratus to<br />
<strong>the</strong> south <strong>of</strong> <strong>the</strong> advancing glaciers, giving rise to A. aldinus, while A. iteratus became<br />
confined to Beringia as a glacial relict. If that were so, A. iteratus or its ancestor probably<br />
reached North America from Asia before <strong>the</strong> land bridge between <strong>the</strong> 2 continents was<br />
overrun by <strong>the</strong> sea during <strong>the</strong> Pliocene.<br />
Asynarchus lapponicus (Zetterstedt) (75)<br />
Variation in <strong>the</strong> superior and intermediate appendages <strong>of</strong> <strong>the</strong> males showed no coherent<br />
pattern correlated with geographic distribution. Among <strong>the</strong> species assigned to <strong>the</strong> lapponicus<br />
group (Schmid 1954b), <strong>the</strong> sister species <strong>of</strong> A. lapponicus would be <strong>the</strong> nor<strong>the</strong>rn North<br />
American species A. montanus Banks. Prominent morphological differences in <strong>the</strong> genitalia<br />
<strong>of</strong> <strong>the</strong> 2 species suggest that <strong>the</strong>y have been separate for some time, perhaps through<br />
inter-continental vicariant speciation, with A. lapponicus dispersing from Asia to North<br />
America across <strong>the</strong> Bering land bridge during <strong>the</strong> Pleistocene. Disjunct distribution between<br />
nor<strong>the</strong>rn and central populations <strong>of</strong> this species in Europe (Malicky 1988, fig. 11) suggests<br />
that it was widely distributed in Europe and Asia before <strong>the</strong> glacial advances <strong>of</strong> <strong>the</strong><br />
Pleistocene. If A. lapponicus was confined to Beringia during Pleistocene glaciation, it has<br />
spread widely in North America following recession <strong>of</strong> <strong>the</strong> ice, in contrast to some o<strong>the</strong>r<br />
Nearctic Beringian species (see category III). The larval habitat for A. lapponicus is littoral<br />
areas <strong>of</strong> lakes, tundra pools, and slow streams (Winchester 1984), and <strong>the</strong> species is<br />
univoltine at <strong>the</strong> latitude <strong>of</strong> Tuktoyaktuk, Northwest Territories (69°29′N).<br />
Limnephilus dispar McLachlan (97); L. externus Hagen (98); L. femoralis Kirby (100);<br />
L. nigriceps (Zetterstedt) (108); L. picturatus McLachlan (113); L. rhombicus (Linnaeus)<br />
(114); L. sericeus (Say) (117).<br />
Phylogenetic relationships within <strong>the</strong> large genus Limnephilus have not been investigated<br />
in sufficient depth to support inferences about <strong>the</strong> geographic origin <strong>of</strong> <strong>the</strong> species.<br />
All <strong>of</strong> <strong>the</strong>se species have a transcontinental distribution in North America, and probably<br />
occurred on this continent before glaciation in <strong>the</strong> Pleistocene. We found morphological<br />
variation in several <strong>of</strong> <strong>the</strong> species, but a congruent pattern was evident only in L. picturatus.<br />
Comparison <strong>of</strong> specimens <strong>of</strong> L. picturatus from all parts <strong>of</strong> <strong>the</strong> range in North America<br />
[64 #, 85 !] with Eurasian material indicates genetic continuity between Nearctic Beringia<br />
and Eurasia, and restriction in gene flow between Beringian populations and those from<br />
o<strong>the</strong>r parts <strong>of</strong> North America. This argues for isolation <strong>of</strong> Nearctic populations <strong>of</strong> L. picturatus<br />
south <strong>of</strong> <strong>the</strong> glaciers while Palaearctic immigrants <strong>of</strong> <strong>the</strong> species entered Beringia.<br />
Accordingly, L. picturatus probably would have occurred in North America before <strong>the</strong><br />
Pleistocene, and its Holarctic distribution would pre-date <strong>the</strong> Pliocene separation between<br />
North America and Asia. On melting <strong>of</strong> <strong>the</strong> glaciers, <strong>the</strong> more sou<strong>the</strong>rly Nearctic populations<br />
appear to have been more successful in colonizing deglaciated territory than have <strong>the</strong><br />
Beringian populations. Analysis <strong>of</strong> Greenland populations for <strong>the</strong>se variable characters<br />
might shed light on <strong>the</strong>ir origin.<br />
Thus, <strong>the</strong> patch <strong>of</strong> stout setae on <strong>the</strong> ventral surface <strong>of</strong> <strong>the</strong> hindwing R2 in males is well<br />
developed in all Eurasian specimens examined and in about 95 per cent <strong>of</strong> Beringian<br />
specimens, but equally well developed in only about one third <strong>of</strong> o<strong>the</strong>r North American<br />
specimens. The pterostigma is light in colour in 90 per cent <strong>of</strong> Beringian and Palaearctic<br />
specimens examined, but light in about 50 per cent <strong>of</strong> o<strong>the</strong>r North American specimens.<br />
Inferior appendages in <strong>the</strong> male genitalia are triangular in dorsal aspect in about 86 per<br />
cent <strong>of</strong> Nearctic Beringian specimens; about 4% <strong>of</strong> specimens from o<strong>the</strong>r parts <strong>of</strong> North<br />
America are similar in this character, where <strong>the</strong> predominant condition is for parallel dorsal
838 G.B. Wiggins and C.R. Parker<br />
margins on <strong>the</strong> inferior appendages. Eurasian specimens examined are, however, about<br />
evenly divided between <strong>the</strong>se 2 conditions. In <strong>the</strong> parameres <strong>of</strong> <strong>the</strong> male genitalia <strong>of</strong> Nearctic<br />
Beringian specimens <strong>the</strong>re are more than 10 long, very fine, setae on <strong>the</strong> dorsal preapical<br />
lobe and <strong>the</strong> setae on <strong>the</strong> mesal surface extend anterad <strong>of</strong> <strong>the</strong> dorsal process. In specimens<br />
from o<strong>the</strong>r parts <strong>of</strong> North America <strong>the</strong>re are fewer than 10 setae on <strong>the</strong> dorsal lobe <strong>of</strong> <strong>the</strong><br />
parameres and <strong>the</strong> setae are coarser, as <strong>the</strong>y are also in Eurasian specimens examined; but<br />
<strong>the</strong> fine setae on <strong>the</strong> mesal surface in Eurasian material extend anterad <strong>of</strong> <strong>the</strong> dorsal lobe as<br />
in Beringian specimens.<br />
In females, <strong>the</strong> dorsal margin <strong>of</strong> segment IX is truncate in 98 per cent <strong>of</strong> Nearctic<br />
Beringian specimens examined and also in Eurasian specimens, differing from <strong>the</strong> rounded<br />
condition found in 87 per cent <strong>of</strong> North American populations generally. Segment X in dorsal<br />
aspect is bifid in only about 17% <strong>of</strong> Nearctic Beringian females examined, but in 90% <strong>of</strong><br />
specimens from elsewhere in North America; in most Nearctic Beringian specimens <strong>the</strong> apex<br />
<strong>of</strong> X is blunt or pointed ra<strong>the</strong>r than bifid.<br />
This species is univoltine at Tuktoyaktuk, Northwest Territories, where larvae live in<br />
tundra ponds (Winchester 1984).<br />
Onocosmoecus unicolor (Banks) (122)<br />
It seems likely that Onocosmoecus is part <strong>of</strong> a complex <strong>of</strong> dicosmoecine genera that<br />
arose in North America (Wiggins and Flint in prep.). Marked variation in this species is<br />
confirmed by no fewer than 6 synonyms, but analysis <strong>of</strong> specimens from many localities<br />
revealed no congruent geographic pattern (Wiggins and Richardson 1987). We infer that<br />
some populations passed <strong>the</strong> Pleistocene glaciation south <strong>of</strong> <strong>the</strong> ice in North America because<br />
<strong>the</strong> only o<strong>the</strong>r species known in <strong>the</strong> genus, O. sequoiae Wiggins and Richardson, is confined<br />
to <strong>the</strong> Sierra Nevada Mountains <strong>of</strong> California and may have originated <strong>the</strong>re as a glacial<br />
disjunct. Widespread occurrence <strong>of</strong> variable populations <strong>of</strong> O. unicolor in North America<br />
from British Columbia to Newfoundland indicates that o<strong>the</strong>r populations <strong>of</strong> this species<br />
extended across a broad front to <strong>the</strong> south <strong>of</strong> <strong>the</strong> glaciers, and that O. unicolor occurred in<br />
North America before Pleistocene glaciation began. This leads to <strong>the</strong> fur<strong>the</strong>r possibility that<br />
o<strong>the</strong>r populations <strong>of</strong> O. unicolor in <strong>the</strong> Beringian refugium dispersed to eastern Asia across<br />
<strong>the</strong> Bering land bridge during <strong>the</strong> Pleistocene glaciation. Larvae <strong>of</strong> this species live in cool<br />
waters <strong>of</strong> slow streams and <strong>the</strong> littoral zone <strong>of</strong> lakes, habitats that would have been readily<br />
available on <strong>the</strong> Bering land bridge; larvae in streams in interior Alaska fed entirely on plant<br />
detritus (Irons 1988).<br />
Molannidae<br />
Molanna flavicornis Banks (127)<br />
This is <strong>the</strong> only North American species <strong>of</strong> Molanna with a transcontinental distribution.<br />
It is very similar to and is perhaps identical with <strong>the</strong> Eurasian Molanna albicans Zett.,<br />
resulting in a circumboreal range through nor<strong>the</strong>rn Eurasia and North America (Fuller 1987).<br />
The albicans group <strong>of</strong> several species evidently arose in Eurasia and only this single Nearctic<br />
extension now exists. Montane populations in Colorado are interpreted as glacial relicts,<br />
indicating that this species ranged widely to <strong>the</strong> south <strong>of</strong> North American glaciers during <strong>the</strong><br />
Pleistocene, and thus its Holarctic distribution would have been established before Asia and<br />
North America were separated in <strong>the</strong> Pliocene. Larvae live on <strong>the</strong> bottom <strong>of</strong> cool lakes.<br />
Molannodes tinctus Zetterstedt (128)<br />
Collections <strong>of</strong> this nor<strong>the</strong>rn Eurasian species in Alaska and <strong>the</strong> <strong>Yukon</strong> have led to <strong>the</strong><br />
entrenched view that it was a glacial relict confined to <strong>the</strong> Beringian refugium. In recent
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 839<br />
years, however, isolated collections <strong>of</strong> <strong>the</strong> species have been made in Saskatchewan and<br />
nor<strong>the</strong>rn Ontario (Fuller 1987). Based on <strong>the</strong> broad distribution <strong>of</strong> M. tinctus in nor<strong>the</strong>rn<br />
Europe and Asia, and on phylogenetic evidence for <strong>the</strong> origin <strong>of</strong> Molannodes in Asia (Fuller<br />
1987), an eastward dispersal by way <strong>of</strong> <strong>the</strong> Bering land bridge is inferred. Evidence is<br />
equivocal as to whe<strong>the</strong>r dispersal occurred in Pleistocene time or earlier.<br />
Phryganeidae<br />
Agrypnia colorata Hagen (129)<br />
Material from Asia has been referred by most authors to A. principalis (Martynov), but<br />
this name is a junior subjective synonym <strong>of</strong> A. colorata Hagen (Wiggins in press). All<br />
Beringian specimens examined and all from <strong>the</strong> Palaearctic portion <strong>of</strong> <strong>the</strong> range are dark in<br />
colour on <strong>the</strong> thorax, legs, and wings; and all have fully developed tibial spurs <strong>of</strong> 2,4,4,<br />
typical for <strong>the</strong> family Phryganeidae (Fig. 18). The single exception in Palaearctic material<br />
that we examined is a light-coloured female from <strong>the</strong> Keriya River area <strong>of</strong> China (Sinkiang<br />
Prov.; ZMAS), although <strong>the</strong> tibial spurs were fully developed. In contrast, most North<br />
American specimens taken outside Beringia are light in colour on <strong>the</strong> thorax, legs, and wings,<br />
and have <strong>the</strong> tibial spurs reduced in some way. The pattern <strong>of</strong> reduction is variable—some<br />
spurs may be absent, o<strong>the</strong>rs reduced to tiny knobs; and in any pair <strong>of</strong> spurs, one might be<br />
reduced or lacking and <strong>the</strong> o<strong>the</strong>r normal; opposite members <strong>of</strong> a pair <strong>of</strong> legs <strong>of</strong>ten have<br />
different spur conditions. Exceptions occur in series from Fort McPherson, Northwest<br />
Territories and Kamloops, British Columbia (ROME), in which specimens are dark as in<br />
Beringian and Palaearctic material, but <strong>the</strong> tibial spurs are variable as in most North<br />
American populations. No congruent variation was found in genitalic characters. [Specimens<br />
examined: N. Am.—58 #, 55 !]<br />
The sister species <strong>of</strong> A. colorata is A. legendrei (Navas) known only from China. We<br />
interpret <strong>the</strong> light-coloured A. colorata to be a vicariant Nearctic form derived from dark<br />
ancestral stock with unmodified spurs following separation <strong>of</strong> Asia from North America,<br />
perhaps in Pliocene time. Nearctic populations would have been isolated to <strong>the</strong> south <strong>of</strong> <strong>the</strong><br />
Pleistocene glaciers when light colour and unstable tibial spurs could have been established;<br />
disjunct montane populations <strong>of</strong> <strong>the</strong> light-coloured form in Wyoming and Utah are consistent<br />
with this interpretation (Wiggins in press). The plesiomorphic dark Palaearctic form with<br />
stable tibial spurs would have dispersed to Nearctic Beringia across <strong>the</strong> land bridge.<br />
Following retreat <strong>of</strong> <strong>the</strong> ice, contact between <strong>the</strong> 2 forms in North America could have given<br />
rise to intergrading populations such as those near Kamloops and Fort McPherson.<br />
Agrypnia pagetana Curtis (135)<br />
No close relative <strong>of</strong> this species is known (Wiggins in press). We infer that A. pagetana<br />
is a Eurasian species that reached North America across <strong>the</strong> Bering land bridge, perhaps<br />
during <strong>the</strong> Pleistocene, and dispersed through nor<strong>the</strong>rn North America following recession<br />
<strong>of</strong> <strong>the</strong> glaciers. It has not been recorded east <strong>of</strong> Hudson Bay. Populations <strong>of</strong> A. pagetana in<br />
Europe range far<strong>the</strong>r south to more temperate climates than in North America. Larvae <strong>of</strong> this<br />
species live in small tundra ponds and slow-flowing streams; <strong>the</strong>y are univoltine at<br />
Tuktoyaktuk, Northwest Territories (lat. 69°29′N) (Winchester 1984).<br />
III. Palaearctic-East Beringian Species<br />
Because <strong>of</strong> <strong>the</strong>ir broad Eurasian range and restricted North American distribution, <strong>the</strong>se<br />
species are inferred to be <strong>of</strong> Palaearctic origin. Dispersal to North America across <strong>the</strong> Bering<br />
land bridge during <strong>the</strong> Pleistocene seems <strong>the</strong> most likely route for a number <strong>of</strong> <strong>the</strong>se species.<br />
The 13 species in category III constitute about 10 per cent <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> fauna.
