Proton Nuclear Magnetic Resonance Spectroscopy
Proton Nuclear Magnetic Resonance Spectroscopy
Proton Nuclear Magnetic Resonance Spectroscopy
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
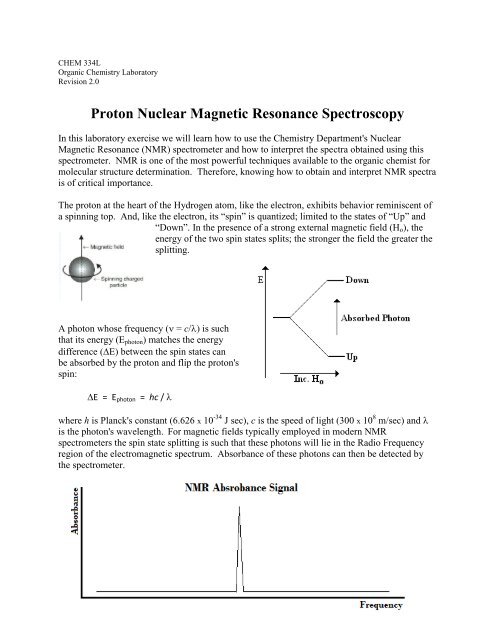
CHEM 334LOrganic Chemistry LaboratoryRevision 2.0<strong>Proton</strong> <strong>Nuclear</strong> <strong>Magnetic</strong> <strong>Resonance</strong> <strong>Spectroscopy</strong>In this laboratory exercise we will learn how to use the Chemistry Department's <strong>Nuclear</strong><strong>Magnetic</strong> <strong>Resonance</strong> (NMR) spectrometer and how to interpret the spectra obtained using thisspectrometer. NMR is one of the most powerful techniques available to the organic chemist formolecular structure determination. Therefore, knowing how to obtain and interpret NMR spectrais of critical importance.The proton at the heart of the Hydrogen atom, like the electron, exhibits behavior reminiscent ofa spinning top. And, like the electron, its “spin” is quantized; limited to the states of “Up” and“Down”. In the presence of a strong external magnetic field (H o ), theenergy of the two spin states splits; the stronger the field the greater thesplitting.A photon whose frequency ( = c/) is suchthat its energy (E photon ) matches the energydifference (E) between the spin states canbe absorbed by the proton and flip the proton'sspin:E = E photon = hc / where h is Planck's constant (6.626 x 10 -34 J sec), c is the speed of light (300 x 10 8 m/sec) and is the photon's wavelength. For magnetic fields typically employed in modern NMRspectrometers the spin state splitting is such that these photons will lie in the Radio Frequencyregion of the electromagnetic spectrum. Absorbance of these photons can then be detected bythe spectrometer.
P a g e | 2Because the splitting energy depends of the size of the NMR spectrometer's magnet field, thefrequency of absorbance will also depend on the machine's magnet "size." Therefore, signalabsorbance is generally reported in terms of a machine independent Chemical Shift (). Thechemical shift of a signal is defined as the signal's frequency "downfield" from a referencecompound's signal (Tetramethylsilane, THS, (CH 3 ) 4 Si), reported in Hertz (Hz) relative to themachine's magnet "size," reported in MegaHertz (MHz): =So, if a proton's signal is 2130 Hz downfield from the TMS signal produced by a 300 MHzNMR, it would have a chemical shift value of: == 7.1 ppmChemical shift values have units of parts per milliom (ppm).Now, this would all be rather uninteresting if all the Hydrogen atoms in a molecule had nucleithat absorbed at exactly the same frequency; we would observe a single absorbance peak in thespectrum. However, locally, within a molecule, each Hydrogen atom will be in a differentmagnetic environment. This is because the electron cloud surrounding a nucleus can act like asmall wire loop within which an electric current induced by the external magnetic fieldestablishes a local magnetic field which acts in opposition to the externally established field.This local field "shields" the nucleus from the external field. Slight molecular differences thenlead to slight differences in the shielding experienced by different protons within a molecule andthis causes their associated NMR signals to exhibit different chemical shifts.For instance, the molecule CH 3 CCl 2 CH 2 Cl has protons in two different "magnetic"environments, CH 3 - and -CH 2 -, and so will produce a spectrum with two signals of slightlydifferent chemical shift. The CH 3 - signal occurs at 2.23 ppm and that of -CH 2 - at 4.00 ppm.
P a g e | 3Introduction to Organic Chemistry, 2 nd Ed.Andrew Streitwiesser & Clayton H. HeathcockNotice also that the CH 3 - signal is "larger," has a greater area, than the -CH 2 - signal. This isbecause it is due to the absorbance of three protons, versus two protons for the -CH 2 - signal.Typical chemical shift values for common proton types are provided below. You should becomefamiliar with these values.
