The Monogenea
The Monogenea is one of the largest groups of parasitic flatworms (Platyhelminthes) with many thousands of species described, and probably a larger number not yet described. The vast majority of species infects the gills, skin and fins of freshwater and marine fishes, some have secondarily adopted an endoparasitic way of life, most of these in the mouth cavity and urinary bladder of turtles and amphibians. Species of one genus infect cephalopods, another species infects the eye of hippopotamus.Most species have a high degree of host specificity, i.e., they infect only a single host species or few related ones. Effects on their hosts are usually insignificant, but occasionally large scale mortalities caused by monogeneans are reported, particularly but not exclusively in aquaculture. For this reason, the economic importance of the group is large, and a considerable amount of work has been devoted to its study. Recent accounts of marine Monogenea are by Hayward (2005 [1]) and Whittington (2005 [2]).
The two subgroups of Monogenea: Poly- and Monopisthocotylea
The two major groups within the Monogenea (= Monogenoidea) can easily be distinguished by the structure of their posterior attachment organ, the haptor or opisthaptor. The opisthaptor of the Polyopisthocotylea possesses a number of clamps (Figure 1), the opisthaptor of the Monopisthocotylea consists either of a large sucker bearing various types of hooks, or it consists entirely of large and small hooks (Figure 2).

Figure 1. Pseudothoracocotyla ovalis (Polyopisthocotylea) from the gills of the Spanish Macherel Scomberomorus commerson on the Great Barrier Reef. Note the opisthaptor bearing a large number of clamps, and posterior large and small hooks. Female reproductive system except vitellarium red. Original Klaus Rohde. © Klaus Rohde

Figure 2.Sprostonia sp. (Monopisthocotylea) from the cod Epinephelus sp. on the Great Barrier Reef. Note the opisthaptor formed by a large sucker bearing hooks. Female reproductive system red. Original Klaus Rohde. © Klaus Rohde
Phylogenetic relationship of the Poly- and Monopisthocotylea
There has been some controversy on whether the Monogenea constitute one monophylum (i.e., whether all Monogenea are derived from one common ancestor, which has given rise to them but to no other species). Studies using cladistic analysis of morphological including ultrastructural characters, as well as molecular biology suggest that the Polyopistocotylea plus Monopisthocotylea are indeed monophyletic (Littlewood et al. 1999 [3]). However, the final verdict may still be out.
Life cycles of Monogenea
Monogeneans, possibly with a few exceptions, have direct life cycles, i.e., there are no intermediate hosts. The adult worm infecting a vertebrate host lays eggs from which ciliated larvae (oncomiracidia) hatch to infect vertebrates, usually of the same species. Larvae may be free swimming or hatch only once the eggs have established contact with the host. Each egg develops to a single adult worm which produces many eggs during its life.
Structure of adult Monogenea
The body surface of adults is formed by a non-cilated neodermis which has replaced the ciliated epidermis of the larva. The digestive system is formed by an anterior sucker or suckers, a pharynx and a blind ending caecum, usually with numerous side branches extending into most of the body. In other words, it is a gastrovascular system, combining the functions of a digestive and a vascular system. The excretory/osmoregulatory system consists of numerous flame bulbs connected to capillaries which join to form larger ducts opening through two separate excretory pores in the anterior part of the body. The flame bulbs do not openly communicate with the surrounding tissue, i.e., the system is a protonephridial system [4]. They consist of a terminal cell and a proximal canal cell, whose cytoplasmic processes (ribs) interdigitate to form the filtration apparatus (=weir) (Figure 3). Examples are described in Rohde et al. 1989 [5].
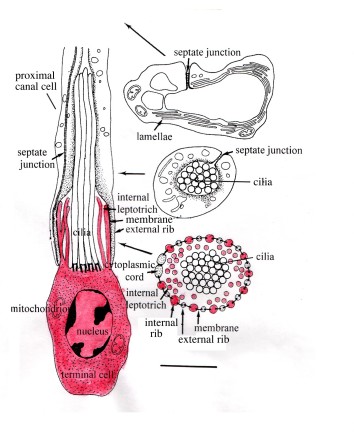
Figure 3. Flame bulb and excretory capillary of a monopistocotylean monogenean reconstructed from serial ultrathin sections. Longitudinal section on the left, cross-sections on the right. Terminal cell drawn red; its ribs (cytoplasmic processes) interdigitate with those of the proximal canal cell with which they are connected by a filtration membrane to form the filtation apparatus (=weir) of the flame bulb. Note that the flame bulb contains a ciliary tuft and does not openly communicate with the surrounding tissue. It is followed by a capillary wih lamellae lining its lumen. Also note some internal leptotriches, cytoplasmic processes extending into the lumen of the flame bulb. The long arrows indicate the location of the cross-sections along the flame bulb and capillary. Scale bar 1 micron. Original Klaus Rohde. © Klaus Rohde
All monogeneans are hermaphroditic, i.e., they possess male and female reproductive systems, often of extraordinary complexity. The biology and morphology of species of the genus Polystomoides from the mouth cavity and bladder of freshwater turtles have been particularly well studied (Rohde 1965 [6]) and are used here to illustrate some important aspects of the structure of Monogenea (Figure 4).