840 G.B. Wiggins and C.R. Parker<br />
FIG. 18. Global distribution <strong>of</strong> variant characters in Agrypnia colorata Hagen (129) (Phryganeidae). Dark-coloured<br />
adults with tibial spurs constant at 2,4,4 occur throughout nor<strong>the</strong>rn Eurasia and Beringia; light-coloured adults with<br />
tibial spurs variably reduced occur through most <strong>of</strong> North America outside Beringia; intermediate dark specimens<br />
with variable tibial spurs occur in northwestern North America between <strong>the</strong> two areas outlined.<br />
Failure <strong>of</strong> <strong>the</strong>se East Beringian species to broaden <strong>the</strong>ir postglacial Nearctic range<br />
perhaps could be attributed to competition for resources from species advancing from <strong>the</strong><br />
south which were better adapted to <strong>the</strong> freshwater habitats <strong>of</strong> <strong>the</strong> deglaciated terrain, or in<br />
any case from species that reached <strong>the</strong> new habitats first. As discussed previously, closure<br />
<strong>of</strong> Beringia by conjunction <strong>of</strong> <strong>the</strong> Laurentide and Cordilleran ice sheets probably would have<br />
caused some extinction in Nearctic Beringia; consequently a wider range <strong>of</strong> ecologically<br />
coordinate species from <strong>the</strong> much larger, and biologically more diverse, West Beringia<br />
would have encountered reduced competition when <strong>the</strong>y dispersed to East Beringia. But this<br />
advantage <strong>of</strong> <strong>the</strong> Palaearctic species might well have been less effective in postglacial time<br />
when Nearctic species were reassembled in more tightly packed communities. For <strong>the</strong>se or
o<strong>the</strong>r reasons, most Palaearctic-East Beringian species <strong>of</strong> category III have functioned<br />
nei<strong>the</strong>r as aggressive colonists nor ecological generalists in North America; but quite clear<br />
is <strong>the</strong> contrast with Europe and Asia where most <strong>of</strong> <strong>the</strong>m are widely distributed, and<br />
apparently are successful generalists. It is one <strong>of</strong> <strong>the</strong> striking paradoxes <strong>of</strong> Beringian distributions<br />
that wide-ranging, and evidently competitively successful, Eurasian species remain<br />
confined to <strong>the</strong>ir East Beringian outpost, evidently unable to disperse much beyond <strong>the</strong>ir<br />
former glacial refuge. This distributional paradox can be added to <strong>the</strong> productivity paradox<br />
(e.g. Hopkins et al. 1982) as significant questions about <strong>the</strong> biological history <strong>of</strong> Beringia.<br />
This issue focusses on species <strong>of</strong> category III because <strong>the</strong>y may differ from category II<br />
essentially in lacking <strong>the</strong> competitive ecological edge required in new communities;<br />
Palaearctic species having that competitive edge now meet <strong>the</strong> distributional criterion <strong>of</strong><br />
category II.<br />
For several <strong>of</strong> <strong>the</strong>m (Ylodes kaszabi, Arctopora trimaculata, Grammotaulius signatipennis,<br />
Limnephilus stigma, and Agrypnia obsoleta), sister-group relationships suggest<br />
intercontinental vicariance, perhaps during <strong>the</strong> Pliocene or earlier, followed later by dispersal<br />
<strong>of</strong> <strong>the</strong> Palaearctic form to East Beringia, probably across <strong>the</strong> Pleistocene land bridge.<br />
In Agraylea cognatella and Limnephilus fenestratus, morphological similarity to <strong>the</strong>ir<br />
respective Nearctic sister species is so close that dichotomy during <strong>the</strong> Pleistocene could be<br />
reasonably inferred. Subdivision <strong>of</strong> <strong>the</strong> range by glacial ice seems likely, with <strong>the</strong> Nearctic<br />
sister species originating in isolation to <strong>the</strong> south <strong>of</strong> <strong>the</strong> glaciers.<br />
Spicipalpia<br />
Hydroptilidae<br />
Agraylea cognatella McLachlan<br />
Rhyacophilidae<br />
Rhyacophila mongolica Schmid, Arefina<br />
and Levanidova<br />
Integripalpia<br />
Leptoceridae<br />
Ylodes kaszabi Schmid<br />
Hydroptilidae<br />
Agraylea cognatella McLachlan (4)<br />
The Palaearctic range <strong>of</strong> A. cognatella appears to be circumscribed by A. multipunctata<br />
Curtis which is widely distributed through Europe and western Asia (BotojAneanu and<br />
Levanidova 1988); in North America, A. cognatella is evidently circumscribed to <strong>the</strong> south<br />
<strong>of</strong> Beringia, not by A. multipunctata as has been <strong>the</strong> traditional interpretation (e.g. Ross<br />
1944), but by a sibling species A. fraterna Banks apparently now widely distributed on this<br />
continent (Vineyard and Wiggins in prep.). It seems reasonable to infer from present<br />
evidence that A. cognatella is a Palaearctic-East Beringian species now confined in North<br />
America to <strong>the</strong> refugium, and that A. fraterna passed <strong>the</strong> glacial period to <strong>the</strong> south <strong>of</strong> <strong>the</strong><br />
ice where it may have arisen.<br />
Rhyacophilidae<br />
Rhyacophila mongolica Schmid, Arefina and Levanidova (14)<br />
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 841<br />
Limnephilidae<br />
Arctopora trimaculata (Zetterstedt)<br />
Dicosmoecus obscuripennis Banks<br />
Grammotaulius signatipennis McLachlan<br />
Grensia praeterita (Walker)<br />
Limnephilus diphyes McLachlan<br />
Limnephilus fenestratus (Zetterstedt)<br />
Limnephilus stigma Curtis<br />
Phryganeidae<br />
Agrypnia obsoleta (Hagen)<br />
Agrypnia sahlbergi (McLachlan)<br />
Oligotricha lapponica (Hagen)
842 G.B. Wiggins and C.R. Parker<br />
FIG. 19. Distribution <strong>of</strong> Arctopora species (71, 72) (Limnephilidae); symbols superimposed indicate occurrence <strong>of</strong><br />
both species at <strong>the</strong> same locality.<br />
In phylogenetic analyses <strong>of</strong> Rhyacophila, some 25 species from eastern and western<br />
North America, Siberia, Japan, and Europe have been assigned to <strong>the</strong> sibirica species group<br />
(Ross 1956; Schmid 1970). This group forms <strong>the</strong> major component <strong>of</strong> Rhyacophila in<br />
Palaearctic Asia, and includes <strong>the</strong> only 2 Holarctic members <strong>of</strong> <strong>the</strong> genus known to<br />
date—R. narvae and R. mongolica (Taxonomic Note 1). Rhyacophila mongolica is considered<br />
<strong>the</strong> sister species <strong>of</strong> R. sibirica McLachlan (Schmid et al. 1993). Among 14 species <strong>of</strong><br />
Rhyacophila occurring in <strong>the</strong> <strong>Yukon</strong>, R. mongolica (Fig. 2) is recorded much far<strong>the</strong>r north<br />
than any <strong>of</strong> <strong>the</strong> o<strong>the</strong>rs (Fig. 1, region 4), indicating that <strong>the</strong> species was ecologically adapted<br />
for dispersal to North America across <strong>the</strong> Bering land bridge during <strong>the</strong> Pleistocene.<br />
Leptoceridae<br />
Ylodes kaszabi (Schmid) (67)<br />
The genus Ylodes appears to have originated in north-central Asia (Manuel and Nimmo<br />
1984), and Y. kaszabi occurs in Mongolia. A sister-species relationship with <strong>the</strong> Nearctic<br />
Y. schmidi suggests <strong>the</strong> possibility <strong>of</strong> intercontinental vicariant origin during <strong>the</strong> Pliocene<br />
or perhaps earlier; thus Y. kaszabi is inferred to have been a Palaearctic species that later<br />
reached North America by way <strong>of</strong> <strong>the</strong> Bering land bridge during <strong>the</strong> Pleistocene.<br />
Limnephilidae<br />
Arctopora trimaculata (Zetterstedt) (72)
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 843<br />
In addition to A. trimaculata, 2 North American sister species are recognized in<br />
Arctopora: A. pulchella (Banks) (71), transcontinental from <strong>Yukon</strong> and Alaska to Newfoundland<br />
and New Hampshire; and A. salmon (Smith) in Idaho (Fig. 19). The evidence<br />
suggests vicariant speciation from a circumboreal common ancestor, followed by derivation<br />
<strong>of</strong> <strong>the</strong> North American species pair from subdivision <strong>of</strong> <strong>the</strong> range, possibly by glacial ice.<br />
This interpretation leads fur<strong>the</strong>r to Pleistocene dispersal <strong>of</strong> A. trimaculata from Asia to North<br />
America across <strong>the</strong> Bering bridge; but <strong>the</strong> species remained confined to East Beringia<br />
following deglaciation, perhaps because A. pulchella was better adapted for colonizing <strong>the</strong><br />
new habitats. In any case, <strong>the</strong> 2 species are now geographically sympatric in East Beringia<br />
(Fig. 19).<br />
Dicosmoecus obscuripennis Banks (81)<br />
Dicosmoecus obscuripennis is <strong>the</strong> only member <strong>of</strong> <strong>the</strong> Palaearctic palatus species group<br />
recorded from North America (Wiggins and Richardson 1982). Study <strong>of</strong> additional collections<br />
provided by I.M. Levanidova (Vladivostok) revealed that this species is actually<br />
widespread in eastern Russia—a situation fur<strong>the</strong>r investigated by Nagayasu and Ito (1993);<br />
Asian specimens examined are larger, <strong>the</strong> length <strong>of</strong> male forewings 20 – 25 mm, in contrast<br />
to 18 – 20 mm for North American specimens. If this species reached North America from<br />
Asia during <strong>the</strong> Pleistocene, passage over <strong>the</strong> Bering land bridge might not have been wholly<br />
dependent upon <strong>the</strong> clear running waters to which larvae <strong>of</strong> species <strong>of</strong> Dicosmoecus are<br />
usually confined because larvae <strong>of</strong> D. obscuripennis have been found along lake margins in<br />
<strong>the</strong> Russian Far East (I.M. Levanidova, pers. comm.). After retreat <strong>of</strong> <strong>the</strong> glaciers, D. obscuripennis<br />
appears not to have penetrated North American habitats much beyond its<br />
glacial-age Beringian range. Possibly a limiting factor in North America is <strong>the</strong> widespread<br />
occurrence <strong>of</strong> D. atripes (80), an ecologically vigorous species <strong>of</strong> <strong>the</strong> western montane region<br />
ranging from California to <strong>Yukon</strong> and Alaska, which appears to have reached Beringia from<br />
<strong>the</strong> south following glacial recession (Fig. 20).<br />
Grammotaulius signatipennis McLachlan (86)<br />
This is <strong>the</strong> sister species <strong>of</strong> G. alascensis Schmid recorded from nor<strong>the</strong>rn North America<br />
(Taxonomic Note 6). From <strong>the</strong> evidence available, G. signatipennis is probably a Palaearctic<br />
species, extended in range to North America across <strong>the</strong> Bering land bridge during <strong>the</strong><br />
Pleistocene.<br />
Grensia praeterita (Walker) (87)<br />
This is one <strong>of</strong> <strong>the</strong> very few truly arctic species <strong>of</strong> Trichoptera, and it probably occurred<br />
in Beringia, at least in part, during glaciation. In contrast to most <strong>of</strong> <strong>the</strong> o<strong>the</strong>r species assigned<br />
to category III, G. praeterita has extended its postglacial range considerably beyond Beringia.<br />
Evidently, <strong>the</strong> species does not occur on <strong>the</strong> mainland <strong>of</strong> North America east <strong>of</strong> Hudson<br />
Bay (Harper 1989), indicating that its dispersal to Greenland was probably by way <strong>of</strong> <strong>the</strong><br />
Arctic Archipelago. Comparison <strong>of</strong> <strong>Yukon</strong> specimens with material from <strong>the</strong> Northwest<br />
Territories and Russia revealed no morphological differences, although <strong>the</strong> latter were<br />
slightly larger.<br />
Limnephilus diphyes McLachlan (96)<br />
This species is recorded from North America for <strong>the</strong> first time (Taxonomic Note 7). Its<br />
affinities are obscure, but since it has not been collected south <strong>of</strong> Alaska and <strong>the</strong> <strong>Yukon</strong>, <strong>the</strong><br />
evidence suggests that L. diphyes is a Palaearctic species that reached North America during<br />
<strong>the</strong> Pleistocene and has not spread beyond East Beringia. Larvae <strong>of</strong> L. diphyes live in<br />
sphagnum bog pools (Johansson et al. 1991).
844 G.B. Wiggins and C.R. Parker<br />
FIG. 20. Distribution <strong>of</strong> Dicosmoecus atripes (Hagen) (80) and D. obscuripennis Banks (81) (Limnephilidae); <strong>the</strong><br />
latter is widely distributed in eastern Asia.
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 845<br />
FIG. 21. Distribution <strong>of</strong> <strong>the</strong> sister species Limnephilus kennicotti Banks (107) and L. fenestratus (Zetterstedt) (101)<br />
(Limnephilidae); symbols superimposed indicate occurrence <strong>of</strong> both species at <strong>the</strong> same locality.<br />
Limnephilus fenestratus (Zetterstedt) (101)<br />
The very similar sister taxon <strong>of</strong> L. fenestratus is L. kennicotti (107) (category I), which<br />
occurs widely through nor<strong>the</strong>rn North America and in Greenland (Fig. 21). We have<br />
identified both taxa in separate collections from <strong>the</strong> <strong>Yukon</strong> (Kluane and Firth River). The<br />
two are most readily separable by male genitalic structure: in L. fenestratus <strong>the</strong> intermediate<br />
appendages are relatively short and do not extend beyond <strong>the</strong> superior appendages in lateral<br />
aspect, but are longer in L. kennicotti and do extend beyond <strong>the</strong> superior appendages in lateral<br />
aspect. Diagnostic characters for males and females <strong>of</strong> both species were illustrated by<br />
Kimmins and Denning (1951, figs. 10, 11, as L. miser McLachlan which is a junior subjective<br />
synonym <strong>of</strong> L. fenestratus proposed by Schmid 1955; and figs. 12, 13, as L. moselyi Kimmins<br />
and Denning from Greenland which is a junior subjective synonym <strong>of</strong> L. kennicotti proposed<br />
by Ross and Merkley 1952).<br />
We presume that records <strong>of</strong> L. fenestratus from Greenland by Fristrup (1942), repeated<br />
by Gislason (1981), were based on Mosely’s (1929) misidentification <strong>of</strong> L. kennicotti as<br />
L. miser (= fenestratus); a series <strong>of</strong> specimens we examined from Greenland (Zoologisk<br />
Museum, Copenhagen, per N.P. Kristensen) proved to be L. kennicotti. From <strong>the</strong> <strong>Yukon</strong>,<br />
Nimmo and Wickstrom (1984) do not record L. fenestratus, but provide several records for
846 G.B. Wiggins and C.R. Parker<br />
L. kennicotti; we have confirmed that <strong>the</strong>ir material from Kluane National Park and Firth<br />
River is L. kennicotti, but that from Burwash Landing is really L. fenestratus. In <strong>the</strong> <strong>Yukon</strong><br />
material examined, we found some indication that male genitalic characters intergrade<br />
between <strong>the</strong>se 2 taxa. Moreover, putative distinction between females (Kimmins and<br />
Denning 1951) has proven unreliable because <strong>of</strong> variation in genitalic characters, culminating<br />
in our material in 2 females collected with a male <strong>of</strong> L. fenestratus (<strong>Yukon</strong>: Old Crow<br />
Flats, ROME 810587a); one female shows characters attributed to L. kennicotti, <strong>the</strong> o<strong>the</strong>r to<br />
L. fenestratus. Fur<strong>the</strong>r evidence on variation is required before <strong>the</strong> status <strong>of</strong> <strong>the</strong>se taxa can<br />
be confirmed. [North American specimens examined: L. fenestratus 26 #, 29 !; L. kennicotti<br />
18 #, 32 !].<br />
Whe<strong>the</strong>r or not <strong>the</strong>se 2 forms are distinguished as species, <strong>the</strong>ir distributional relationships<br />
are informative in a Beringian context. If <strong>the</strong> very close morphological similarity<br />
between <strong>the</strong> 2 taxa is interpreted to indicate separation during <strong>the</strong> Pleistocene, L. kennicotti<br />
could have diverged as a disjunct population along <strong>the</strong> sou<strong>the</strong>rn margin <strong>of</strong> <strong>the</strong> North<br />
American glaciers. By this interpretation, L. fenestratus would have been <strong>the</strong> Holarctic<br />
ancestor, and its present East Beringian population would be a glacial relict. As <strong>the</strong> ice<br />
receded, widespread recolonization by L. kennicotti over nor<strong>the</strong>rn North America evidently<br />
led to its dispersal to Greenland, approaching <strong>the</strong> western limit <strong>of</strong> its ancestral stock<br />
represented by L. fenestratus in Iceland (Fig. 21). Judging from material we have examined,<br />
L. fenestratus has extended its Nearctic range little if at all beyond unglaciated Beringia;<br />
L. kennicotti has shown marked capacity for colonizing deglaciated habitats, evidently far<br />
into <strong>the</strong> nor<strong>the</strong>rn <strong>Yukon</strong> where <strong>the</strong> 2 forms are now apparently sympatric. Some intergradation<br />
in <strong>the</strong> morphological characters distinguishing <strong>the</strong> two (see above) suggests that this<br />
postglacial sympatry has yet to reach some equilibrium.<br />
Limnephilus stigma Curtis (118)<br />
Records for <strong>the</strong> close sister species L. indivisus Walker, now widespread over much <strong>of</strong><br />
North America, approach <strong>the</strong> Nearctic limit <strong>of</strong> L. stigma in <strong>the</strong> Northwest Territories (Fort<br />
Smith, CNCI) and nor<strong>the</strong>rn British Columbia (Alaska Hwy. km 359, Prophet R. Prov. Park,<br />
ROME). Specimens from Kluane in <strong>the</strong> <strong>Yukon</strong> were identified as L. indivisus by Nimmo<br />
and Wickstrom (1984), but our examination <strong>of</strong> that same material indicates that <strong>the</strong>y are<br />
L. stigma. In <strong>the</strong> continued absence <strong>of</strong> intermediates, we infer that <strong>the</strong> 2 species do not<br />
hybridize. It has long been recognized that L. stigma (Figs. 22 – 25) is very similar morphologically<br />
to <strong>the</strong> widespread North American species L. indivisus Walker (e.g. Betten and<br />
Mosely 1940). Males <strong>of</strong> L. indivisus are distinguished by <strong>the</strong> much longer intermediate<br />
appendages and by <strong>the</strong> ventral gap in <strong>the</strong> mesal dentation <strong>of</strong> <strong>the</strong> superior appendages<br />
(Fig. 26); females <strong>of</strong> L. indivisus are distinguished by <strong>the</strong> narrow and deeply incised apex<br />
<strong>of</strong> segment X, especially evident in ventral aspect (Fig. 27).<br />
Material <strong>of</strong> L. stigma from <strong>Yukon</strong> and Alaska [26 #, 24 !] differs slightly in genitalic<br />
characters from specimens examined from Europe and nor<strong>the</strong>rn Asia [18 #, 16 !]. In East<br />
Beringian males (Fig. 24b), <strong>the</strong> peripheral dentate ridge on <strong>the</strong> superior appendages bears a<br />
large point underlying <strong>the</strong> intermediate appendages which is lacking from European specimens<br />
(Fig. 22b). Males in our Nearctic material <strong>of</strong> L. stigma bear a prominent sclerotized<br />
point on each paramere (Fig. 24d); this sclerotized point is lacking in specimens we examined<br />
from Europe (Fig. 22c; and Malicky 1983, p. 188), but is present in specimens examined<br />
from Kamchatka. In East Beringian females (Fig. 25), <strong>the</strong> apex <strong>of</strong> segment X is more<br />
narrowly tapered than in Eurasian material (Fig. 23), especially in ventral aspect.
