The Aspidogastrea, a “primitive”, i.e. very ancient group of flukes, has been studied extensively using experimental techniques as well as molecular biology, transmission and scanning electron microscopy. They belong to the best known groups of helminths (parasitic worms) and can therefore serve as a model group for parasitological studies. In three knols, we first discuss their morphology, taxonomy, phylogeny and life cycles, second the light they cast on the supposed “retrograde” evolution of parasites, the so-called sacculinisation, and third their functional morphology and ecology. Reviews of work on the group were given by Rohde (1972,1994, 1999, 2005) [1][2][3][4] and a key to the genera of the group can be found in Rohde (2002) [5]. All these papers, in particular [1][2][3], contain detailed lists of references.
The Trematoda, main characteristics
Trematodes (flukes) comprise one of the largest groups of helminths (parasitic worms), with many thousands of species. Liver flukes of sheep and cattle and blood flukes of man, to mention only some, are of great economic or medical importance. Most species of trematodes belong to the Digenea. Adult Digenea usually possess one or two suckers located anteriorly and ventrally/posteriorly, and their life cycles are complex, involving at least two hosts. In almost all species, the final or definitive host, i.e., the host harbouring the adult stage, is a vertebrate, whereas the first intermediate host, i.e., the host harbouring larval, sexually immature stages, usually is a mollusc. In addition to this first intermediate host, further intermediate hosts may be incorporated in the life cycle. Importantly, larval stages in the first intermediate host multiply, i.e., a single egg develops to a first larva (the so-called miracidium transforming to a sporocyst), which typically gives rise to a very large number of later larval stages, the rediae (or daughter sporocysts) which in turn produce cercariae. Final hosts become infected by the cercariae or metacercariae (= encapsulated cercariae).
However, there is one group of trematodes, the Aspidogastrea (=Aspidobothria, Aspidobothrea), which differs markedly from the Digenea in morphology and life cycles. Investigations using molecular biology and electron-microscopy have shown that the Aspidogastrea are the sister group of the Digenea, i.e., the two groups had a common ancestor and have split very early in evolutionary history (probably several hundred million years ago).The Aspidogastrea are discussed in detail below.
The Aspidogastrea, morphology
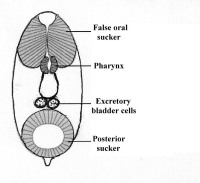
Larvae always have a posterior sucker, and may have an anterior so-called “pseudo- or false sucker”, which is not separated from the surrounding tissue by a genuine connective tissue sheath, may also be present (Fig.1).
Figure 1. Larva of Lobatostoma manteri. Note the false anterior sucker, the parynx, the blind ending caecum, and the two excretory bladder cells. The posterior end is drawn out into a short appendage of unknown function. Original Klaus Rohde. © Klaus Rohde
Adults do not have a posterior or ventral sucker, but an adhesive (ventral) disk consisting of transverse grooves (rugae), a single row of well separated small suckers (suckerlets) or three to four rows of alveoli (suckerlets) on a ventral disk (see Figures 2-4), and – although hosts include vertebrates and molluscs – there is no multiplication of larval stages in the mollusc, i.e., a single egg produces a single adult.
Figure 2. A ventral view of Rugogaster hydrolagi, an aspidogastrean from the rectal glands of the elephant shark Hydrolagus in Tasmania, Australia. Note the row of transverse grooves (rugae). Original Klaus Rohde. © Klaus Rohde
Figure 3. Part of the aspidogastrean Multicalyx sp, from the intestine of a shark. Note the single row of alveoli (suckerlets) separated by transverse septa. Original Klaus Rohde. © Klaus Rohde
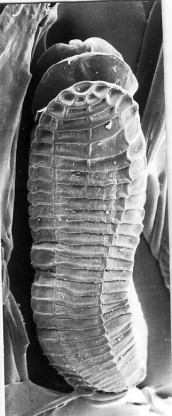
Figure 4. Scanning electron-micrograph of the aspidogastrean Lobatostoma manteri from the intestine of the teleost fish Trachinotus blochi, ventral view. Note the anterior “head” and the adhesive disk consisting of four rows of alveoli. Original Klaus Rohde. © Klaus Rohde
All aspidogastreans are hermaphroditic, i.e., the adults possess male as well as female reproductive systems.This is demonstrated below by the example of Multicotyle purvisi from the stomach and intestine of freshwater turtles in Southeast Asia. It reaches a length of about 10 mm and contains both a fully mature male as well as a fully mature and gravid female reproductive system (Fig.5).