Figure 4. Polystomoides malayi (Polyopisthocotylea) from the urinary bladder of the freshwater turtle Cuora amboinensis in Malaysia. Female reproductive system except vitellarium drawn red. Original Klaus Rohde. © Klaus Rohde
The nervous system is in the form of a “ladder”, consisting of longitudinal connectives and transverse commissures. The dorsal part of one anterior commissure is particularly well developed, functioning as a brain (Rohde 1968 [7]). The reproductive system is of extraordinary complexity. The egg-forming part of the female system consists of various ducts and glands which guarantee effective production of numerous eggs in the lifetime of the individual parasite (Rohde and Ebrahimzadeh 1969 [8], Figure 5).

Figure 5. Egg-forming part of reproductive system of Polystomoides malayi. Egg cells are formed in the ovary, the largest egg cell is discharged into the oocapt, from there into the oviduct and ovovitelline duct, the latter also carrying yolk cells. A cluster of mucous gland cells (specific to certain histological stains) opens into the entrance into the ootype, a compact mass of serous gland cells open into the ootype; both, together with the yolk cells, probably contribute to the formation of the egg shell. At any particular time, only a single egg is found in the ootype. It is discharged into the uterus which opens to the outside through the common (both male and female) gonopore. Original Klaus Rohde. © Klaus Rohde.
Sensory receptors are of various kinds. There are non-cilated sensilla, sensilla with a single or several cilia, and probably specialized larger sensory organs (Figures 6 and 7).

Figure 6. Uniciliate receptor of Pricea multae (Polyopisthocotylea). The receptor is the terminal swelling of a dendrite (nerve fibre) supported by a spirally arranged dense collar and terminating in a single cilium. Original electron micrograph by Klaus Rohde. © Klaus Rohde

Figure 7. Cross-section through multiciliate receptor of Pricea multae (Polyopisthocotylea). Note four cilia and one basal body of a cilium. Original electron micrograph by Klaus Rohde. © Klaus Rohde
Photoreceptors are generally found in the larvae, but also in some adults. Some photoreceptors consist of cilia modified to form complex arrangements of lamellae, thought to be light sensitive (Figure 8).

Figure 8. Lamellated photoreceptor of Gyrodactylus (Monopisthocotylea). Original electron micrograph by N. Watson and Klaus Rohde. © Klaus Rohde
The so-called oral sucker (buccal organ) of many species in fact combines the funtions of a sucker, secretory and complex sensory organ (Rohde 1979 [9], Rohde and Watson 1996 [10]), although its structure and function need further investigation (Figure 9).

Figure 9. Anterior end of Pricea multae (Polyopisthocotylea) from the Spanish Mackerel Scomberomorus commerson on the Great Barrier Reef. Note the buccal organ (sucker) with some “buccal (glandular) ducts” and large extension across the lumen of the “sucker” (“bridge”). Electron-microscopic studies of some species have detected involved structures in such suckers suggesting a sensory (taste?) function. Original Klaus Rohde. © Klaus Rohde
The oncomiracidium
Eggs often have filaments which entangle them in the gills or weed, or which increase their flotation ability. An operculum (egg cover) opens to allow escape of the larva (Figure 10).