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 847<br />
FIGS. 22 – 25. Limnephilus stigma Curtis (111) (Limnephilidae). 22, Male genitalia <strong>of</strong> specimen from Europe: a,<br />
lateral; b, caudal; c, phallus, lateral; 23, Female genitalia <strong>of</strong> specimen from Europe: a, lateral; b, dorsal; c, ventral;<br />
24, Male genitalia <strong>of</strong> specimen from <strong>Yukon</strong>: a, lateral; b, caudal; c, superior appendage, mesal; d, phallus, tip <strong>of</strong><br />
paramere; 25, Female genitalia <strong>of</strong> specimen from <strong>Yukon</strong>: a, lateral; b, dorsal; c, ventral.
848 G.B. Wiggins and C.R. Parker<br />
FIGS. 26 – 27. Limnephilus indivisus Walker (Limnephilidae), specimens from Ontario. 26, Male genitalia: a, lateral;<br />
b, caudal; c, superior appendage, mesal; d, phallus with detail <strong>of</strong> tip <strong>of</strong> paramere; 27, Female genitalia: a, lateral;<br />
b, dorsal; c, ventral.<br />
The sister species could have arisen through vicariant speciation <strong>of</strong> <strong>the</strong> circumboreal<br />
range <strong>of</strong> a common Pliocene ancestor, giving rise to L. stigma in Eurasia and L. indivisus in<br />
North America; during <strong>the</strong> last glacial advance, L. indivisus probably would have been<br />
restricted to <strong>the</strong> south <strong>of</strong> <strong>the</strong> ice, while L. stigma could have entered <strong>the</strong> East Beringian<br />
refugium but did not extend its range. Larvae <strong>of</strong> <strong>the</strong>se species inhabit small marshy water<br />
bodies, including temporary pools (Wiggins 1973).<br />
Phryganeidae<br />
Agrypnia obsoleta (Hagen) (134)<br />
We have material <strong>of</strong> this widely distributed Eurasian species from <strong>the</strong> nor<strong>the</strong>rn <strong>Yukon</strong>.<br />
Its Nearctic sister species, A. deflata (Milne) (130), is common over much <strong>of</strong> nor<strong>the</strong>rn and<br />
western montane North America; although designated as a subspecies A. obsoleta deflata<br />
by some authors (e.g. Milne 1934; Fischer 1964; Nimmo and Wickstrom 1984), <strong>the</strong> evidence<br />
does not support this interpretation (Wiggins in press). Specimens from British Columbia<br />
identified as A. obsoleta (Nimmo and Scudder 1983: Glacier Nat. Park, 1# 1!) have been<br />
examined, and are A. deflata.<br />
Two successive events seem to have been involved: vicariant subdivision <strong>of</strong> <strong>the</strong><br />
circumboreal ancestor from which <strong>the</strong> sister species A. obsoleta and deflata were derived in<br />
Eurasia and North America respectively, perhaps during <strong>the</strong> Pliocene or earlier; and<br />
subsequent Pleistocene dispersal <strong>of</strong> A. obsoleta to East Beringia during a recent glacial<br />
advance, perhaps with A. deflata restricted to <strong>the</strong> south <strong>of</strong> <strong>the</strong> Laurentide and Cordilleran<br />
glaciers. Outlying montane populations <strong>of</strong> A. deflata, for example in Colorado (Wiggins in
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 849<br />
press), can be interpreted as relicts from this time. With recession <strong>of</strong> <strong>the</strong> ice, A. deflata now<br />
occurs in nor<strong>the</strong>rn and montane North America to <strong>the</strong> <strong>Yukon</strong> and Alaska, but evidently<br />
A. obsoleta has dispersed little beyond Beringia.<br />
Agrypnia sahlbergi (McLachlan) (136)<br />
This species is widespread from Scandinavia through nor<strong>the</strong>rn and eastern Asia. It is<br />
recorded here from <strong>the</strong> <strong>Yukon</strong> and also from Alaska, and recently from British Columbia<br />
(Nimmo and Scudder 1978). We infer that it reached this continent by way <strong>of</strong> <strong>the</strong> Bering<br />
land bridge during <strong>the</strong> Pleistocene, and evidently has dispersed southward to a limited extent.<br />
Oligotricha lapponica (Hagen) (139)<br />
All 5 species <strong>of</strong> Oligotricha are Palaearctic (Wiggins and Kuwayama 1971), but only<br />
O. lapponica extends to North America where records are confined to <strong>the</strong> <strong>Yukon</strong> and Alaska.<br />
From this evidence, we infer a Eurasian origin for O. lapponica, and it probably reached<br />
North America across <strong>the</strong> Bering land bridge during <strong>the</strong> Pleistocene. The forewings usually<br />
bear reticulate dark markings on a lighter background (Wiggins and Kuwayama 1971), but<br />
in some populations from nor<strong>the</strong>rn Lapland <strong>of</strong> Sweden and Finland <strong>the</strong> wings are uniform<br />
dark brown—O. lapponica var. hyperborea (Forsslund 1933). Some specimens we have<br />
seen from East Beringia also have uniform dark brown wings, o<strong>the</strong>rs have normal reticulate<br />
wings. The brown-winged form <strong>of</strong> O. lapponica in Europe and in East Beringia probably<br />
arose from independent changes in <strong>the</strong> genotype. This condition suggests a parallel with<br />
melanic forms that occur in some butterflies, apparently as <strong>the</strong>y approach <strong>the</strong>ir ecological<br />
limits (Downes 1966); but in one species <strong>of</strong> <strong>the</strong> genus, O. striata (Linnaeus) <strong>of</strong> Europe, <strong>the</strong><br />
wings are consistently uniform dark brown (Wiggins and Kuwayama 1971).<br />
This species is a good example <strong>of</strong> <strong>the</strong> Beringian distributional paradox—a widespread<br />
and successful species throughout Eurasia, but evidently still circumscribed in North<br />
America within <strong>the</strong> borders <strong>of</strong> unglaciated Beringia (cf. Asynarchus lapponicus (75),<br />
category II). Biotic factors responsible for this situation could include <strong>the</strong> closely related<br />
phryganeid Banksiola crotchi (Banks) (138)—a common and locally abundant transcontinental<br />
Nearctic species that is clearly aggressive ecologically (Wiggins 1956); Oligotricha<br />
and Banksiola are sister genera (Wiggins in press). Larvae <strong>of</strong> both genera live in shallow<br />
waters <strong>of</strong> lakes, marshes, and slow streams; consequently, suitable habitats that might<br />
o<strong>the</strong>rwise accommodate O. lapponica in North America beyond Beringia are likely to have<br />
been pre-empted by B. crotchi or possibly by some o<strong>the</strong>r species in <strong>the</strong> Phryganeidae.<br />
Coupled with this is <strong>the</strong> probability <strong>of</strong> some genetic drift in <strong>the</strong> populations isolated in<br />
Nearctic Beringia, perhaps altering <strong>the</strong>ir competitive potential; some change in <strong>the</strong> genome<br />
can be inferred from reappearance <strong>of</strong> <strong>the</strong> uniform brown forewings.<br />
IV. Beringian Species<br />
These species share distributional and biological characteristics indicating that <strong>the</strong>y<br />
were confined to Beringia during <strong>the</strong> Pleistocene glacial period. Five are Nearctic species<br />
recorded from <strong>the</strong> <strong>Yukon</strong>, Alaska, or <strong>the</strong> Northwest Territories. On present evidence <strong>the</strong>se<br />
species appear to be East Beringian endemics or glacial relicts from a broader pre-Pleistocene<br />
range in North America. Two species, Lenarchus expansus and Asynarchus innuitorum,<br />
occur in both Nearctic and Palaearctic Beringia, but are not known elsewhere. Most <strong>of</strong> <strong>the</strong>se<br />
species appear to be confined to arctic or alpine tundra, and are consistent with <strong>the</strong> general<br />
pattern in o<strong>the</strong>r groups in which <strong>the</strong> arctic species were derived from Beringia. One Nearctic<br />
species, Limnephilus pallens, is assigned provisionally to category IV because some records
850 G.B. Wiggins and C.R. Parker<br />
are anomalous with <strong>the</strong> general pattern. Two species (†) not yet recorded from <strong>the</strong> <strong>Yukon</strong><br />
may occur <strong>the</strong>re.<br />
Integripalpia<br />
Leptoceridae<br />
Ylodes schmidi Manuel and Nimmo<br />
Apataniidae<br />
Allomyia picoides (Ross) (†)<br />
Limnephilidae<br />
Asynarchus innuitorum (Nimmo) (†)<br />
Grammotaulius alascensis Schmid<br />
Lenarchus expansus Martynov<br />
Limnephilus fumosus Banks<br />
Limnephilus pallens Banks<br />
Sphagnophylax meiops Wiggins and<br />
Winchester<br />
Leptoceridae<br />
Ylodes schmidi Manuel and Nimmo (69)<br />
This species is known only from <strong>the</strong> type locality in <strong>the</strong> <strong>Yukon</strong>, and was proposed as<br />
<strong>the</strong> sister species <strong>of</strong> Y. kaszabi (67) (Manuel and Nimmo 1984). Under that species (category<br />
III), it was suggested that Y. schmidi may have originated as <strong>the</strong> Nearctic vicariant from a<br />
common ancestor that occurred in both northwestern North America and eastern Asia.<br />
Apataniidae<br />
Allomyia picoides (Ross) (†)<br />
This species has not been recorded outside <strong>of</strong> Alaska (Katmai; Ross 1950); although its<br />
range may or may not extend to <strong>the</strong> <strong>Yukon</strong>, it can be considered a Nearctic (East) Beringian<br />
endemic or relict.<br />
Limnephilidae<br />
Asynarchus innuitorum (Nimmo) (†)<br />
Although this species has not been recorded yet from <strong>the</strong> <strong>Yukon</strong>, it was described from<br />
a tundra stream in <strong>the</strong> vicinity <strong>of</strong> Tuktoyaktuk, Northwest Territories, a short distance from<br />
<strong>the</strong> <strong>Yukon</strong> border (Fig. 1) (Winchester 1984: Limnephilus species A); and it has been found<br />
also across <strong>the</strong> Bering Strait in Chukotka (A.P. Nimmo, pers. comm.). Assigned originally<br />
to Limnephilus, this species was transferred to Asynarchus by Ruiter (1995:23-24). On <strong>the</strong><br />
basis <strong>of</strong> existing information, we infer that A. innuitorum is a Beringian endemic, and<br />
probably also a glacial relict.<br />
Grammotaulius alascensis Schmid (84)<br />
This species appears to be <strong>the</strong> Nearctic sister species <strong>of</strong> <strong>the</strong> originally Palaearctic<br />
G. signatipennis (86) (category III). Present evidence suggests that G. alascensis passed<br />
glaciation in East Beringia, and dispersed eastward to Hudson Bay as <strong>the</strong> ice receded (See<br />
Taxonomic Note 6).<br />
Lenarchus expansus Martynov (90)<br />
Records for this species indicate that it is known only from <strong>the</strong> Beringian refugium in<br />
both Asia (Schmid 1952: Kolyma delta, Jana plains) and North America (Alaska, see also<br />
Nimmo 1986; <strong>Yukon</strong>, see above). In West Beringian specimens <strong>the</strong> forewings are light tan<br />
in colour with only faintly contrasting markings (Martynov 1914); but in all except one<br />
specimen (YT: Firth R.; CNCI) in <strong>the</strong> Nearctic material that we have seen, <strong>the</strong> dark markings<br />
<strong>of</strong> <strong>the</strong> forewing contrast strongly with <strong>the</strong> light base colour. Diversification in colour is<br />
probably a consequence <strong>of</strong> <strong>the</strong> isolation <strong>of</strong> <strong>the</strong> 2 populations. Since we found no morphological<br />
differences, <strong>the</strong> amphi-Beringian range could have been achieved by way <strong>of</strong> <strong>the</strong>
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 851<br />
Pleistocene Bering land bridge. The species was assigned to <strong>the</strong> subgenus L. (Lenarchus)<br />
(Schmid 1952), <strong>the</strong> only Holarctic member <strong>of</strong> <strong>the</strong> group <strong>of</strong> 6 Nearctic and Palaearctic species.<br />
Although L. expansus is an amphi-Beringian species, its origin in ei<strong>the</strong>r Asia or North<br />
America remains unresolved.<br />
Larvae <strong>of</strong> most Lenarchus live in standing waters, but those <strong>of</strong> L. expansus have been<br />
found in water-saturated tundra sod (MacLean and Pitelka 1971). Under <strong>the</strong>se conditions,<br />
restriction <strong>of</strong> this species to Beringia is noteworthy because, although wet tundra habitat<br />
seems scarcely limiting now, it is thought to have been rare in Pleistocene Beringia, when<br />
much drier conditions prevailed (Schweger et al. 1982). Consequently, wet tundra must have<br />
been available in Beringia throughout <strong>the</strong> Pleistocene, at least in localized patches. (See also<br />
Sphagnophylax meiops.)<br />
Limnephilus fumosus Banks (102)<br />
Present information on distribution <strong>of</strong> <strong>the</strong> two very similar North American sister<br />
species, L. fumosus and L. santanus, suggests that <strong>the</strong>y may be <strong>the</strong> products <strong>of</strong> populations<br />
that became disjunct during <strong>the</strong> formation <strong>of</strong> glaciers. If Limnephilus fumosus is confined to<br />
nor<strong>the</strong>rn latitudes, it probably passed <strong>the</strong> Pleistocene glacial period in Beringia, although<br />
<strong>the</strong>re is no evidence for dispersal to Asia across <strong>the</strong> Bering land bridge. Limnephilus santanus<br />
is known from Oregon and probably occurs in Washington (Banks 1900). Oregon was not<br />
covered by ice, but lies at <strong>the</strong> sou<strong>the</strong>rn edge <strong>of</strong> <strong>the</strong> maximum extent <strong>of</strong> glaciation; thus records<br />
from British Columbia are critical in interpreting <strong>the</strong> postglacial history <strong>of</strong> <strong>the</strong>se 2 species<br />
(see Taxonomic Note 8). All <strong>of</strong> this indicates that L. fumosus is probably a glacial relict<br />
confined to East Beringia.<br />
Limnephilus pallens Banks (109)<br />
This species was assigned to <strong>the</strong> asiaticus group (Schmid 1955) with 3 o<strong>the</strong>r North<br />
American and 8 Eurasian species. Judging from <strong>the</strong> male genitalic morphology (Nimmo<br />
1991), L. pallens appears to be more closely related to several <strong>of</strong> <strong>the</strong> Eurasian species,<br />
especially L. tricalcaratus Mosely (1936) from Tibet, than to North American species.<br />
Larvae <strong>of</strong> this species live in tundra ponds (Lehmkuhl and Kerst 1979), and thus its<br />
adaptation to far nor<strong>the</strong>rn conditions during Pleistocene glaciation seems assured. If this<br />
species passed <strong>the</strong> glacial period in a nor<strong>the</strong>rn refugium such as East Beringia, evidently it<br />
did not become established in West Beringia. If L. pallens is a Nearctic Beringian endemic<br />
species, it must have dispersed eastward to Hudson Bay (Lehmkuhl and Kerst 1979: Rankin<br />
Inlet) following glacial recession. However, a recent record for L. pallens from <strong>the</strong> Michigan<br />
shore <strong>of</strong> Lake Huron (Ruiter 1995) raises <strong>the</strong> possibility that this eastern extension could<br />
have been derived from <strong>the</strong> sou<strong>the</strong>rn margin <strong>of</strong> <strong>the</strong> glaciers, as <strong>the</strong> ice retreated. Because <strong>of</strong><br />
<strong>the</strong>se discrepancies, assignment <strong>of</strong> Limnephilus pallens to category IV is provisional.<br />
Sphagnophylax meiops Wiggins and Winchester (126)<br />
This species (Frontispiece, Fig. 28) has been collected in <strong>the</strong> <strong>Yukon</strong> and in adjacent<br />
parts <strong>of</strong> <strong>the</strong> Northwest Territories (Aklavik, Tuktoyaktuk), but is o<strong>the</strong>rwise unknown.<br />
Sphagnophylax is a monotypic genus, which on morphological grounds appears to represent<br />
an aberrant lineage in <strong>the</strong> limnephiline tribe Limnephilini (Winchester et al. 1993); and<br />
Sphagnophylax is <strong>the</strong> only trichopteran genus known that is confined to Beringia. Consequently,<br />
S. meiops would be both a phylogenetic and a geographic relict, apparently<br />
preserved from extinction only by <strong>the</strong> unglaciated Beringian refugium. The larval habitat <strong>of</strong><br />
wet moss in transient tundra pools is significant because larvae <strong>of</strong> some o<strong>the</strong>r phylogenetically<br />
relict Trichoptera also occur in wet edaphic sites; a low incidence <strong>of</strong> competitors and
852 G.B. Wiggins and C.R. Parker<br />
FIG. 28. Adult <strong>of</strong> Sphagnophylax meiops Wiggins and Winchester (126) (Limnephilidae). Brachyptery and<br />
anomalous venation suggest that this species is probably flightless, and perhaps was preserved from extinction only<br />
by <strong>the</strong> unglaciated Beringian refugium. Forewing length 4 mm. (From Canadian Journal <strong>of</strong> Zoology)<br />
predators in <strong>the</strong>se sites may be a factor in <strong>the</strong> survival <strong>of</strong> relict Trichoptera (Wiggins 1984).<br />
Occurrence <strong>of</strong> this species in wet tundra is also interesting because <strong>the</strong> Beringian refugium<br />
for at least part <strong>of</strong> <strong>the</strong> Pleistocene is inferred to have been a region <strong>of</strong> low precipitation<br />
throughout <strong>the</strong> year and a predominantly dry upland region with little muskeg where<br />
Sphagnum was absent (Schweger et al. 1982). Survival <strong>of</strong> S. meiops, and also <strong>of</strong> Lenarchus<br />
expansus (see above) which evidently lives under similar conditions, indicates that areas <strong>of</strong><br />
wet tundra persisted in Beringia.<br />
Reduced wings (Fig. 28) and anomalous venation suggest that this species has limited<br />
ability to fly and perhaps is even flightless; and <strong>the</strong> eyes are unusually small for adult<br />
Trichoptera. Similar modifications occur in a number <strong>of</strong> arctic Lepidoptera (Downes 1964).<br />
V. Holarctic Species Not in Beringia<br />
Because categories II, III and IV include almost all <strong>of</strong> <strong>the</strong> North American Trichoptera<br />
now recognized as occurring in Europe and Asia as well, it is useful for comparison to add<br />
here <strong>the</strong> few remaining Holarctic species. Six species are assigned to category V, and none<br />
has been recorded from <strong>the</strong> <strong>Yukon</strong>; most <strong>of</strong> <strong>the</strong>m probably dispersed from one continent to<br />
<strong>the</strong> o<strong>the</strong>r under more equable conditions <strong>of</strong> climate some time before <strong>the</strong> separation <strong>of</strong> Asia<br />
and North America in <strong>the</strong> Pliocene. With Holarctic distribution established long ago, <strong>the</strong>se<br />
species appear now to be disjunct relicts, still resistant to cladogenetic divergence.