P a g e | 4Finally, neighboring protons can influence the"magnetic" environment of each other via a mechanismcalled spin-spin coupling. Consider two neighboringprotons A and B. A will observe that B can occupy itstwo possible spin states. Whether B is spin Up or Downwill influence the spin states of A; the coupling is throughthe chemical bonds connecting the Hydrogen atoms. Onecase will cause A to absorb at a slightly higher frequencyand the other a slightly lower frequency. This will leadto a splitting of A’s absorbance into a “doublet”.If A couples to two Hydrogen atoms, then the splitting will occur again and a “triplet” will beobserved; etc.The relative intensities of simple multiplets are:Multiplet IntensitiesDoublet 1:1Triplet 1:2:1Quartet 1:3:3:1Quintet 1:4:6:4:1Sextet 1:5:10:10:5:1Septet 1:6:15:20:15:6:1For example, the molecule CH 3 CH 2 Cl should exhibit two NMR signals. The first is due to theCH 3 - protons and will be split into a triplet due to the neighboring two protons. The other signal,due to the -CH 2 - protons, should be split into a quartet. As seen in the spectrum below, this is infact the case:
P a g e | 5Introduction to Organic Chemistry, 2 nd Ed.Andrew Streitwiesser & Clayton H. HeathcockSo, spectral information concerning Chemical Shift and Coupling will provide important cluesabout the molecular environment of each Hydrogen atom in a molecule. This information isextracted from the spectrum via:The number of NMR signals tells us how many different "types" of protons occur withinthe molecule.The relative intensity of an NMR signal tells us the relative number of protons of that"type" which occur within the molecule.The chemical shift of an NMR signal tells us the nature of the environment the protonsfind themselves in within the molecule.The splitting pattern of the NMR signal tells us about the neighboring protons within themolecule.Let us consider the following spectrum from Streitwiesser and Heathcock:
P a g e | 6A separate analysis of the compound producing this spectrum indicates it has the chemicalformula C 2 H 4 Br 2 . The spectrum exhibits two signals, indicating the molecule possesses twotypes of protons. The integrated areas of the signals are in a 3:1 ratio, indicating one signal(~2.25 ppm) is due to 3 protons and the other (~5.75 ppm) is due to one proton. Thus, we havesignals due to CH 3 - and -CH- moieties. The signal due to the CH 3 - moiety is split into a doublet,indicating it neighbors a -CH- moiety. The signal due to the -CH- moiety is split into a quartet,indicating it neighbors a -CH 3 moiety. Thus, the molecular structure that produced this spectrumshould be: CH 3 -CHBr 2 . In this way, NMR spectra can be used to elucidate molecular structure.The Department's NMR is a Bruker 400 MHz machne, similar to the one pictured below.Sample Insertion Pt.Liquid N 2 Fill PortLiquid N 2 DewarSurrounding theElectromagnet(http://www.chem.mq.edu.au/~vislab/galleries/equipment/imagepages/400NMR.html)The magnetic field in which the sample is inserted is generated by a 400 MHz superconductingsolenoid. The solenoid is cooled in liquid He jacketed with a liquid Nitrogen dewar such that asufficiently low temperature can be achieved to allow the windings ofthe solenoid to exhibit superconductivity. This electromagnet iscapable of producing very large magnetic fields, thereby increasing theresolution of the instrument significantly. A solution of the sample isplaced in a small glass tube and is inserted into the magnet. It is thenpulsed with RF radiation and the resulting signal is FourierTransformed into the type of spectrum we have been examining.
P a g e | 7In this lab exercise, you will obtain and examine the spectra of some simple compounds. Youwill then be asked to correlate the spectral peaks with the Hydrogen atoms in the molecules ofthese compounds. You should become familiar with how to interpret NMR spectra in terms ofmolecular structure.
P a g e | 8ProcedureYour laboratory instructor will demonstrate how to:prepare a sample for NMR analysis.take an NMR spectrum.Special Deuterated solvents are available with which solutions of your compounds can beprepared. The deuterated solvent with then interfere with the proton NMR spectrum minimally.These solvents typically come with the TMS reference already added.You will be expected to master the use of the Department's spectrometer such that you canindependently take spectra without assistance.Each person in the laboratory class should take the spectrum of one of the following compounds:Ethyl Aminet-Butyl ChlorideEthyl Benzoatep-Bromo Toluene2-Bromo Butane1,2-Dichloro EthaneThen, as a class, you should collectively assign all the peaks in each spectrum to the Hydrogenatoms of the molecules comprising the compounds.