Figure 5. Adult Multicotyle purvisi. Note the single and blind ending caecum (intestine), the ventral (adhesive) disk consisting of four rows of alveoli, the male genital system consisting of two large testes opening into the vas deferens (sperm duct) and the terminal cirrus pouch (copulatory organ), as well as the female genital system with a single ovary and vitellarium (yolk gland). The common (to both the male and female systems) genital opening is located at the anterior end. The marginal bodies are located between the outer rows of alveoli and have a glandular function. Laurer’s canal extends from the female reproductive system to the dorsal surface where it opens to the outside. Original Klaus Rohde. © Klaus Rohde
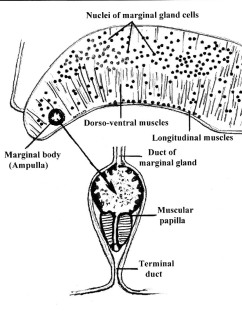
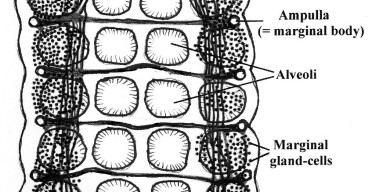
Unique feature of the Aspidogastrea include a septate oviduct (i.e., the oviduct carrying the egg cells from the ovary has a number of concentric constrictions), and the marginal bodies, which were long considered to be sensory in nature but are in fact secretory organs ([6]) (Figs. 6 and 7).
Figure 6. Cross-section through the lateral part of the adhesive disk of Lobatostoma manteri showing the nuclei and the terminal ampulla, papilla and terminal duct of a marginal gland. Original Klaus Rohde. © Klaus Rohde
Figure 7. Ventral view of part of the ventral disk of Lobatostoma manteri showing the marginal glands and bodies connected by longitudinal and transverse ducts. Original Klaus Rohde. © Klaus Rohde
The Aspidogastrea, life cycles
The life cycles of several species have been worked out. Based on the knowledge available to date we can distinguish two kinds: in one type, the entire life cycle can be completed in molluscs, although vertebrates may act as facultative (not obligate) hosts, in the other, both a mollusc and a vertebrate are required for completion of the life cycle. An example of the first kind is Aspidogaster conchicola, whose life cycle has been studied by many authors beginning in the 19th century (references in [1][3]) (Fig.8).
Figure 8. Life cycle of Aspidogaster conchicola. Only molluscs (freshwater bivalves or snails) are necessary for the completion of the life cycle. Adult worms produce eggs in which a non-ciliated larva with an anterior and posterior sucker develops. There are conflicting reports on how mollusks become infected: either by eggs containing larvae or by hatched larvae. The life cycle can be repeated without involvement of a vertebrate host, but if a fish eats an infected mollusc, adults can produce eggs in it. Original Klaus Rohde. © Klaus Rohde
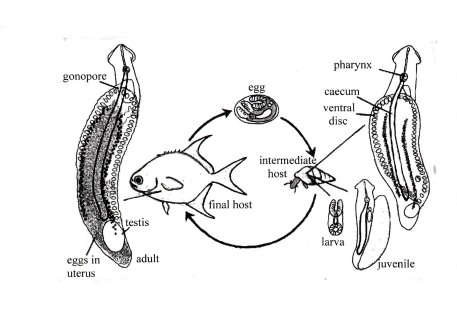
An example of the second kind of life cycle is Lobatostoma manteri from the small intestine of the marine teleost fish Trachinotus blochi ([8]) (Figs.9 and 10).