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 853<br />
Hydroptilidae<br />
Ithytrichia clavata Morton<br />
This species is widely distributed in North America from British Columbia to California,<br />
through Texas, Kansas, Oklahoma to Quebec and New Hampshire; it is widespread through<br />
nor<strong>the</strong>rn and western Europe (L. BotojAneanu, pers. comm.). It is not now a far nor<strong>the</strong>rn<br />
species in ei<strong>the</strong>r North America or Europe, and would seem unlikely to have dispersed across<br />
<strong>the</strong> Bering land bridge during a glacial climate regime. Moreover, <strong>the</strong> larvae live in running<br />
water, a habitat used by only very few successful Pleistocene dispersants. This evidence<br />
suggests that Holarctic distribution was achieved before <strong>the</strong> Quaternary glacial periods<br />
occurred, and perhaps <strong>the</strong> Nearctic and Palaearctic forms have diverged to some extent not<br />
yet recognized, as in <strong>the</strong> Agraylea multipunctata complex (Vineyard and Wiggins in prep.).<br />
In any event, <strong>the</strong> name I. clavata was based initially on populations in New York (Ithaca),<br />
and fur<strong>the</strong>r taxonomic resolution is focussed on European populations.<br />
Oxyethira mirabilis Morton<br />
The range <strong>of</strong> this species in nor<strong>the</strong>rn Europe has been extended to eastern Canada with<br />
recognition <strong>of</strong> O. barnstoni Harper as a junior subjective synonym (Kelley 1984). The<br />
pattern <strong>of</strong> distribution, if now adequately understood, suggests a north Atlantic dispersal. It<br />
is <strong>the</strong> only European representative in <strong>the</strong> aeola group <strong>of</strong> O. (Oxytrichia), which is o<strong>the</strong>rwise<br />
entirely confined to North and South America (Kelley 1984).<br />
Polycentropodidae<br />
Polycentropus picicornis Stephens<br />
This species occurs through most <strong>of</strong> Europe (BotojAneanu and Malicky 1978), Siberia<br />
and Mongolia to Kamchatka (Lepneva 1964; Mey and Dulmaa 1985). It is known locally in<br />
North America from <strong>the</strong> Northwest Territories to New Hampshire and may yet be recorded<br />
from <strong>the</strong> <strong>Yukon</strong>. Larvae live in small bodies <strong>of</strong> standing water and in slow currents <strong>of</strong> rivers<br />
(Lepneva 1964). This pattern <strong>of</strong> nor<strong>the</strong>rn distribution suggests that P. picicornis could have<br />
passed between Asia and North America more recently than o<strong>the</strong>r species <strong>of</strong> category V,<br />
possibly during <strong>the</strong> Pleistocene.<br />
Psychomyiidae<br />
Psychomyia flavida Hagen<br />
This species was described from North America where it is transcontinental and<br />
abundant in running waters. The Asian P. composita Martynov was proposed as a junior<br />
subjective synonym <strong>of</strong> P. flavida (Schmid 1965b); consequently, a wide distribution through<br />
Siberia, Mongolia, and North America now has to be attributed to P. flavida. In this context,<br />
<strong>the</strong> absence <strong>of</strong> records <strong>of</strong> any Psychomyia from <strong>the</strong> Russian Far East (e.g. Levanidova 1982)<br />
is <strong>of</strong> interest. In North America, P. flavida has not been recorded in <strong>the</strong> extreme northwest,<br />
but only to <strong>the</strong> edge <strong>of</strong> treeline at Churchill, Manitoba (Lehmkuhl and Kerst 1979), and<br />
ranges from British Columbia to California and Nova Scotia to North Carolina. Thus,<br />
whatever its place <strong>of</strong> origin, dispersal <strong>of</strong> P. flavida across <strong>the</strong> Bering land connection between<br />
North America and Asia probably occurred in a climate more moderate than <strong>the</strong> Pleistocene<br />
glacial periods.<br />
Limnephilidae<br />
Grammotaulius betteni Hill-Griffin<br />
Known originally from Oregon, this species has also been recorded from China (Schmid<br />
1950a). Larvae live in slow streams and small marshy ponds (Hill-Griffin 1912), some <strong>of</strong>
854 G.B. Wiggins and C.R. Parker<br />
which are probably temporary (Wiggins 1977); however, <strong>the</strong> species is not part <strong>of</strong> <strong>the</strong><br />
nor<strong>the</strong>rn Nearctic fauna.<br />
Hydatophylax variabilis Martynov<br />
Widely distributed through nor<strong>the</strong>rn Eurasia from Sweden to Kamchatka (Schmid<br />
1950b) and Chukotka (Levanidova 1982) at <strong>the</strong> eastern extremity <strong>of</strong> Siberia, this species has<br />
also been collected at several localities in sou<strong>the</strong>astern Alaska. It has not been recorded from<br />
<strong>the</strong> <strong>Yukon</strong> or elsewhere in North America. The species is part <strong>of</strong> an Asian complex (Schmid<br />
1950b), and on present evidence represents dispersal across <strong>the</strong> land connection between<br />
North America and Asia—ei<strong>the</strong>r in Pleistocene or Pliocene time. This is <strong>the</strong> only Holarctic<br />
trichopteran known to occur in <strong>the</strong> coastal extension <strong>of</strong> sou<strong>the</strong>astern Alaska; several Nearctic<br />
species appear to have moved northward to this area after retreat <strong>of</strong> <strong>the</strong> glaciers, but are not<br />
known elsewhere in Alaska or in <strong>the</strong> <strong>Yukon</strong>. This pattern <strong>of</strong> distribution might reflect a<br />
coastal glacial refugium for aquatic insects (e.g. Kavanaugh 1988), but streams with ample<br />
organic detritus would be required for survival <strong>of</strong> <strong>the</strong> larvae (e.g. Irons 1988).<br />
Ecological Considerations<br />
Aquatic insects have major roles in <strong>the</strong> cycling <strong>of</strong> nutrients and energy which underlies<br />
<strong>the</strong> natural productivity <strong>of</strong> freshwater systems. At temperate latitudes, aquatic insect larvae<br />
are diverse and abundant; and in individual systems, Trichoptera are usually high in both<br />
diversity and abundance in relation to o<strong>the</strong>r aquatic insects (Wiggins and Mackay 1978).<br />
However, our analysis has shown that species diversity in Trichoptera shows a marked<br />
decline at higher latitudes. Because <strong>the</strong> fauna is now fairly well defined, <strong>the</strong> <strong>Yukon</strong><br />
Trichoptera provide a promising focus for inquiry into <strong>the</strong> exploitation <strong>of</strong> aquatic habitats<br />
by caddisflies at higher latitudes and into <strong>the</strong> biological factors responsible for this latitudinal<br />
decline in diversity.<br />
We examine <strong>the</strong>se questions by contrasting patterns <strong>of</strong> geographic distribution and <strong>the</strong><br />
use <strong>of</strong> resources in different behavioural and ecological groups <strong>of</strong> <strong>Yukon</strong> Trichoptera with<br />
<strong>the</strong> same groups at more temperate latitudes in North America. Considering first <strong>the</strong> generic<br />
level, approximately 150 genera are recognized in North American Trichoptera—case-makers,<br />
retreat-makers, and cocoon-making species combined (Wiggins 1996). Comparison<br />
between genera <strong>of</strong> running-water (lotic) and standing-water (lentic) forms in <strong>the</strong> North<br />
American Trichoptera yields a ratio for lotic genera to lentic genera <strong>of</strong> about 4:1. This is a<br />
valid biological comparison because <strong>the</strong> genus is an ecological as well as a morphological<br />
unit for Trichoptera (Wiggins and Mackay 1978); with few exceptions, habitats for species<br />
at this broad level <strong>of</strong> discrimination are consistent within genera <strong>of</strong> Trichoptera. A ratio <strong>of</strong><br />
4:1 demonstrates that in North America overall <strong>the</strong>re are, by a substantial margin, more<br />
ecological niches accessible to Trichoptera in running waters than in standing waters.<br />
A similar comparison between lotic and lentic genera for <strong>the</strong> 51 genera <strong>of</strong> Trichoptera<br />
recorded from <strong>the</strong> <strong>Yukon</strong> yields a ratio <strong>of</strong> approximately 1:1. The contrast between 4:1 for<br />
North America and 1:1 for <strong>the</strong> <strong>Yukon</strong> substantiates a marked decline in lotic-dwelling taxa<br />
with increasing latitude. This decline could be attributed ei<strong>the</strong>r to reduction in <strong>the</strong> resources<br />
available to lotic Trichoptera, or to diminishing ability <strong>of</strong> Trichoptera to exploit lotic niches<br />
at higher latitudes; this issue is best approached at <strong>the</strong> species level.<br />
To examine <strong>the</strong> decline in diversity <strong>of</strong> Trichoptera at <strong>the</strong> species level in <strong>the</strong> north, <strong>the</strong><br />
<strong>Yukon</strong> fauna <strong>of</strong> 145 species can be contrasted with that <strong>of</strong> British Columbia immediately to<br />
<strong>the</strong> south, where 279 species have been recorded (Nimmo and Scudder 1978, 1983). In broad
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 855<br />
TABLE 1. Numbers <strong>of</strong> species in families <strong>of</strong> Trichoptera: data for <strong>Yukon</strong> from this study; for British Columbia from<br />
Nimmo and Scudder (1978, 1983), and for Alaska from Nimmo (1986), with taxonomic emendations.<br />
Family British Columbia<br />
lat. 49° to 60°N<br />
Alaska <strong>Yukon</strong><br />
lat. 60° to 70°N lat. 67° to 70°N<br />
Spicipalpia 70 23 22 4<br />
Glossosomatidae 13 4 3 1<br />
Hydroptilidae 14 4 5 2<br />
Rhyacophilidae 43 15 14 1<br />
Annulipalpia 41 14 17 7<br />
Hydropsychidae 21 7 10 2<br />
Philopotamidae 7 1 1 1<br />
Polycentropodidae 13 6 6 4<br />
Integripalpia 168 95 106 49<br />
Apataniidae 7 5 4 2<br />
Brachycentridae 5 4 3 2<br />
Calamoceratidae 1 - - -<br />
Goeridae 1 1 1 1<br />
Lepidostomatidae 11 3 6 -<br />
Leptoceridae 22 12 16 7<br />
Limnephilidae 98 57 57 26<br />
Molannidae 1 2 2 2<br />
Phryganeidae 14 8 13 9<br />
Rossianidae 1 - - -<br />
Uenoidae 8 3 4 -<br />
Totals 279 132 145 60<br />
terms, <strong>the</strong>se figures demonstrate that from latitude 49°N on <strong>the</strong> sou<strong>the</strong>rn border <strong>of</strong> British<br />
Columbia to 60°N at <strong>the</strong> sou<strong>the</strong>rn border <strong>of</strong> <strong>the</strong> <strong>Yukon</strong>, <strong>the</strong> trichopteran fauna decreases by<br />
134 species—approximately 50 per cent (Table 1). An adjunct to this database is available<br />
from a list <strong>of</strong> <strong>the</strong> Trichoptera <strong>of</strong> Alaska (Nimmo 1986). These 3 adjacent areas have similar<br />
montane topography and all have been affected by glaciation to some extent, ei<strong>the</strong>r directly<br />
or indirectly.<br />
To extend <strong>the</strong> analysis, <strong>the</strong> nor<strong>the</strong>rn terminus <strong>of</strong> this latitudinal gradient in <strong>the</strong> <strong>Yukon</strong><br />
can be represented by ecogeographic regions 1 through 5 (Fig. 1), from approximately<br />
latitude 67°N at <strong>the</strong> Arctic Circle to 70°N, extending through treeline and coastal tundra to<br />
<strong>the</strong> Arctic shoreline. Sixty species <strong>of</strong> Trichoptera are recorded within that area (Table 1),<br />
although o<strong>the</strong>rs will likely still be found. Consequently, <strong>the</strong> <strong>Yukon</strong> Trichoptera fauna<br />
declines from 145 species to approximately 60 species, about 59 per cent, through <strong>the</strong><br />
latitudinal gradient <strong>of</strong> 60° to 70°N.<br />
Numbers <strong>of</strong> species in each family <strong>of</strong> Trichoptera compiled for <strong>the</strong>se areas are summarized<br />
in Table 1. Through approximately <strong>the</strong> same latitudinal range, total numbers <strong>of</strong> species<br />
in <strong>the</strong> faunas <strong>of</strong> <strong>Yukon</strong> and Alaska are close, as are <strong>the</strong> figures for all 3 suborders. The<br />
trichopteran fauna <strong>of</strong> British Columbia is approximately twice that <strong>of</strong> ei<strong>the</strong>r <strong>Yukon</strong> or<br />
Alaska; and within <strong>the</strong> overall latitudinal range <strong>of</strong> 49° to 70°N <strong>the</strong> fauna decreases from 279<br />
species to 60—approximately 78 per cent overall. Changes in proportions <strong>of</strong> <strong>the</strong> 3 major<br />
groups <strong>of</strong> Trichoptera in <strong>the</strong>se regional faunas are shown in Table 2; <strong>the</strong>re is a general decline<br />
in proportions <strong>of</strong> species <strong>of</strong> <strong>the</strong> Spicipalpia and Annulipalpia, and an increase in <strong>the</strong><br />
proportion <strong>of</strong> species <strong>of</strong> <strong>the</strong> case-making Integripalpia.