Figure 10. Encotyllabe chironemi (Monopisthocotylea), larva escaping from the egg. Original scanning electron-micrograph by Klaus Rohde. © Klaus Rohde
Typically, larvae have a pharynx and single caecum, as well as a number of flame bulbs opening into excretory capillaries which open to the outside through two anteriorly located excretory pores (Figure 11). The posterior end may carry a ciliated cone and terminal globule (Figures 11 and 12) [11]. Ciliated cells have a number and a distribution characteristic for a particular taxon, they are not distributed over the entire surface of the larva (Figure 13). Likewise, sense receptors (sensilla) have a pattern characteristic of a taxon (Whittington et al. 2000 [12]). Complex multiciliate receptors have been demonstrated in some species (Figure 14). Eyes are of various types, most common is one consisting of a light sensitive rhabdomere, a lens and a pigmented cup (Figure 15). Others may have double rhabdomeres, lack a lens or pigment, or consist of a number of lamellated substructures. – The terminal globule may play a role in the reaction to magnetic stimuli (see Rothsey and Rohde 2002 [13]), although attempts to demonstrate magnetite in the inclusions failed.

Figure 11. Oncomiracidium of Plectanocotyle gurnardi (Polyopisthocotylea). Note the pharynx and caecum, anterior glands, flame bulbs, capillaries and excretory pores of the excretory system, the opisthaptor with large hooks and hooklets, and the posteriorly located ciliated cone and terminal globule. Original Klaus Rohde. © Klaus Rohde

Figure 12. Electron micrograph of horizontal section through ciliated cone and terminal globule of the oncomiracidium of Zeuxapta seriolae (Polyopisthocotylea). Note the large vacuoles in the terminal globule, some with dense (cristalline) inclusions in the vacuoles, suggesting a possible function in reacting to magnetic stimuli, although attempts to demonstrate magnetite in the inclusions have failed. Scale bar 2 microns. Original electron micrograph by Klaus Rohde. © Klaus Rohde

Figure 13. Scanning electron micrograph of oncomiracidium of Zeuxapta seriolae (lateral view). Note ciliated and non-ciliated parts of the surface. Original Klaus Rohde. © Klaus Rohde

Figure 14. Encotyllabe chironemi (Monopistocotylea). Uni- and multiciliate receptors at anterior end of larva. Original scanning electron-micrograph by Klaus Rohde. © Klaus Rohde