Figure 9. Life cycle of Lobatostoma manteri. Adults live in the small intestine of the marine teleost fish Trachinotus blochi (Carangidae). They possess fully mature male and female reproductive systems with one large posterior testis, and a uterus filled with eggs which are shed through the gonopore at the anterior end. They are deposited with the faeces of the fish on the sea floor, where they are eaten by snails such as Cerithium (Clypeomorus) moniliferum. In the snail, the posterior sucker of the larva develops to the adhesive disk, and reproductive organs develop to (almost) the final state, without – however – maturing and producing sperm and eggs. Original Klaus Rohde, based on Rohde, K. 2001, Parasitism. Encyclopedia of Biodiversity volume 4, Academic Press [9]. © Klaus Rohde
At Heron Island on the Great Barrier Reef, only juvenile Trachinotus (a few cm long) were found to be infected. They crush the very thick-shelled snails between their well developed pharyngeal plates (Fig.10).
Figure 10. The head of juvenile Trachinotus blochi (A) opened along the dotted line. Snails (B) are crushed between the pharyngeal plates (red) which are moved by strongly developed muscles responsible for the peculiarly shaped head of the fish species (“Snub-nosed dart”). The pointed vomer (arrow) prevents the snail from slipping out of the mouth. On the right a close relative of Trachinotus blochi of about the same size (C): note the “normal’ pharyngeal plates. This fish species cannot become infected because it cannot crush the snails. Original Klaus Rohde. © Klaus Rohde
Larvae hatch in the stomach of the snails but move into the digestive gland where they grow up (Fig.11).
Figure 11. A snail, Cerithium moniliferum, dissected out of the shell. A juvenile Lobatostoma manteri (red) is coiled up in the digestive gland of the snail. An expanded worm is drawn beside the snail to show its true size. Rarely, two worms are found. Other snails of larger size, Planaxis sulcatus and Peristernia australiensis, may harbour several worms. Worms may move from the digestive gland into the stomach and back. Original Klaus Rohde. © Klaus Rohde
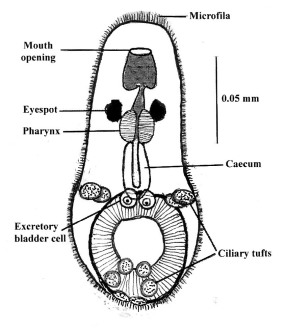
Like Lobatostoma manteri, Multicotyle purvisi (Fig.5) also needs a mollusc and vertebrate host for the completion of its life cycle. However, infection of the mollusc is not by an egg that is ingested, but by a larva that hatches in freshwater, swims for hours by means of its 10 ciliary tufts (Fig. 12) and supported by a flotation mechanism, a thick sheath of so-called microfila (Figs.12 and 13). Larvae are inhaled by snails, and migrate into the kidneys where they grow to the stage infective to turtles [10].
Figure 12. Larva of Multicotyle purvisi. Note the anterior mouth not surrounded by an anterior sucker, followed by the pharynx and caecum. As in the larva of Lobatostoma manteri (Fig.1), there are a posterior sucker and two excretory bladder cells. In addition, the surface is covered by a thick sheath of microfila which appear to help in flotation. Altogether 10 ciliary tufts enable the larva to actively swim, and a pair of eyespots (ocelli) facilitate reaction to light. Original Klaus Rohde. © Klaus Rohde
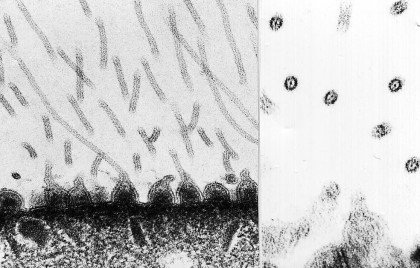
Figure 13. Transmission electron-micrographs of the tegument of larval Multicotyle purvisi. Note the tubercles at the surface of the tegument drawn out into long microfila. Left: longitudinally sectioned; right: transversely sectioned. Original Klaus Rohde. © Klaus Rohde
It is possible that other species of aspidogastreans have more complex life cycles. Thus, larvae of Stichocotyle nephropis, adults of which infect elasmobranchs, were found encapsulated in the intestinal wall of lobsters, and immature Multicalyx , adults of which infect holocephalans and elasmobranchs, have been recorded from the intestine of teleost fish. This suggests that, in addition to the intermediate and final hosts, a further host acting as a transport host (i.e., a host containing immature stages which do not develop in it), may be involved.