856 G.B. Wiggins and C.R. Parker<br />
TABLE 2. Percentages <strong>of</strong> <strong>the</strong> regional trichopteran faunas in major groups, based on data from Table 1.<br />
Major group British Columbia Alaska <strong>Yukon</strong><br />
lat. 49° to 60°N lat. 60° to 70°N lat. 67° to 70°N<br />
Spicipalpia<br />
(mainly carnivores and<br />
algal feeders in lotic<br />
habitats)<br />
25% 17% 15% 7%<br />
Annulipalpia<br />
(fixed retreats in lotic<br />
habitats)<br />
15% 11% 10% 12%<br />
Integripalpia<br />
(portable cases, mainly in<br />
lentic habitats)<br />
60% 72% 75% 81%<br />
100% 100% 100% 100%<br />
In far nor<strong>the</strong>rn parts <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> from latitude 67° to 70°N (Table 2), <strong>the</strong> Spicipalpia<br />
decline substantially from <strong>the</strong>ir representation over <strong>the</strong> <strong>Yukon</strong> and Alaska as a whole, due<br />
mainly to a drastic reduction in <strong>the</strong> lotic and predatory Rhyacophilidae. The Annulipalpia<br />
maintain about <strong>the</strong> same proportion as for <strong>the</strong> <strong>Yukon</strong> generally, with a large decline in<br />
filter-feeding lotic Hydropsychidae, but a small reduction in <strong>the</strong> Polycentropodidae which<br />
are mainly predacious species tolerant <strong>of</strong> slow currents and lentic waters. Correspondingly,<br />
<strong>the</strong> proportion <strong>of</strong> case-making Integripalpia in <strong>the</strong> far nor<strong>the</strong>rn trichopteran fauna increases<br />
somewhat to 81 per cent (Table 2). Species <strong>of</strong> <strong>the</strong> Limnephilidae decline from 57 in <strong>the</strong><br />
<strong>Yukon</strong> generally to 26 in <strong>the</strong> far nor<strong>the</strong>rn section (Table 1). The genus Limnephilus is<br />
represented in <strong>the</strong> <strong>Yukon</strong> overall by 27 species, but declines to 15 in <strong>the</strong> extreme nor<strong>the</strong>rn<br />
part. Limnephilid species <strong>of</strong> <strong>the</strong> far nor<strong>the</strong>rn part <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> occur almost entirely in<br />
standing waters or slow currents in streams <strong>of</strong> low gradient; Dicosmoecus obscuripennis<br />
(81) is perhaps <strong>the</strong> sole exception, and occurs largely in lotic waters.<br />
Reduction in species <strong>of</strong> <strong>the</strong> Spicipalpia and Annulipalpia in <strong>the</strong> <strong>Yukon</strong> and Alaska<br />
(Table 1) translates biologically into a decrease <strong>of</strong> Trichoptera in running-water habitats at<br />
<strong>the</strong>se latitudes, consistent with <strong>the</strong> generic analysis. Each <strong>of</strong> <strong>the</strong> 3 Spicipalpian families<br />
represented is substantially reduced (Table 1); and to whatever extent trophic factors are<br />
responsible for <strong>the</strong> decline, <strong>the</strong>y do differ for each family. Larvae in <strong>the</strong> Glossosomatidae<br />
are grazers <strong>of</strong> diatoms and fine organic particles. In <strong>the</strong> Hydroptilidae, larvae in almost all<br />
<strong>of</strong> <strong>the</strong> genera represented feed on filamentous algae. Larvae <strong>of</strong> most species in <strong>the</strong> Rhyacophilidae<br />
are believed to be predacious, although <strong>the</strong>re is some evidence for specific restriction<br />
to certain prey groups, and a few feed on algae and vascular plant tissue; for this family a<br />
decline <strong>of</strong> prey organisms in lotic habitats at higher latitudes could have some influence on<br />
diversity.<br />
In <strong>the</strong> Annulipalpia through <strong>the</strong> latitudinal gradient <strong>of</strong> 49° to 70°, species decrease by<br />
about 50 per cent in both <strong>the</strong> Hydropsychidae and Polycentropodidae. The Philopotamidae<br />
show a marked decline in species by about 85 per cent, and are rare in collections from<br />
<strong>the</strong> <strong>Yukon</strong>; larvae in this family are filter-feeders, but consume finer organic particulates<br />
than do o<strong>the</strong>r filter-feeding Trichoptera. The fine particulate organic matter consumed by<br />
filter-feeders and by Annulipalpia generally is largely produced by larvae <strong>of</strong> <strong>the</strong> shredder<br />
guild <strong>of</strong> aquatic insects, which includes most case-making Trichoptera (Integripalpia).<br />
Decline in filter-feeding Hydropsychidae could be caused by a decrease in fine particulate<br />
organic matter carried in suspension by <strong>the</strong> current; reduced populations <strong>of</strong> lotic insects
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 857<br />
generally could affect <strong>the</strong> supply. Part <strong>of</strong> <strong>the</strong> nutrient value <strong>of</strong> this resource is aquatic<br />
hyphomycete fungi growing on faecal particles <strong>of</strong> o<strong>the</strong>r aquatic invertebrates; <strong>the</strong> fungi are<br />
known to grow at low temperatures (Bärlocher and Kendrick 1973). However, studies by<br />
Irons et al. (1994) suggest that microbial populations are physiologically less able to maintain<br />
optimal metabolic rates at <strong>the</strong> cold temperatures <strong>of</strong> high latitudes. O<strong>the</strong>r suspended materials<br />
including ultra-fine particulate matter may be involved, but <strong>the</strong> decline in filter-feeding<br />
Trichoptera raises <strong>the</strong> likelihood that nutritional resources from suspended particulates in<br />
general may be marginal for supporting Trichoptera. These factors suggest that filter-feeding<br />
species <strong>of</strong> <strong>the</strong> Hydropsychidae which are successful at higher latitudes may derive a larger<br />
proportion <strong>of</strong> <strong>the</strong>ir food from insect prey than do those at lower latitudes, and perhaps are<br />
preadapted through a tendency toward increased predation; <strong>the</strong> hydropsychid subfamily<br />
Arctopsychinae (Arctopsyche, Parapsyche) is such a group, and is relatively well represented<br />
at higher latitudes. Filter-feeding larvae <strong>of</strong> <strong>the</strong> Polycentropodidae (Polycentropus,<br />
Neureclipsis) also derive a larger part <strong>of</strong> <strong>the</strong>ir food from insect prey than do Hydropsyche<br />
and Cheumatopsyche (e.g. Wiggins 1977, 1996). This difference could underlie <strong>the</strong> more<br />
nor<strong>the</strong>rly <strong>Yukon</strong> distribution for polycentropodids among Annulipalpia, and is ano<strong>the</strong>r<br />
question to which more detailed study <strong>of</strong> <strong>Yukon</strong> Trichoptera might be directed.<br />
Our analysis shows that within <strong>the</strong> latitudinal gradient <strong>of</strong> 49° to 70°N, <strong>the</strong>re is a marked<br />
decline in lotic Trichoptera, chiefly Spicipalpia and Annulipalpia, in all <strong>of</strong> <strong>the</strong>ir niches <strong>of</strong><br />
resource exploitation. Changing trophic resources in lotic habitats will be responsible for<br />
some <strong>of</strong> this decline, but physical changes in running-water habitats must be taken into<br />
account. The range <strong>of</strong> lotic habitats at higher latitudes does not differ significantly from that<br />
available to <strong>the</strong> south, and <strong>the</strong>se habitats are highly diverse in <strong>the</strong> <strong>Yukon</strong> and Alaska; but<br />
colder temperatures influence winter survival <strong>of</strong> lotic Trichoptera in smaller streams that<br />
freeze to <strong>the</strong> bottom (e.g. Harper 1981), including <strong>the</strong> hyporheic zone. Larval Trichoptera<br />
do not overwinter in streams that freeze to <strong>the</strong> bottom in subarctic interior Alaska (Irons et<br />
al. 1993), but survive only in sections that do not freeze (Irons 1988). Surveys <strong>of</strong> Arctic slope<br />
streams show that communities <strong>of</strong> benthic invertebrates are more diverse and <strong>of</strong> higher<br />
density in spring streams than in tundra streams, which in turn are richer than mountain<br />
streams (Craig and McCart 1975). The differences were attributed largely to <strong>the</strong> perennial<br />
flow from groundwater in spring streams, contrasted with interrupted flow when <strong>the</strong> o<strong>the</strong>r<br />
streams are frozen to <strong>the</strong> bottom during <strong>the</strong> long winter period. Trichoptera occurred in 68<br />
per cent <strong>of</strong> 59 spring streams sampled, but in only 21 per cent <strong>of</strong> 98 tundra streams, and 1<br />
per cent <strong>of</strong> 137 mountain streams. Taxonomic refinement below <strong>the</strong> ordinal level was not<br />
provided, but would have been highly informative even at <strong>the</strong> family level; in any event,<br />
both Plecoptera and Ephemeroptera maintained relatively high occurrence <strong>of</strong> 95 to 63 per<br />
cent in all 3 stream types in <strong>the</strong> same survey. In <strong>the</strong> larger rivers, water would remain unfrozen<br />
beneath <strong>the</strong> ice. Consequently, <strong>the</strong> low extremes <strong>of</strong> temperature in rivers would not differ<br />
greatly from lotic waters at more sou<strong>the</strong>rly latitudes, although <strong>the</strong> longer duration <strong>of</strong> <strong>the</strong> ice<br />
cover might reduce <strong>the</strong> annual period suitable for growth. However, bottom substrates <strong>of</strong><br />
large nor<strong>the</strong>rn rivers tend to be mainly unstable shifting sediments (Barton 1986; Soluk<br />
1985), and inappropriate for stationary, filter-feeding annulipalpian Trichoptera. For example,<br />
<strong>the</strong> low number <strong>of</strong> caddisflies colonizing streams following <strong>the</strong> Mt. St. Helens eruption<br />
in Washington was attributed by Anderson (1992) to shifting substrata and high mobility <strong>of</strong><br />
<strong>the</strong> stream bed. In a glacier-fed river in Alaska, Trichoptera were one <strong>of</strong> <strong>the</strong> last insect orders<br />
to appear at <strong>the</strong> progression <strong>of</strong> sampling sites from <strong>the</strong> headwaters, downstream (Slack et al.<br />
1979). Studying development <strong>of</strong> freshwater communities following rapid recession <strong>of</strong> a<br />
neoglacial ice sheet in Alaska, Milner (1987) found that Chironomidae, Ephemeroptera, and
858 G.B. Wiggins and C.R. Parker<br />
Plecoptera colonized streams but Trichoptera had a minimal role in <strong>the</strong> formation <strong>of</strong> new<br />
communities. Marked decline in <strong>the</strong> proportion <strong>of</strong> Trichoptera in lotic systems <strong>of</strong> Alaska<br />
generally was found by Oswood (1989). Therefore, compared to <strong>the</strong>ir dominant role in lotic<br />
systems at temperate latitudes (Wiggins and Mackay 1978), Trichoptera are ill-suited to<br />
running waters at high latitudes, where larvae are exposed to encasement in ice, unstable<br />
substrates, and suspended flow.<br />
However, larvae <strong>of</strong> several species <strong>of</strong> case-making Trichoptera (Integripalpia: Limnephilidae,<br />
Phryganeidae) in a slow arctic tundra stream near Tuktoyaktuk, Northwest<br />
Territories (lat. 69°N), remained frozen in <strong>the</strong> ice from October through May, when <strong>the</strong>y<br />
resumed development to complete <strong>the</strong>ir univoltine life cycle (Winchester 1984). Tundra<br />
streams support lentic species for <strong>the</strong> most part. These observations suggest that certain lentic<br />
Trichoptera are physiologically capable <strong>of</strong> tolerating freezing <strong>of</strong> body fluids, even though<br />
some evidence indicates that avoiding freezing by supercooling is unlikely for most aquatic<br />
insects (Oswood et al. 1991). In Norway, Solem (1981) found larvae <strong>of</strong> Agrypnia obsoleta<br />
(Phryganeidae) to survive enclosure in solid ice for 6 months to –11°C; laboratory experiments<br />
confirmed freezing resistance for A. obsoleta, but larvae <strong>of</strong> Phryganea bipunctata<br />
were dead after several weeks in ice. Larvae <strong>of</strong> Agrypnia obsoleta (Phryganeidae), Oecetis<br />
ochracea (Leptoceridae), and 2 species <strong>of</strong> Molanna (Molannidae) which survived freezing<br />
in ice in a Swedish river had blocked <strong>the</strong> openings <strong>of</strong> <strong>the</strong>ir cases, although <strong>the</strong>y were not in<br />
prepupal or pupal stages (Olsson 1981). These observations raise <strong>the</strong> critical question<br />
whe<strong>the</strong>r <strong>the</strong> portable integripalpian case confers some physiological advantage for overwintering<br />
trichopteran larvae embedded in ice? In Chironomidae, larvae constructing winter<br />
cocoons have a higher survival rate in frozen habitats than do larvae without cocoons (Danks<br />
1971). Tolerance to <strong>the</strong> freezing <strong>of</strong> body fluids appears to be a requirement <strong>of</strong> Trichoptera<br />
living at high latitudes, but <strong>the</strong>se observations fur<strong>the</strong>r suggest that <strong>the</strong>re may be behavioural<br />
as well as physiological components to that tolerance. Resistance to low levels <strong>of</strong> oxygen is<br />
ano<strong>the</strong>r aspect <strong>of</strong> <strong>the</strong> survival <strong>of</strong> aquatic insects encased in ice (Moore and Lee 1991).<br />
A wholly different approach to cold winter temperatures is shown in 2 species <strong>of</strong> <strong>the</strong><br />
Limnephilidae, Glyphopsyche irrorata and Psychoglypha subborealis (83, 125). Adults <strong>of</strong><br />
both species collected from October through May near Juneau, Alaska by Ellis (1978a)<br />
became sexually mature in spring. This unusual strategy for surviving cold winter conditions<br />
raises <strong>the</strong> question whe<strong>the</strong>r larvae <strong>of</strong> <strong>the</strong>se species are tolerant <strong>of</strong> freezing; larvae <strong>of</strong><br />
G. irrorata occur in lentic habitats, P. subborealis in lotic. Both species are assigned to<br />
category I, and are inferred to have reached <strong>the</strong> <strong>Yukon</strong> from more sou<strong>the</strong>rly areas following<br />
retreat <strong>of</strong> <strong>the</strong> glaciers.<br />
Among <strong>the</strong> 3 suborders, <strong>the</strong>re is a considerably smaller latitudinal decline in species <strong>of</strong><br />
<strong>the</strong> case-making Integripalpia <strong>of</strong> about 40 per cent through 49° to 70°N (Table 1). Again,<br />
trophic characteristics in <strong>the</strong> families <strong>of</strong> Integripalpia are not uniform. Larvae <strong>of</strong> Apataniidae,<br />
Goeridae, Uenoidae, and Brachycentridae in part, feed mainly by grazing diatoms from rock<br />
surfaces; this is <strong>the</strong> same trophic guild to which <strong>the</strong> Glossosomatidae (Spicipalpia, see above)<br />
belong. All <strong>of</strong> <strong>the</strong>se groups occur in lotic habitats, demonstrating that in running waters food<br />
resources for grazing larvae do support Trichoptera at latitudes <strong>of</strong> 60° to 70°N.<br />
O<strong>the</strong>r groups <strong>of</strong> Integripalpia in streams at high latitudes are detritivorous: Lepidostomatidae;<br />
and Limnephilidae (Chyranda, Dicosmoecus, Hesperophylax, Onocosmoecus,<br />
Psychoglypha). As members <strong>of</strong> <strong>the</strong> functional group <strong>of</strong> shredders, larvae <strong>of</strong> <strong>the</strong>se species<br />
feed mainly on allochthonous plant debris supporting microbial growth. This food resource<br />
may be limiting; <strong>the</strong> supply <strong>of</strong> detritus in a subarctic Alaskan stream was meagre compared<br />
with that in temperate streams (Cowan and Oswood 1984), and was believed to influence