Figure 15. Pigmented eye of Neoheterocotyle rhinobatidis (Monopisthocotylea). Note lens, rhabdomere and pigment cup. Scale bar 1 micron. Original electron micrograph by Klaus Rohde. © Klaus Rohde
Monogenea as ecological models
Monogenea have proven their worth as ecological models, useful for studying a variety of problems, as shown in the following.
Speciation within or between host species?
Many host species are infected by entire clusters of congeneric species of Monogenea, i.e., by species belonging to the same genus. The question arises whether these species have evolved on that particular host or whether they have evolved separately on different host species and only secondarily “come together”. This is indeed an important question with relevance for ecology as a whole (sympatric vs. allopatric speciation !). Polystome monogeneans of turtles have been used to solve the problem for this group. Evidence from DNA as well as from morphology clearly shows that species pairs infecting different sites (mouth cavity and urinary bladder) of the same turtle species are less closely related to each other than to species from the same infection site of different host species (Littlewood et al. 1997 [14]).
Niche restriction and segregation: Interspecific competition or reinforcement of reproductive barriers?
Very detailed studies of communities of Monogenea parasitic on the gills of marine and freshwater fishes by several authors have shown the great importance of reinforcement of reproductive barriers for niche segregation. Species use strictly defined microhabitats on the gills and body surface of fish and have very complex copulatory organs. This and the fact that fish replicas are available in almost unlimited numbers, makes them ideal ecological models. Many congeners (species belonging to the same genus) and non-congeners of Monogenea were found on single host species. The maximum number of congeners was nine species. The only limiting factor is space for attachment, since food (blood, mucus, fast regenerating epithelial cells) is in unlimited supply as long as the fish is alive. Various authors, using a variety of statistical methods, have consistently found that species with different copulatory organs may co-occur in the same microhabitat, whereas congeners with identical or very similar copulatory organs are spatially segregated, convincing evidence that reinforcement and not competition is responsible for niche segregation in Monogenea [15][16][17][18][19][20]. For a detailed discussion, especially of competition and reinforcement of reproductive barriers, see [21][22][23]. A knol also deals with the problem in greater detail.
Monogeneans as agents of fish disease
Ogawa (2005) [24] has recently given a detailed account of parasitic diseases of marine fish in aquaculture, and Jones (2005) [25] has discussed mass mortalities in the oceans, some of them caused by monogeneans. Older discussions can be found in Rohde (1984,1993) [26][27]. Marine mass mortalities have not been documented frequently for a number of reasons, among them the huge spaces of the oceans which make exact quantitative studies difficult, and the possibility that fish die soon after infection. Nevertheless, a mass mortality of the anchovy Engraulis japonica in the Sea of Iyo, which killed over 87 000 fish, was attributed to the monogenean Pseudacanthocotyloide ssp. The introduction of the monogenean Nitzschia sturionis into the Aral Sea with its host, the sturgeon Accipenser stellatus, led to huge fish mortalities among the local sturgeon Accipenser nudiventris and a collapse of the sturgeon/caviar industry in the Aral Sea for many years in the 1930’s. The monogenean Neoheterobothrium hirame killed large numbers of juvenile flounder, Paralichthys olivaceus, in the western Sea of Japan, where it was first noticed in 1993.The aquaculture industry is worth billions of Dollars every year. Thus, the commercial value of cultured salmonid fishes alone in 2001 was estimated to be around US $3.84 billion, and of all finfish about US $7.44 billion. Disease, much of it caused by parasites, is the most significant factor threatening the aquaculture industry. Ogawa (2005) [24] mentions several monogeneans among the important parasitic diseases of cultured marine fish. They included Benedenia seriolaeon the body surface of yellowtail and amberjack, Neobenedenia girellae on various fish species, Heteraxine heterocerca on yellowtail, and Zeuxapta japonica on amberjack and golden amberjack. Neobenedenia melleni infects cultured barramundi in Australia. Various capsalid monogeneans (Monopisthocotylea) cause mortality in groupers, and several other species have been found to cause disease for example in cultured tiger puffers (Takifugu rubripes), flounders etc. It is important to realize that quite frequently mortalities are due to disease agents introduced with seed stock. Efficient quarantine measures and checks for parasites before introduction are therefore important control measures. Important for disease outbreaks are synergistic effects, i.e., not only the parasites but also adverse effects such as lack of oxygen contribute, which shows the importance of optimal aquaculture conditions.Ornamental fish, i.e., fish kept in freshwater or marine aquaria, also have very great economic importance. They are often killed by monogeneans, in particular small species of Gyrodactylus.