In all species of Aspidogastrea that have been studied, the posterior sucker of the larva is transformed into the adhesive disk: in Rugogaster, for example, the rugae are formed by the posterior wall of the sucker [10], in species of Lobatostoma and Multicotyle, among others, alveoli are formed within the sucker. Detailed studies of Stichocotyle have not been made.
The Aspidogastrea, taxonomy
About 80 species of aspidogastreans in 13 genera have been described. There has been some controversy about the relationships of the various genera of aspidogastreans, but according to the prevailing view, four families are distinguished as follows ([11]):
1) Rugogastridae (two caeca, single row of transverse rugae) comprising a single genus Rugogaster with two species from the rectal glands of holocephalan fishes;
2) Stichocotylidae (one caecum, single row of well separated suckerlets) with a single species, Stichocotyle nephropis, from the intestine of elasmobranchs;
3) Multicalycidae (one caecum, single ventral row of alveoli separated by transverse septa) with a single genus Multicalyx from the intestine of holocephalan fishes and elsamobranchs;
4) Aspidogastridae (one caecum, ventral disk with three or four rows of alveoli) with nine genera in three subfamilies from molluscs, turtles and teleost fishes.
4a) Subfamily Rohdellinae (terminal part of male and female reproductive ducts united to form a hermaphroditic duct) with a single species Rohdella siamensis from freshwater teleosts.
4b) Subfamily Cotylaspidinae (three rows of alveoli) with three genera, Cotylogaster, Cotylaspis and Lissemysia which differ in the number of testes (one or two) and the absence or presence of a cirrus pouch.
4c) Subfamily Aspidogastrinae (four rows of alveoli) with six genera, Multicotyle, Lobatostoma, Aspidogaster, Lophotaspis,Sychnocotyle and Neosychnocotyle, which differ in the number of testes (one or two), the absence or presence of a cirrus pouch and the absence or presence of head lobes and/or papillae on the ventral disk.
The Aspidogastrea, phylogeny
Whereas the Aspidogastridae have an adhesive disk bearing three or four rows of alveoli and use teleosts/turtles as hosts, all the other families share the characters (synapomorphies) rugae or a single row of deep suckers/alveoli, as well as the use of elasmobranchs/holocephalans as hosts. Gibson and Chinabut (1984) therefore distinguished two orders, the Aspidogastrida with the single family Aspidogastridae, and the Stichocotylida with the other families ([12]).The following diagram illustrates the likely relationship of the aspidogastrean families with each other:
The sister group of the Aspidogastrea is the very large group Digenea, with thousands of species and many families. The ancestor of the Digenea split from the ancestor of the Aspidogastrea early in evolutionary history, probably more than 400 million years ago ([13]). Comparative studies using 18S rDNA ([14]) and 28S rDNA ([15]), as well as extensive electron-microscopic studies, have demonstrated that the Trematoda (Aspidogastrea plus Digenea), the Cestoda, Gyrocotylidea and Amphilinidea, as well as the Monogenea, i.e., all the major groups of parasitic Platyhelminthes, have one common ancestor, i.e., form one monophylum, the so-called Neodermata ([16][17]).
References
- Rohde, K. 1972. The Aspidogastrea, especially Multicotyle purvisi Dawes, 1941. Advances in Parasitology, 10, 77-151.
- Rohde, K. 1994. The minor groups of parasitic Platyhelminthes. Advances in Parasitology, 33, 145-234.
- Rohde, K. 1999. Aspidogastrea, In: Tree of Life (eds. Maddison, D.R. and Maddison, W.P.). http://tolweb.org/tree?group=Aspidogastrea&contgroup=Platyhelminthes
- Rohde, K. 2005. Aspidogastrea. In: K.Rohde (ed.). Marine Parasitology. CSIRO Publishing, Melbourne and CABI Publishing, Wallingford, Oxon, 72-76, 460.
- Rohde K. 2002. Subclass Aspidogastrea Faust & Tang, 1936. In: Keys to the Trematoda vol. 1, pp. 5-14 (eds. Gibson, D.I., Jones, A. and Bray, R.A.). CABI Publishing and the Natural History Museum, Wallingford, Oxon.