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 859<br />
strongly <strong>the</strong> spatial and temporal patterns <strong>of</strong> detritivores. Moreover, microbial processing <strong>of</strong><br />
plant detritus in high latitude streams was held to be impeded at low temperatures (Irons<br />
et al. 1994).<br />
Although <strong>the</strong> success <strong>of</strong> case-making Trichoptera in subarctic running waters is constrained<br />
by ice and by low levels <strong>of</strong> allochthonous detritus, biological interactions in<br />
subarctic ponds and lakes appear to be ra<strong>the</strong>r different because it is in <strong>the</strong>se lentic habitats<br />
that Trichoptera flourish at higher latitudes. Case-making Integripalpia constitute 75 per cent<br />
<strong>of</strong> <strong>the</strong> species <strong>of</strong> <strong>Yukon</strong> Trichoptera (Table 2), and more than half <strong>of</strong> <strong>the</strong>se (56 per cent) live<br />
in lentic habitats. Since inputs <strong>of</strong> allochthonous detritus are relatively lower in ponds and<br />
lakes because <strong>the</strong> ratio <strong>of</strong> shoreline to water area is much lower than it is in streams,<br />
autochthonous plant matter seems to be <strong>the</strong> energy resource underlying <strong>the</strong> success <strong>of</strong><br />
detritivorous Trichoptera in <strong>the</strong>se habitats. A number <strong>of</strong> species <strong>of</strong> both submerged and<br />
emergent vascular aquatic plants occur in sou<strong>the</strong>rn parts <strong>of</strong> <strong>the</strong> arctic tundra, and considerably<br />
more in <strong>the</strong> subarctic (e.g. Hobbie 1973; I.L. Wiggins and Thomas 1962).<br />
Case-making larvae in <strong>the</strong> Phryganeidae, Molannidae, and Leptoceridae (Ceraclea,<br />
Mystacides, Oecetis) are predacious on insects and o<strong>the</strong>r invertebrates; overall, <strong>the</strong>se groups<br />
decrease ra<strong>the</strong>r little at higher latitudes (Table 1).<br />
The latitudinal gradient analyzed here reflects <strong>the</strong> scale <strong>of</strong> environmental constraints<br />
met by species <strong>of</strong> Trichoptera dispersing to <strong>the</strong> north in <strong>the</strong> wake <strong>of</strong> receding glacial ice. The<br />
gradient also reveals a pattern in <strong>the</strong>ir use <strong>of</strong> <strong>the</strong> resources <strong>of</strong> aquatic systems under<br />
conditions imposed by increasing latitude. However, <strong>the</strong> relatively smaller decline in<br />
case-making Integripalpia within this latitudinal gradient can also be interpreted as a<br />
reflection <strong>of</strong> <strong>the</strong> capacity <strong>of</strong> some species <strong>of</strong> <strong>the</strong> Limnephilidae and Phryganeidae to exploit<br />
lentic habitats at high latitudes. Both families appear to have originated in running waters,<br />
but most extant species have been derived subsequently in lentic habitats. Thus, some groups<br />
<strong>of</strong> Limnephilidae and Phryganeidae can be seen as preadapted through origin to low<br />
temperatures and nor<strong>the</strong>rly photoperiods. If a number <strong>of</strong> species in <strong>the</strong>se families have <strong>the</strong><br />
physiological capability to live under conditions <strong>of</strong> extreme cold, <strong>the</strong> failure <strong>of</strong> most o<strong>the</strong>r<br />
families to do so may reflect <strong>the</strong> warmer climatic conditions in which <strong>the</strong>y originated.<br />
Consequently, although reduced resources in aquatic communities may account for some <strong>of</strong><br />
<strong>the</strong> depletion in species diversity, most groups <strong>of</strong> Trichoptera may have evolved at more<br />
temperate latitudes and probably still lack <strong>the</strong> physiological capability to persist under<br />
relatively recent regimes <strong>of</strong> cold climates at high latitudes (see above, Origin <strong>of</strong> <strong>the</strong> Beringian<br />
and Holarctic Trichoptera).<br />
Diptera are one <strong>of</strong> <strong>the</strong> most successful insect orders in <strong>the</strong> far north, particularly <strong>the</strong><br />
family Chironomidae (e.g. Oliver 1968). Because most chironomid larvae are aquatic, and<br />
adults feed sparingly if at all, <strong>the</strong> success <strong>of</strong> this family in <strong>the</strong> far north has been attributed<br />
to <strong>the</strong>ir independence from plants and o<strong>the</strong>r insects (Downes 1962). Trichoptera, however,<br />
are biological analogues <strong>of</strong> Chironomidae but are represented in <strong>the</strong> far north by far fewer<br />
species, fur<strong>the</strong>r indicating that intrinsic limitations <strong>of</strong> Trichoptera influence <strong>the</strong> penetration<br />
<strong>of</strong> <strong>the</strong>se insects into far nor<strong>the</strong>rn latitudes. Larvae <strong>of</strong> Chironomidae and <strong>of</strong> Empididae survive<br />
freezing in Alaskan subarctic streams (Irons et al. 1993).<br />
Larval habitat and food must also have governed <strong>the</strong> success <strong>of</strong> Trichoptera in <strong>the</strong>ir<br />
passage across <strong>the</strong> Bering land bridge. Among <strong>the</strong> present Holarctic and Beringian Trichoptera<br />
are 43 lentic species but only 8 lotic species—a ratio <strong>of</strong> approximately 5 lentic : 1<br />
lotic—indicating that caddisflies inhabiting standing waters have been far more successful<br />
as colonizers bridging <strong>the</strong> gaps between larval habitats, and in moving between Asia and<br />
North America. During glacial maxima, Beringian species dispersing across <strong>the</strong> land bridge
860 G.B. Wiggins and C.R. Parker<br />
would have had to cross cold, dry land <strong>of</strong> low relief (Schweger et al. 1982). The clear, stable<br />
and well-oxygenated streams required by some species probably would have been sparse,<br />
although lakes and marshes would have occurred in low-lying areas.<br />
A related aspect is that larvae in Apatania, Ecclisomyia, and Micrasema are mainly<br />
grazers confined to cool streams at lower latitudes, but in <strong>the</strong> far north also occur in lakes.<br />
If ice conditions at higher latitudes render stream habitats unsuitable for larvae in <strong>the</strong>se<br />
genera (see <strong>the</strong> foregoing), <strong>the</strong>ir transfer to cold lakes would provide an ecological alternative,<br />
which in Apatania may underlie extension <strong>of</strong> A. zonella to Ellesmere Island (Lake<br />
Hazen 81°49′N; Corbet 1966)—<strong>the</strong> most nor<strong>the</strong>rly record for Trichoptera.<br />
Because many Holarctic and Beringian species are colonizers, it is significant that 13<br />
<strong>of</strong> <strong>the</strong> 51 species (25 per cent) belong to <strong>the</strong> single genus Limnephilus, a dominant group in<br />
standing waters through much <strong>of</strong> <strong>the</strong> nor<strong>the</strong>rn hemisphere. Although <strong>the</strong> life histories <strong>of</strong> few<br />
Nearctic Limnephilus are known in detail, a good deal <strong>of</strong> <strong>the</strong> success <strong>of</strong> <strong>the</strong>se species in<br />
standing waters is probably due to <strong>the</strong> specialized characteristics by which many <strong>of</strong> <strong>the</strong>m<br />
successfully exploit temporary pools (Wiggins 1973): marked tendency to disperse as adults;<br />
eggs deposited not in water but on damp substrates in a gelatinous matrix resistant to<br />
desiccation; and rapid larval development. Moreover, 6 additional Beringian species belong<br />
to o<strong>the</strong>r nor<strong>the</strong>rn limnephilid genera that also occur in transient waters—2 in Asynarchus, 2<br />
in Grammotaulius, and 1 in each <strong>of</strong> Arctopora and Lenarchus. Species in <strong>the</strong>se genera share<br />
most <strong>of</strong> <strong>the</strong> same developmental characteristics found in Limnephilus (e.g. Wiggins 1973),<br />
and in all <strong>the</strong>y account for 37 per cent <strong>of</strong> <strong>the</strong> Holarctic Trichoptera in North America.<br />
Adaptations to transient waters are clearly apomorphic specializations in Trichoptera (Wiggins<br />
et al. 1980) and occur mainly in <strong>the</strong> family Limnephilidae; transient pools are a dominant<br />
aquatic biotope at high latitudes.<br />
One characteristic <strong>of</strong> species <strong>of</strong> Limnephilidae in transient waters at temperate latitudes<br />
is deferral <strong>of</strong> oviposition through diapause until <strong>the</strong> height <strong>of</strong> <strong>the</strong> dry phase <strong>of</strong> <strong>the</strong> pool basins<br />
has passed (Wiggins 1973). Females <strong>of</strong> <strong>the</strong>se species are inactive until sexual maturity is<br />
induced by decreasing photoperiod (Novak and Sehnal 1963); at higher elevations temperature<br />
was also found to be a factor in breaking diapause. At high latitudes, temperatures<br />
conducive to larval development are available for only a few months, and yet species <strong>of</strong><br />
Limnephilidae appear to be univoltine in tundra streams and ponds near Tuktoyaktuk at<br />
latitude 69°29′N (e.g. Winchester 1984). These species appear to have no diapause during<br />
<strong>the</strong>ir life cycle, development proceeding until inhibited by low temperature. Development<br />
deferred by obligate diapause in <strong>the</strong> short growing season would be biologically costly for<br />
Trichoptera at high latitudes if a second year <strong>of</strong> larval growth was <strong>the</strong>reby required,<br />
suggesting that <strong>the</strong> ecological strategy in species <strong>of</strong> Trichoptera successful at high latitudes<br />
is uninterrupted larval development initiated from a low temperature threshold. At temperate<br />
latitudes, diapause in <strong>the</strong> life cycle <strong>of</strong> <strong>the</strong>se species <strong>of</strong> Limnephilidae makes existence in<br />
transient pools possible by imposing a delay in development while <strong>the</strong> pool and its basin are<br />
dry; this delay can be accommodated because <strong>the</strong> annual growth period is still adequate for<br />
univoltine development. By contrast, life cycles with uninterrupted development have a<br />
selective advantage for rapid growth in transient waters at high latitudes, suggesting that <strong>the</strong><br />
incidence <strong>of</strong> diapause in congeneric species <strong>of</strong> Limnephilidae must change between temperate<br />
and arctic latitudes.<br />
At high latitudes within <strong>the</strong> zone <strong>of</strong> continuous permafrost, basins <strong>of</strong> transient surface<br />
pools probably do not become desiccated by exposure to <strong>the</strong> sun, as do basins <strong>of</strong> temporary<br />
pools at more sou<strong>the</strong>rly latitudes. Although exposed surface waters in transient tundra pools<br />
do evaporate during <strong>the</strong> brief summer (e.g. Winchester et al. 1993), a supply <strong>of</strong> water is
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 861<br />
assured from melting permafrost, and <strong>the</strong> meltwater is shielded from evaporation by moss<br />
and o<strong>the</strong>r plant materials; underlying substrates are not likely to become desiccated because<br />
<strong>the</strong> frozen permafrost below prevents <strong>the</strong> water from draining away. These are <strong>the</strong> classic<br />
factors producing muskeg in <strong>the</strong> far north (e.g. Pielou 1991); and this ra<strong>the</strong>r paradoxical<br />
relationship between transient tundra pools, permafrost, and insolation could underlie a<br />
unique type <strong>of</strong> habitat for aquatic insect larvae adapted to an arctic climate. Among <strong>the</strong><br />
Trichoptera, only case-making detritivorous species occur in transient tundra pools. Three<br />
species, all members <strong>of</strong> <strong>the</strong> Limnephilidae, are now known in <strong>the</strong>se habitats: Lenarchus<br />
expansus and Sphagnophylax meiops (category IV), and Asynarchus lapponicus (II) (90,<br />
126, 75); but to what extent do larvae <strong>of</strong> o<strong>the</strong>r species use water-saturated tundra habitats in<br />
<strong>the</strong> basins <strong>of</strong> transient tundra pools? Moreover, if because <strong>of</strong> <strong>the</strong> relatively recent origin <strong>of</strong><br />
<strong>the</strong> arctic biome (see Biogeographic Analysis), time has been insufficient for <strong>the</strong> evolutionary<br />
potential <strong>of</strong> aquatic insects to fully exploit <strong>the</strong>se tundra pools with <strong>the</strong>ir constant supply<br />
<strong>of</strong> water from permafrost, <strong>the</strong>se habitats may provide an evolutionary plateau <strong>of</strong> <strong>the</strong> future.<br />
Perhaps it is not coincidental that Sphagnophylax meiops (Frontispiece, Fig. 28), a relict<br />
species on both geographic and phylogenetic grounds, and <strong>the</strong> sole trichopteran genus<br />
confined to Beringia, still persists only in just such an arctic tundra habitat.<br />
Acknowledgements<br />
Field work for this study was supported by a Co-operative Grant (1981– 84) from<br />
<strong>the</strong> Natural <strong>Sciences</strong> and Engineering Research Council <strong>of</strong> Canada to G.G.E. Scudder<br />
and G.B. Wiggins. Analysis <strong>of</strong> <strong>the</strong> material, including participation by C.R. Parker and<br />
additional field work, was supported by an NSERC Operating Grant (G5707) to G.B.<br />
Wiggins. Participants in ROM field parties included H.E. Frania, E.R. Fuller, R.<br />
Jaagumagi, and B.D. Marshall. Assistance in analysis <strong>of</strong> <strong>the</strong> collections was provided<br />
by J. Conn, H.E. Frania, E.R. Fuller, R. Jaagumagi, J.D. Kerr, J. Thomson-Delaney, and<br />
by P.W. Schefter who also identified material <strong>of</strong> Hydropsyche and Cheumatopsyche.<br />
Information on particular species was provided by T.J. Arefina, L. BotojAneanu, O.S.<br />
Flint, W.K. Gall, V.D. Ivanov, N.P. Kristensen, I.M. Levanidova, J. Lukyanchenko, H.<br />
Malicky, W. Mey, A.P. Nimmo, and R.N. Vineyard who also translated literature in <strong>the</strong><br />
Russian language. Critical comments on <strong>the</strong> manuscript were provided by L. BotojAneanu,<br />
J.A. Downes, O.S. Flint, H.E. Frania, I.M. Levanidova, R.J. Mackay, J.V.<br />
Mat<strong>the</strong>ws, A.P. Nimmo, V.H. Resh, and R.A. Ring. Illustrations were prepared by P.<br />
Stephens-Bourgeault, except for Figs. 2 and 3 by Z. Zichmanis and 12 –15, 17 –18 by<br />
C.R. Parker. C. Rutland and R. Darling rendered valuable assistance in preparing <strong>the</strong><br />
manuscript. Additional collections <strong>of</strong> Trichoptera were made available from <strong>the</strong> Canadian<br />
National Collection <strong>of</strong> Insects maintained by <strong>the</strong> <strong>Biological</strong> Resources Division <strong>of</strong><br />
Agriculture Canada (F. Schmid); <strong>the</strong> Illinois Natural History Survey (J.D. Unzicker);<br />
<strong>the</strong> Royal British Columbia Museum (R.A. Cannings); <strong>the</strong> Spencer Museum, University<br />
<strong>of</strong> British Columbia (G.G.E. Scudder and S.G. Cannings); <strong>the</strong> Strickland Museum,<br />
University <strong>of</strong> Alberta (G.E. Ball and A.P. Nimmo); <strong>the</strong> <strong>Department</strong> <strong>of</strong> Entomology,<br />
United States National Museum <strong>of</strong> Natural History (O.S. Flint); D.G. Huggins <strong>of</strong> <strong>the</strong><br />
Kansas State <strong>Biological</strong> Survey; and N.N. Winchester and R.A. Ring <strong>of</strong> <strong>the</strong> <strong>Department</strong><br />
<strong>of</strong> Biology, University <strong>of</strong> Victoria.