References
Hayward, C. (2005). Monogenea Polyopisthocotylea (ectoparasitic flukes). In: K. Rohde ed. Marine Parasitology. CSIRO Publishing Melburne and CABI Wallingford Oxon., pp.55 – 63.
Whittington, I.D. (2005). Monogenea Monopisthocotylea (ectoparasitic flukes). In: K.Rohde ed. Marine Parasitology. CSIRO Melbourne and CABI Wallingford, Oxon, pp.63 – 72.
Littlewood D.T.J., Rohde, K., Bray, R.A. and Herniou, E.A. (1999). Phylogeny of the Platyhelminthes and the evolution of parasitism. Biological Journal of the Linnean Society , 68, 257-287.
Rohde, K. (2001). Protonephridia as phylogenetic characters. In: Interrelationships of the Platyhelminthes, pp. 203-216. (eds. D.T.J. Littlewood and R.A.Bray). Taylor & Francis., London and New York.
Rohde, K. and Watson, N. and Roubal, F. (1989). Ultrastructure of the protonephridial system of Dactylogyrus sp. and an unidentified ancyrocephaline (Monogenea: Dactylogyridae). International Journal for Parasitology, 19, 859-864.
Rohde, K. (1965). Studies on the genus Polystomoides Ward, 1917 (Monogenea). I. Description of 4 Malayan species, a key to the known species and a comparison of the subcuticular layers in Polystomoides and some digenetic trematodes. Festschrift 65. Geburtstag B. Rensch. Zoologische Jahrbücher, Abteilung für Systematik, 92, 345-368.
Rohde, K. (1968). Das Nervensystem der Gattung Polystomoides Ward, 1967 (Monogenea). Zeitschrift für Morphologie der Tiere, 62, 58-76.
Rohde, K. and Ebrahimzadeh, A. (1969). Das weibliche Geschlechtssystem der Gattung Polystomoides Ward, 1917 (Monogenea). Zeitschrift für Parasitenkunde, 33, 110-134.
Rohde, K. (1979). The buccal organ of some Monogenea Polyopisthocotylea. Zoologica Scripta, 8, 161-170.
Rohde, K. and Watson, N.A. (1996). Ultrastructure of the buccal complex of Pricea multae (Monogenea, Polyopisthocotylea, Gastrocotylidae). Folia Parasitologica, 43, 117-132.
Rohde, K. (1998). Ultrastructure of the terminal globule of the oncomiracidium of Zeuxapta seriolae (Meserve, 1938) (Monogenea, Polyopisthocotylea, Axinidae). Parasitology Research, 84, 157-159.
Whittington, I. D., Chisholm L.A. and Rohde, K. (2000). The larvae of Monogenea (Platyhelminthes). Advances in Parasitology, 44, 139-232.
Rothsey, S. and Rohde, K. (2002). The responses of larval copepods and monogeneans to light, gravity and magnetic fields. Acta Parasitologica 47, 167-172.
Littlewood, D.T.J., Rohde, K. and Clough, K.A. (1997). Parasite speciation within or between host species?- phylogenetic evidence from site-specific polystome monogeneans. International Journal for Parasitology, 27, 1289-1297.
Rohde, K. (1991). Intra- and interspecific interactions in low density populations in resource-rich habitats. Oikos 60, 91-104.
Rohde, K. (1994). Niche restriction in parasites: proximate and ultimate causes. Parasitology 109, S69-S84.
Simkova, A., Desdevises,Y.,Gelnar,M. and Morand, S. (2000). Co-existence of nine gill ectoparasites (Dactylogyus: Monogenea) parasitising the roach Rutilus rutilus (L.): history and present ecology. International Journal for Parasitology 30, 1077-.
Simkova, A., Gelnar, M. and Morand, S. (2001). Order and disorder in ectoparasite communities: the case of congeneric gill monogeneans (Dactylogyrus spp.). International Journal for Parasitology 31, 1205-1210.
Simkova, A., Gelnar, M. and Sasal, P. (2001). Aggregation of congeneric parasites (Monogenea: Dactylogyrus). Parasitology 123, 599-607.
Simkova, A., Desdevises,Y., Gelnar,M. and Morand, S. (2001). Morphometric correlates of host specificity in Dactylogyrus species (Monogenea) parasites of European Cyprinid fish. Parasitology 123, 169-177.
Rohde, K. (2005). Nonequilibrium Ecology, Cambridge University Press, Cambridge, 2005b, 223 pp. auf http://www.cambridge.org/9780521674553
Rohde, K. (1980). Warum sind ökologische Nischen begrenzt? Zwischenartlicher Antagonismus oder innerartlicher Zusammenhalt? Naturwissenschaftliche Rundschau, 33, 98-102.
Rohde, K. (2005). Eine neue Ökologie. Aktuelle Probleme der evolutionären Ökologie. Naturwissenschaftliche Rundschau, 58, 420-426.
Ogawa, K. (2005). Effects in finfish culture. In: K.Rohde: Marine Parasitology. CSIRO Publishing Melbourne and CABI Wallingford Oxon., pp.378-391.
Jones, B. (2005). Mass mortalities in the oceans. In: K.Rohde: Marine Parasitology. CSIRO Publishing Melbourne and CABI Wallingford Oxon., pp.371-374.
Rohde, K. (1984). Helminth Diseases of Marine Fishes. In Diseases of Marine Animals, vol. IV (ed. O. Kinne.). Biol. Anst. Helgoland, 193-320, 435-501.
Rohde, K. (1993). Ecology of Marine Parasites. 2nd edition. CAB – International (Commonwealth Bureaux of Agriculture), Wallingford, Oxon, U.K. 298 pp.
Rohde, K. (1976). Monogenean gill parasites of Scomberomorus commersoni Lacepede and other mackerel on the Australian east coast. Zeitschrift für Parasitenkunde 51, 49-69.
Rohde, K. (1977). Marine taxonomic research. Search, 8, 221-222.