862 G.B. Wiggins and C.R. Parker<br />
References<br />
Ager, T.A. 1982. Vegetational history <strong>of</strong> western Alaska during <strong>the</strong> Wisconsin glacial interval and <strong>the</strong> Holocene.<br />
pp. 75 – 83 in D.M. Hopkins, J.V. Mat<strong>the</strong>ws Jr., C.E. Schweger, and S.B. Young (Eds.), Paleoecology <strong>of</strong><br />
Beringia. Academic Press, New York. 489 pp.<br />
Anderson, N.H. 1992. Influence <strong>of</strong> disturbance on insect communities in Pacific Northwest streams. Hydrobiologia<br />
248:79 – 92.<br />
Banks, N. 1900. Neuropteroid insects. In Papers from <strong>the</strong> Harriman Alaska Expedition. Proc. Acad. Sci. Wash.<br />
2:465 – 473.<br />
______ 1914. American Trichoptera—notes and descriptions. Can. Ent. 46:149 –156, 201– 205, 252 – 258, 261–<br />
268.<br />
Bärlocher, F. and B. Kendrick. 1973. Fungi and food preferences <strong>of</strong> Gammarus pseudolimnaeus. Arch. Hydrobiol.<br />
72:501– 516.<br />
Barton, D.R. 1986. Invertebrates <strong>of</strong> <strong>the</strong> Mackenzie system. pp. 473 – 492 in B.R. Davies, and K.F. Walker (Eds.),<br />
The Ecology <strong>of</strong> River Systems. W. Junk, The Hague. 793 pp.<br />
Baumann, R.W. and J.D. Unzicker. 1981. Preliminary checklist <strong>of</strong> Utah caddisflies. Encyclia 58:25 – 29.<br />
Betten, C. and M.E. Mosely. 1940. The Francis Walker types <strong>of</strong> Trichoptera in <strong>the</strong> British Museum. British Museum<br />
(Natural History), London.<br />
BotojAneanu, L. 1988. A superspecies, or Formenkreis, in caddisflies: Micrasema (superspecies gelidum) McLachlan<br />
(Trichoptera). Populational thinking versus Hennigian fundamentalism. Riv. Idrobiol. 27:181– 210.<br />
BotojAneanu, L. and I.M. Levanidova. 1988. Trichoptera Hydroptilidae (Insecta) from Soviet Union Far-Eastern<br />
Territories. Bull. zool. Mus. Univ. Amsterdam 11:169 –176.<br />
BotojAneanu, L. and H. Malicky. 1978. Trichoptera. pp. 333 – 359 in J. Illies (Ed.), Limn<strong>of</strong>auna Europaea. Gustav<br />
Fischer Verlag, Stuttgart, New York; Swets and Zeitlinger B.V., Amsterdam. 474 pp.<br />
Briggs, J.C. 1966. Zoogeography and evolution. Evolution 20:282 – 289.<br />
Buckland, P.C., D.W. Perry, G.M. Gislason, and A.J. Dugmore. 1986. The pre-Landnám fauna <strong>of</strong> Iceland: a<br />
palaeontological contribution. Boreas 15:173 –184.<br />
Corbet, P.S. 1966. Par<strong>the</strong>nogenesis in caddisflies (Trichoptera). Can. J. Zool. 44:981– 982.<br />
Cowan, C.A. and M.W. Oswood. 1984. Spatial and seasonal associations <strong>of</strong> benthic macroinvertebrates and detritus<br />
in an Alaskan subarctic stream. Polar Biol. 3:211– 215.<br />
Craig, P.C. and P.J. McCart. 1975. Classification <strong>of</strong> stream types in Beaufort Sea drainages between Prudhoe Bay,<br />
Alaska, and <strong>the</strong> Mackenzie Delta, N.W.T., Canada. Arct. Alp. Res. 7:183 –198.<br />
Danks, H.V. 1971. Overwintering <strong>of</strong> some north temperate and arctic Chironomidae. II. Chironomid biology. Can.<br />
Ent. 103:1875 –1910.<br />
______ 1981. Arctic Arthropods. A review <strong>of</strong> systematics and ecology with particular reference to <strong>the</strong> North<br />
American fauna. Entomological Society <strong>of</strong> Canada, Ottawa. 608 pp.<br />
Downes, J.A. 1962. What is an arctic insect? Can. Ent. 94:143 –162.<br />
______ 1964. Arctic insects and <strong>the</strong>ir environment. Can. Ent. 96:279 – 307.<br />
______ 1966. The Lepidoptera <strong>of</strong> Greenland; some geographic considerations. Can. Ent. 98:1135 –1144.<br />
Downes, J.A. and D.H. Kavanaugh. 1988. Origins <strong>of</strong> <strong>the</strong> North American insect fauna. Introduction and commentary.<br />
pp. 1 –11 in J.A. Downes and D.H. Kavanaugh (Eds.), Origins <strong>of</strong> <strong>the</strong> North American Insect Fauna.<br />
Mem. ent. Soc. Can. 144. 168 pp.<br />
Ellis, R.J. 1978a. Over-winter occurrence and maturation <strong>of</strong> gonads in adult Psychoglypha subborealis (Banks)<br />
and Glyphopsyche irrorata (Fabricius). Pan-Pacif. Ent. 54:178 –180.<br />
______ 1978b. Seasonal abundance and distribution <strong>of</strong> adult caddisflies <strong>of</strong> Sashin Creek, Baran<strong>of</strong> Island, sou<strong>the</strong>astern<br />
Alaska. Pan-Pacif. Ent. 54:199 – 206.<br />
Fischer, F.C.J. 1964. Trichopterorum Catalogus, vol. 5. Nederlandsche Entomologische Vereeniging, Amsterdam.<br />
214 pp.<br />
______ 1968. Trichopterorum Catalogus, vol. 9. Nederlandsche Entomologische Vereeniging, Amsterdam.<br />
363 pp.<br />
Flint, O.S., Jr. 1964. Two species <strong>of</strong> Limnephilidae new to North America. Proc. ent. Soc. Wash. 66:60.<br />
______ 1984. The genus Brachycentrus in North America, with a proposed phylogeny <strong>of</strong> <strong>the</strong> genera <strong>of</strong> Brachycentridae<br />
(Trichoptera). Smithsonian Contribs Zool. 398. 58 pp.<br />
Foote, C.J., J.W. Clayton, C.C. Lindsey, and R.H. Bodaly. 1992. Evolution <strong>of</strong> lake whitefish (Coregonus<br />
clupeaformis) in North America during <strong>the</strong> Pleistocene: evidence for a Nahanni glacial refuge race in <strong>the</strong><br />
nor<strong>the</strong>rn Cordillera region. Can. J. Fish. aquat. Sci. 49:760 – 768.<br />
Forsslund, K.H. 1932. Zur Kenntniss der Trichopteren Grönlands. Ent. Tidskr. 53:56 – 59.<br />
______ 1933. Eine neue melanistiche Neuronia - Varietät (Trich.). Ent. Tidskr. 54:36.<br />
Frania, H. E. and G. B. Wiggins. 1997. Analysis <strong>of</strong> morphological and behavioural evidence for <strong>the</strong> phylogeny and<br />
higher classification <strong>of</strong> Trichoptera. Contr. Life Sci. Div. R. Ont. Mus. 160.<br />
Fristrup, B. 1942. Neuroptera and Trichoptera. Zool. Iceland 3:1– 23.<br />
Fuller, E.R. 1987. Systematics <strong>of</strong> <strong>the</strong> caddisfly family Molannidae (Trichoptera). M.Sc. Thesis (unpublished), Dept.<br />
Zoology, Univ. Toronto.
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 863<br />
Gislason, G.M. 1981. Distribution and habitat preferences <strong>of</strong> Icelandic Trichoptera. pp. 99 –109 in G.P. Moretti<br />
(Ed.), Proc. Third Int. Symp. on Trichoptera. Series Entomologica 20. W. Junk, The Hague. 472 pp.<br />
Harper, P.P. 1981. Ecology <strong>of</strong> streams at high latitudes. pp. 313 – 337 in M.A. Lock and D.D. Williams (Eds.),<br />
Perspectives in Running Water Ecology. Plenum, New York. 450 pp.<br />
______ 1989. Zoogeographical relationships <strong>of</strong> aquatic insects (Ephemeroptera, Plecoptera, and Trichoptera) from<br />
<strong>the</strong> eastern James Bay drainage. Can. Fld-Nat. 103:535 – 546.<br />
Harper, P.P. and G. Méthot. 1975. Goera radissonica n. sp., nouveau Trichoptère de la région de la baie James.<br />
Naturaliste can. 102:593 – 595.<br />
Hill-Griffin, A.L. 1912. New Oregon Trichoptera. Ent. News 23:17 – 21.<br />
Hobbie, J.E. 1973. Arctic limnology: a review. pp. 127 –168 in M.E. Britton (Ed.), Alaskan Arctic Tundra. Arct.<br />
Inst. N. Am. Tech. Pap. 25. 224 pp.<br />
Hopkins, D.M., J.V. Mat<strong>the</strong>ws, J.A. Wolfe, and M.L. Silberman. 1971. A Pliocene flora and insect fauna from <strong>the</strong><br />
Bering Strait region. Palaeogeogr. Palaeoclimatol. Palaeoecol. 9:211– 231.<br />
Hopkins, D.M., J.V. Mat<strong>the</strong>ws Jr., C.E. Schweger, and S.B. Young (Eds.). 1982. Paleoecology <strong>of</strong> Beringia.<br />
Academic Press, New York. 489 pp.<br />
Irons, J.G. 1988. Life history patterns and trophic ecology <strong>of</strong> Trichoptera in two Alaskan (U.S.A.) subarctic streams.<br />
Can. J. Zool. 66:1258 –1265.<br />
Irons, J.G., L.K. Miller, and M.W. Oswood. 1993. Ecological adaptations <strong>of</strong> aquatic macroinvertebrates to<br />
overwintering in interior Alaska (U.S.A.) subarctic streams. Can. J. Zool. 71:98 –108.<br />
Irons, J.G., M.W. Oswood, R.J. Stout, and C.M. Pringle. 1994. Longitudinal patterns in leaf litter breakdown: is<br />
temperature really important? Freshwat. Biol. 32:401– 411.<br />
Johansson, A., A.N. Nilsson, and B.W. Svensson. 1991. Larval morphology, habitat and distribution <strong>of</strong> Limnephilus<br />
diphyes (Trichoptera, Limnephilidae). Ent. Tidskr. 112:19 – 25.<br />
Kaneshiro, K.Y. 1993. Introduction, colonization, and establishment <strong>of</strong> exotic insect populations: fruit flies in<br />
Hawaii and California. Am. Ent. 39:23 – 29.<br />
Karlstrom, T.N.V. and G.E. Ball (Eds.). 1969. The Kodiak Island Refugium; its Geology, Flora, Fauna and History.<br />
Boreal Institute, Univ. Alberta. Ryerson Press, Toronto. 262 pp.<br />
Kavanaugh, D.H. 1988. The insect fauna <strong>of</strong> <strong>the</strong> Pacific Northwest coast <strong>of</strong> North America: present patterns and<br />
affinities and <strong>the</strong>ir origins. pp. 125 –149 in J.A. Downes and D.H. Kavanaugh (Eds.), Origins <strong>of</strong> <strong>the</strong> North<br />
American Insect Fauna. Mem. ent. Soc. Can. 144. 168 pp.<br />
Kelley, R.W. 1984. Phylogeny, morphology and classification <strong>of</strong> <strong>the</strong> micro-caddisfly genus Oxyethira Eaton<br />
(Trichoptera: Hydroptilidae). Trans. Am. ent. Soc. 110:435 – 463.<br />
Kimmins, D.E. and D.G. Denning. 1951. The McLachlan types <strong>of</strong> North American Trichoptera in <strong>the</strong> British<br />
Museum. Ann. ent. Soc. Am. 44:111 –140.<br />
Labandeira, C.C. and J.J. Sepkoski Jr. 1993. Insect diversity and <strong>the</strong> fossil record. Science 261:310 – 315.<br />
Lafontaine, J.D. and D.M. Wood. 1988. A zoogeographic analysis <strong>of</strong> <strong>the</strong> Noctuidae (Lepidoptera) <strong>of</strong> Beringia, and<br />
some inferences about past Beringian habitats. pp. 109 –123 in J.A. Downes and D.H. Kavanaugh (Eds.),<br />
Origins <strong>of</strong> <strong>the</strong> North American Insect Fauna. Mem. ent. Soc. Can. 144. 168 pp.<br />
Lehmkuhl, D.M. and C.D. Kerst. 1979. Zoogeographical affinities and identification <strong>of</strong> central Arctic caddisflies<br />
(Trichoptera). Musk-Ox 25:1– 28.<br />
Lepneva, S.G. 1964. Fauna <strong>of</strong> <strong>the</strong> U.S.S.R.; Trichoptera, vol. 2, no. 1. Larvae and Pupae <strong>of</strong> Annulipalpia. (Original<br />
in Russian, translated into English, Israel Program for Scientific Translations, 1970. 638 pp.)<br />
______ 1966. Fauna <strong>of</strong> <strong>the</strong> U.S.S.R.; Trichoptera, vol. 2, no. 2. Larvae and Pupae <strong>of</strong> Integripalpia. (Original in<br />
Russian, translated into English, Israel Program for Scientific Translations, 1971. 700 pp.)<br />
Levanidova, I.M. 1975. The caddisflies (Trichoptera) <strong>of</strong> Kamchatka; an ecological-faunistic outline (In Russian).<br />
Bull. Pacif. Sci. Inst. Fish. and Oceanogr. 97:83 –114.<br />
______ 1982. Amphibiotic insects <strong>of</strong> <strong>the</strong> mountainous regions <strong>of</strong> <strong>the</strong> Soviet Far East (Faunistics, ecology,<br />
zoogeography <strong>of</strong> <strong>the</strong> Ephemeroptera, Plecoptera and Trichoptera) (In Russian). U.S.S.R. Academy <strong>of</strong> Science,<br />
Far Eastern Science Centre. Biology - Soils Institute. 214 pp.<br />
Lindsey, C.C. and J.D. McPhail. 1986. Zoogeography <strong>of</strong> fishes <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> and Mackenzie basins. pp. 639 – 674<br />
in C.H. Hocutt and E.O. Wiley (Eds.), The Zoogeography <strong>of</strong> North American Freshwater Fishes. Wiley, New<br />
York. 866 pp.<br />
MacLean, S.F. and F.A. Pitelka. 1971. Seasonal patterns <strong>of</strong> abundance <strong>of</strong> tundra arthropods near Barrow. Arctic<br />
24:19 – 40.<br />
Malicky, H. 1979. Notes on some caddisflies (Trichoptera) from Europe and Iran. Aquat. Insects 1:3 –16.<br />
______ 1983. Atlas <strong>of</strong> European Trichoptera. Series Entomologica 24. W. Junk, The Hague. 298 pp.<br />
______ 1988. Spuren der Eiszeit in der Trichopterenfauna Europas (Insecta, Trichoptera). Riv. Idrobiol. 27(2 – 3):247 –<br />
297.<br />
Manuel, K.L. and A.P. Nimmo. 1984. The caddisfly genus Ylodes in North America (Trichoptera: Leptoceridae).<br />
pp. 219 – 224 in J.C. Morse (Ed.), Proc. Fourth Int. Symp. on Trichoptera. Series Entomologica 30. W. Junk,<br />
The Hague. 486 pp.<br />
Martynov, A.V. 1909. Les Trichoptères de la Sibérie et des régions adjacentes. I. Ezheg. zool. Muz. [Annls Mus.<br />
Zool. Acad. Imp. Sci. St. Petersbourg] 14:223 – 255.
864 G.B. Wiggins and C.R. Parker<br />
______ 1910. Les Trichoptères de la Sibérie et des régions adjacentes. II. Ezheg. zool. Muz. [Annls Mus. Zool. Acad.<br />
Imp. Sci. St. Petersbourg] 15:351– 429.<br />
______ 1914. Les Trichoptères de la Sibérie et des régions adjacentes. IV, Limnophilinae. Ezheg. zool. Muz. [Annls<br />
Mus. Zool. Acad. Imp. Sci. St. Petersbourg] 19:173 – 285.<br />
______ 1924a. Practical entomology, vol. 5. Trichoptera (In Russian). Gosudarstvennoe Izdatelstvo, Leningrad.<br />
______ 1924b. Notice sur les Trichoptères de la District de Minoussinsk (In Russian). Ezheg. gosud. Muz. N.M.<br />
Mart’yanova [Jb. Martjanovischen Staats Mus. Minoussinsk] 2(3):62 –107.<br />
______ 1935. Trichoptera <strong>of</strong> <strong>the</strong> Amur region. I. Trudy zool. Inst., Leningr. [Trav. Inst. Zool. Acad. Sci. URSS]<br />
2:205 – 395.<br />
Mat<strong>the</strong>ws, J.V., Jr. 1979a. Tertiary and Quaternary environments: historical background for an analysis <strong>of</strong> <strong>the</strong><br />
Canadian insect fauna. pp. 31– 86 in H.V. Danks (Ed.), Canada and its Insect Fauna. Mem. ent. Soc. Can.<br />
108. 573 pp.<br />
______ 1979b. Fossil beetles and <strong>the</strong> late Cenozoic history <strong>of</strong> <strong>the</strong> tundra environment. pp. 371– 378 in J. Gray and<br />
A.J. Boucot (Eds.), Historical Biogeography, Plate Tectonics, and <strong>the</strong> Changing Environment. Oregon State<br />
Univ. Press, Corvallis. 500 pp.<br />
McLachlan, R. 1874 –1880. A monographic revision and synopsis <strong>of</strong> <strong>the</strong> Trichoptera <strong>of</strong> <strong>the</strong> European fauna. Pt. 1,<br />
1874:1– 46, pls. 1– 5. Pt. 2, 1875:47 –108, pls. 6 –11. Pt. 3, 1875:109 –144, pls. 12 –15. Pt. 4, 1876:145 – 220,<br />
pls. 16 – 23. Pt. 5, 1876:221– 280, pls. 24 – 31, w. supplement I – XII. Pt. 6, 1877:281– 348, pls. 32 – 37., Pt. 7,<br />
1878:349 – 428, pls. 38 – 44. Pt. 8, 1879:429 – 500, pls. 45 – 51. Pt. 9, 1880:501– 23, w. supplement XIII –<br />
LXXXIV, pls. 52 – 59.<br />
Mey, W. and A. Dulmaa. 1985. Die Köcherfliegenfauna der Mongolei (Insecta, Trichoptera). Mitt. zool. Mus. Berl.<br />
61:79 –104.<br />
Milne, L.J. 1934. Studies in North American Trichoptera, 1. Publ. by author, Cambridge, Mass. 19 pp.<br />
Milner, A.M. 1987. Colonization and ecological development <strong>of</strong> new streams in Glacier Bay National Park, Alaska.<br />
Freshwat. Biol. 18:53 – 70.<br />
Moore, M.V. and R.E. Lee Jr. 1991. Surviving <strong>the</strong> big chill: overwinter strategies <strong>of</strong> aquatic and terrestrial insects.<br />
Am. Ent. 37:111 –118.<br />
Morse, J.C. 1975. A phylogeny and revision <strong>of</strong> <strong>the</strong> caddisfly genus Ceraclea (Trichoptera, Leptoceridae). Contr.<br />
Am. Ent. Inst. 11(2). 97 pp.<br />
Mosely, M.E. 1929. Trichoptera and Ephemeroptera <strong>of</strong> Greenland. Additional records made by <strong>the</strong> Oxford<br />
University Expedition to Kugssuk, Godthaab Fjord, W. Greenland, 1928. Ann. Mag. nat. Hist. (ser. 10)<br />
4:501– 509.<br />
______ 1932. Oxford University Greenland expedition, 1928: a correction. Ann. Mag. nat. Hist. (ser. 10) 9:573 – 74.<br />
______ 1936. The Indian caddis-flies (Trichoptera). Pt. IV. J. Bombay nat. Hist. Soc. 38(3):447 – 478.<br />
Nagayasu, Y. and T. Ito. 1993. The caddisfly genus Dicosmoecus in Asia (Trichoptera, Limnephilidae). I. Males.<br />
pp. 123 –127 in C. Otto (Ed.), Proc. Seventh Int. Symp. on Trichoptera. Backhuys, Leiden. 312 pp.<br />
Nimmo, A.P. 1971. The adult Rhyacophilidae and Limnephilidae (Trichoptera) <strong>of</strong> Alberta and eastern British<br />
Columbia and <strong>the</strong>ir post-glacial origin. Quaest. ent. 7:3 – 234.<br />
______ 1986. Preliminary annotated checklist <strong>of</strong> <strong>the</strong> Trichoptera (Insecta) <strong>of</strong> Alaska. Contr. nat. Sci., Br. Columb.<br />
Prov. Mus. 5:1– 7.<br />
______ 1991. Seven new species <strong>of</strong> Limnephilus from western North America with description <strong>of</strong> female <strong>of</strong><br />
L. pallens (Banks) (Trichoptera, Limnephilidae, Limnephilinae, Limnephilini). Proc. ent. Soc. Wash. 93:499 –<br />
508.<br />
Nimmo, A.P. and G.G.E. Scudder. 1978. An annotated checklist <strong>of</strong> <strong>the</strong> Trichoptera (Insecta) <strong>of</strong> British Columbia.<br />
Syesis 11:117 –133.<br />
______ 1983. Supplement to an annotated checklist <strong>of</strong> <strong>the</strong> Trichoptera (Insecta) <strong>of</strong> British Columbia. Syesis<br />
16:71– 83.<br />
Nimmo, A.P. and R. Wickstrom. 1984. Preliminary annotated checklist <strong>of</strong> <strong>the</strong> Trichoptera (Insecta) <strong>of</strong> <strong>the</strong> <strong>Yukon</strong>.<br />
Syesis 17:3 – 9.<br />
Novak, K. and F. Sehnal. 1963. The development cycle <strong>of</strong> some species <strong>of</strong> <strong>the</strong> genus Limnephilus (Trichoptera).<br />
$ as. % sl. Spol. ent. 60:68 – 80.<br />
Oliver, D.R. 1968. Adaptations <strong>of</strong> Arctic Chironomidae. Annls zool. fenn. 5:111 –118.<br />
Olsson, T.I. 1981. Overwintering <strong>of</strong> benthic invertebrates in ice and frozen sediment in a North Swedish river.<br />
Holarct. Ecol. 4:161 –166.<br />
Oswood, M.W. 1989. Community structure <strong>of</strong> benthic invertebrates in interior Alaskan (USA) streams and rivers.<br />
pp. 97 –110 in W.F. Vincent and J.C. Ellis-Evans (Eds.), High Latitude Limnology. Kluwer Academic<br />
Publishers, Dordrecht, Ne<strong>the</strong>rlands.<br />
Oswood, M.W., L.K. Miller, and J.G. Irons. 1991. Overwintering <strong>of</strong> freshwater benthic invertebrates. pp. 360 – 375<br />
in R.E. Lee Jr. and D.L. Denlinger (Eds.), Insects at Low Temperature. Chapman and Hall, New York. 513 pp.<br />
Parker, C.R. and G.B. Wiggins. 1985. The Nearctic caddisfly genus Hesperophylax Banks (Trichoptera: Limnephilidae).<br />
Can. J. Zool. 63:2443 – 2472.<br />
Pielou, E.C. 1991. After <strong>the</strong> Ice Age. The Return <strong>of</strong> Life to Glaciated North America. Univ. Chicago Press, Chicago.<br />
366 pp.
<strong>Caddisflies</strong> <strong>of</strong> <strong>the</strong> <strong>Yukon</strong> 865<br />
Reeves, B.O.K. 1973. The nature and age <strong>of</strong> <strong>the</strong> contact between <strong>the</strong> Laurentide and Cordilleran ice sheets in <strong>the</strong><br />
western interior <strong>of</strong> North America. Arct. Alp. Res. 5:1 –16.<br />
Resh, V.H. 1976. The biology and immature stages <strong>of</strong> <strong>the</strong> caddisfly genus Ceraclea in eastern North America<br />
(Trichoptera: Leptoceridae). Ann. ent. Soc. Am. 69:1039 –1061.<br />
Resh, V.H., J.C. Morse, and I.D. Wallace. 1976. The evolution <strong>of</strong> <strong>the</strong> sponge feeding habit in <strong>the</strong> caddisfly genus<br />
Ceraclea (Trichoptera: Leptoceridae). Ann. ent. Soc. Am. 6:937 – 941.<br />
Ross, H.H. 1944. The caddis flies, or Trichoptera, <strong>of</strong> Illinois. Bull. Ill. nat. Hist. Surv. 23(1). 326 pp.<br />
______ 1949. Descriptions <strong>of</strong> some western Limnephilidae (Trichoptera). Pan-Pacif. Ent. 25:119 –128.<br />
______ 1950. Synoptic notes on some Nearctic limnephilid caddisflies (Trichoptera, Limnephilidae). Am. midl.<br />
Nat. 43:410 – 429.<br />
______ 1956. Evolution and Classification <strong>of</strong> <strong>the</strong> Mountain <strong>Caddisflies</strong>. Univ. Illinois Press, Urbana. 213 pp.<br />
______ 1958. Affinities and origins <strong>of</strong> <strong>the</strong> nor<strong>the</strong>rn and montane insects <strong>of</strong> western North America. pp. 231-252 in<br />
C.L. Hubbs (Ed.), Zoogeography. Am. Assoc. Advance. Sci. Publ. 51. 509 pp.<br />
______ 1965. Pleistocene events and insects. pp. 583 – 596 in D.G. Frey and H.E. Wright (Eds.), The Quaternary<br />
<strong>of</strong> <strong>the</strong> United States. Princeton Univ. Press, Princeton. 922 pp.<br />
Ross, H.H. and D.R. Merkley. 1952. An annotated key to <strong>the</strong> Nearctic males <strong>of</strong> Limnephilus (Trichoptera:<br />
Limnephilidae). Am. midl. Nat. 47:435 – 455.<br />
Ross, H.H. and J.C. Morse. 1973. Micrasema kluane, a probable stepping stone to <strong>the</strong> Arctic (Trichoptera,<br />
Brachycentridae). Ent. News 84:291– 293.<br />
Roy, D. and P.P. Harper. 1979. Liste préliminaire des Trichoptères (insectes) du Québec. Annls Soc. ent. Québec<br />
24:148 –172.<br />
Ruiter, D. E. 1995. The adult Limnephilus Leach (Trichoptera: Limnephilidae) <strong>of</strong> <strong>the</strong> New World. Bull. Ohio Biol.<br />
Surv. (n.s.) 11(1). 200 pp.<br />
Schmid, F. 1950a. Monographie du genre Grammotaulius Kolenati (Trichoptera, Limnophilidae). Revue suisse<br />
Zool. 57:317 – 352.<br />
______ 1950b. Le genre Hydatophylax Banks (Trichoptera, Limnophilidae). Mitt. Schweiz. ent. Ges. 23:265 – 296.<br />
______ 1952. Le groupe de Lenarchus Mart. (Trichoptera, Limnophilidae). Mitt. Schweiz. ent. Ges. 25:157 – 210.<br />
______ 1954a. Contribution à l’étude de la sous-famille des Apataniinae II. Tijdschr. Ent. 97:1– 74.<br />
______ 1954b. Le genre Asynarchus McL. Mitt. Schweiz. ent. Ges. 27:57 – 96.<br />
______ 1955. Contribution à l’étude des Limnophilidae (Trichoptera). Université de Lausanne, Suisse. 245 pp.<br />
______ 1964. Some Nearctic species <strong>of</strong> Grammotaulius Kolenati (Trichoptera, Limnephilidae). Can. Ent. 96:914 –<br />
917.<br />
______ 1965a. Quelques Trichoptères asiatiques II. Ent. Tidskr. 86:28 – 35.<br />
______ 1965b. Trichoptera. Ergebnisse der zoologischen Forschungen von Dr. Z. Kaszab in der Mongolei.<br />
Reichenbachia 7:201– 203.<br />
______ 1968. La Famille des Arctopsychides (Trichoptera). Mem. Soc. ent. Québec 1. 84 pp.<br />
______ 1970. Le Genre Rhyacophila et la Famille des Rhyacophilidae (Trichoptera). Mem. ent. Soc. Can. 66. 230<br />
pp. + 52 pl.<br />
______ 1982. Revision des Trichoptères canadiens. II. Les Glossosomatidae et Philopotamidae (Annulipalpia).<br />
Mem. ent. Soc. Can. 122. 76 pp.<br />
______ 1983. Revision des Trichoptères canadiens. III. Les Hyalopsychidae, Psychomyiidae, Goeridae, Brachycentridae,<br />
Sericostomatidae, Helicopsychidae, Beraeidae, Odontoceridae, Calamoceratidae et Molannidae.<br />
Mem. ent. Soc. Can. 125. 116 pp.<br />
Schmid, F., T.J. Arefina, and I.M. Levanidova. 1993. Contribution to <strong>the</strong> knowledge <strong>of</strong> <strong>the</strong> Rhyacophila (Trichoptera)<br />
<strong>of</strong> <strong>the</strong> sibirica group. Bull. Inst. r. Sci. nat. Belg., Ent. 63:161 –172.<br />
Schweger, C.E., J.V. Mat<strong>the</strong>ws, D.M. Hopkins, and S.B. Young. 1982. Paleoecology <strong>of</strong> Beringia—a syn<strong>the</strong>sis.<br />
pp. 425 – 444 in D.M. Hopkins, J.V. Mat<strong>the</strong>ws Jr., C.E. Schweger, and S.B. Young (Eds.), Paleoecology <strong>of</strong><br />
Beringia. Academic Press, New York. 489 pp.<br />
Scudder, G.G.E. 1997. Environment <strong>of</strong> <strong>the</strong> <strong>Yukon</strong>. pp. 13 – 57 in H.V. Danks and J.A. Downes (Eds.), Insects <strong>of</strong><br />
<strong>the</strong> <strong>Yukon</strong>. <strong>Biological</strong> Survey <strong>of</strong> Canada (Terrestrial Arthropods), Ottawa.<br />
Slack, K.V., J.W. Naumann, and L.J. Tilley. 1979. Benthic invertebrates in a north-flowing stream and a<br />
south-flowing stream, Brooks Range, Alaska. Wat. Resour. Bull. 15:108 –135.<br />
Smith, S.D. and K.L. Manuel. 1984. Reconsideration <strong>of</strong> <strong>the</strong> Nearctic species <strong>of</strong> <strong>the</strong> Rhyacophila acropedes subgroup<br />
based on adults (Trichoptera: Rhyacophilidae). pp. 369 – 374 in J.C. Morse (Ed.), Proc. Fourth Int. Symp. on<br />
Trichoptera. Series Entomologica 30. W. Junk, The Hague. 486 pp.<br />
Solem, J.O. 1981. Overwintering strategies in some Norwegian caddisflies. Summary. pp. 321– 322 in G.P. Moretti<br />
(Ed.), Proc. Third Int. Symp. on Trichoptera. Series Entomologica 20. W. Junk, The Hague. 472 pp.<br />
Solem, J.O. and V.H. Resh. 1981. Larval and pupal description, life cycle, and adult flight behaviour <strong>of</strong> <strong>the</strong><br />
sponge-feeding caddisfly Ceraclea nigronervosa (Retzius) in central Norway (Trichoptera). Entomologica<br />
scand. 12:311– 319.<br />
Soluk, D.A. 1985. Macroinvertebrate abundance and production <strong>of</strong> psammophilous Chironomidae in shifting sand<br />
areas <strong>of</strong> a lowland river. Can. J. Fish. aquat. Sci. 42:1296 –1302.<br />
Stanley, S.M. 1986. Earth and Life Through Time. W.H. Freeman, New York. 690 pp.
866 G.B. Wiggins and C.R. Parker<br />
Ulmer, G. 1907. Trichopteren, Fasc. VI(I). Collections Zoologiques du baron Edm. de Selys Longchamps. Hayez,<br />
Impr. des Académies, Bruxelles. 102 pp.<br />
______ 1927. Entomologische Ergebnisse der schwedischen Kamtchatka-Expedition 1920-1922. 11. Trichopteren<br />
und Ephemeropteren. Ark. Zool. 19A (8). 17 pp.<br />
Vermeij, G.J. 1991. When biotas meet: understanding biotic interchange. Science 253:1099 –1104.<br />
Vineyard, R.N. and G.B. Wiggins. 1988. Fur<strong>the</strong>r revision <strong>of</strong> <strong>the</strong> caddisfly family Uenoidae (Trichoptera): evidence<br />
for inclusion <strong>of</strong> Neophylacinae and Thremmatidae. Syst. Ent. 13:361– 372.<br />
Wiggins, G.B. 1956. A revision <strong>of</strong> <strong>the</strong> North American caddisfly genus Banksiola (Trichoptera: Phryganeidae).<br />
Contr. R. Ont. Mus. Zool. Palaeont. 43. 13 pp.<br />
______ 1973. A contribution to <strong>the</strong> biology <strong>of</strong> caddisflies (Trichoptera) in temporary pools. Contr. Life Sci. Div.<br />
R. Ont. Mus. 88. 28 pp.<br />
______ 1977. Larvae <strong>of</strong> <strong>the</strong> North American Caddisfly Genera (Trichoptera). First edition. Univ. Toronto Press,<br />
Toronto. 401 pp.<br />
______ 1984. Trichoptera - some concepts and questions, Keynote address. pp. 1 –12 in J.C. Morse (Ed.), Proc.<br />
Fourth Int. Symp. on Trichoptera. Series Entomologica 30. W. Junk, The Hague. 486 pp.<br />
______ 1996. Larvae <strong>of</strong> <strong>the</strong> North American Caddisfly Genera (Trichoptera). Second edition, revised. Univ.<br />
Toronto Press, Toronto. 457 pp.<br />
______ in press. The Caddisfly Family Phryganeidae (Trichoptera). Univ. Toronto Press, Toronto.<br />
Wiggins, G.B. and S. Kuwayama. 1971. A new species <strong>of</strong> <strong>the</strong> caddisfly genus Oligotricha from nor<strong>the</strong>rn Japan and<br />
Sakhalin, with a key to <strong>the</strong> adults <strong>of</strong> <strong>the</strong> genus (Trichoptera: Phryganeidae). Kontyu 39:340 – 346.<br />
Wiggins, G.B. and R.J. Mackay. 1978. Some relationships between systematics and trophic ecology in Nearctic<br />
aquatic insects with special reference to Trichoptera. Ecology 59:1211 –1220.<br />
Wiggins, G.B., R.J. Mackay, and I.M. Smith. 1980. Evolutionary and ecological strategies <strong>of</strong> animals in annual<br />
temporary pools. Arch. Hydrobiol. Suppl. 58:97 – 206.<br />
Wiggins, G.B. and J.S. Richardson. 1982. Revision and synopsis <strong>of</strong> <strong>the</strong> caddisfly genus Dicosmoecus (Trichoptera:<br />
Limnephilidae). Aquat. Insects 4:181– 217.<br />
______ 1987. Revision <strong>of</strong> <strong>the</strong> Onocosmoecus unicolor group (Trichoptera: Limnephilidae, Discosmoecinae).<br />
Psyche 93:187 – 216 (1986).<br />
Wiggins, G.B. and W. Wichard. 1989. Phylogeny <strong>of</strong> pupation in Trichoptera, with proposals on <strong>the</strong> origin and<br />
higher classification <strong>of</strong> <strong>the</strong> order. J. N. Am. benthol. Soc. 8:260 – 276.<br />
Wiggins, G.B. and N.N. Winchester. 1984. A remarkable new caddisfly genus from northwestern North America<br />
(Trichoptera, Limnephilidae, Limnephilinae). Can. J. Zool. 62:1853 –1858.<br />
Wiggins, G.B. and R.W. Wisseman. 1992. New North American species in <strong>the</strong> genera Neothremma and Farula,<br />
with hypo<strong>the</strong>ses on phylogeny and biogeography (Trichoptera: Uenoidae). Can. Ent. 124:1063 –1074.<br />
Wiggins, I.L. and J.H. Thomas. 1962. A Flora <strong>of</strong> <strong>the</strong> Alaskan Arctic Slope. Univ. Toronto Press, Toronto. Arct.<br />
Inst. N. Am. Publ. 4. 425 pp.<br />
Winchester, N.N. 1984. Life histories and post-glacial origins <strong>of</strong> tundra caddisflies (Trichoptera) from <strong>the</strong><br />
Tuktoyaktuk Peninsula, Northwest Territories. M.Sc. Thesis (unpublished), <strong>Department</strong> <strong>of</strong> Biology, Univ.<br />
Victoria.<br />
Winchester, N.N., G.B. Wiggins, and R.A. Ring. 1993. The immature stages and biology <strong>of</strong> <strong>the</strong> unusual North<br />
American arctic caddisfly Sphagnophylax meiops, with consideration <strong>of</strong> <strong>the</strong> phyletic relationships <strong>of</strong> <strong>the</strong> genus<br />
(Trichoptera: Limnephilidae). Can. J. Zool. 71:1212 –1220.<br />
Yamamoto, T. and H.H. Ross. 1966. A phylogenetic outline <strong>of</strong> <strong>the</strong> caddisfly genus Mystacides (Trichoptera:<br />
Leptoceridae). Can. Ent. 98:627 – 632.<br />
Yamamoto, T. and G.B. Wiggins. 1964. A comparative study <strong>of</strong> <strong>the</strong> North American species in <strong>the</strong> caddisfly genus<br />
Mystacides (Trichoptera: Leptoceridae). Can. J. Zool. 42:1105 –1126.<br />
Yang, L.-F. and J.C. Morse. 1988. Ceraclea <strong>of</strong> <strong>the</strong> People’s Republic <strong>of</strong> China (Trichoptera; Leptoceridae). Contr.<br />
Am. ent. Inst. 23. 69 pp.