- Rohde, K. and Watson, N. 1989. Ultrastructure of the marginal glands of Lobatostoma manteri (Trematoda, Aspidogastrea). Zoologischer Anzeiger, 223, 301-310.
- Rohde, K. 1971. Untersuchungen an Multicotyle purvisi Dawes, 1941 (Trematoda, Aspidogastrea). V. Licht- und elektronenmikroskopischer Bau der Randkörper. Zoologische Jahrbücher, Abteilung für Anatomie, 88, 387-398.
- Rohde, K. 1973. Structure and development of Lobatostoma manteri sp. nov. (Trematoda, Aspidogastrea) from the Great Barrier Reef, Australia. Parasitology, 66, 63-83.
- Rohde, K. 2001, Parasitism. Encyclopedia of Biodiversity volume 4, Academic Press.
- Rohde, K. 1971. Untersuchungen an Multicotyle purvisi Dawes, 1941 (Trematoda, Aspidogastrea). I. Entwicklung und Morphologie. Zoologische Jahrbücher, Abteilung für Anatomie, 88, 138-187.
- Rohde, K. 1968. Die Entwicklung von Multicotyle purvisi Dawes, 1941 (Trematoda, Aspidogastrea). Zeitschrift für Parasitenkunde, 30, 278-280.
- Rohde, K. and Watson, N.A. 1992. Ultrastructure of tegument, ventral sucker and rugae of Rugogaster hydrolagi (Trematoda, Aspidogastrea). International Journal for Parasitology, 22, 967-974.
- Gibson, D.I. 1987. Questions in digenean systematics and evolution. Parasitology 95, 429-460.
- Gibson, D.I. and Chinabut, S. 1984. Rohdella siamensis gen. et sp. nov. (Aspidogastridae: Rohdellinae subfam. nov.) from freshwater fishes in Thailand, with a reorganization of the classification of the subclass Aspidogastrea. Parasitology 88, 383-393.
- Littlewood, D.T.J., Rohde, K., Bray, R.A. and Herniou, E.A. 1999. Phylogeny of the Platyhelminthes and the evolution of parasitism. Biological Journal of the Linnean Society , 68, 257-287.
- Littlewood, D.T.J., Rohde, K. and Clough, K.A. 1999. The interrelationships of all major groups of Platyhelminthes – phylogenetic evidence from morphology and molecules. Biological Journal of the Linnean Society, 66, 75-114.
- Litvaitis, M.K. and Rohde, K. 1999. A molecular test of platyhelminth phylogeny: inferences from partial 28S rDNA sequences. Invertebrate Biology, 118, 42-56.
- Ehlers, U. 1985. Das phylogenetische System der Plathelminthes. Gustav Fischer Verlag, Stuttgart.
- Rohde, K.1997. The origins of parasitism in the Platyhelminthes: a summary interpreted on the basis of recent literature. International Journal for Parasitology, 27, 739-746. 630.
Related Knols
See also the following knols
Parasitism
Aspidogastrea II
Aspidogastrea III
Meeresparasiten
Amphilinidea: http://knol.google.com/k/klaus-rohde/the-amphilinidea-a-small-group-of/xk923bc3gp4/21#
and the Tree of Life webpage:
Tree of Life page on the Aspidogastrea.







Anonymous
Knols at its best! — The three knols about Aspidogastrea are an intricately interwoven but skillfully subdivided body of information about the small sister group of the much larger Digenea – about 80 versus many thousand species – gathered in extensive studies performed at many levels including transmission microscopy, scanning electron microscopy, molecular biology and experimental techniques. A profoundly known, markedly different and comparatively small taxon at a high taxonomic level like this is per se highly welcome as a model group for parasitological studies, taking into account that the Digenea (forming together with the Aspidogastrea the Trematoda) are only one group, albeit one of the largest groups of the helminths comprising tens of thousands of parasitic worms in need of parasitological study. But if on top of it theoretical conclusions of general importance have been drawn out of the few species comprising the group – as is the case here with the Aspidogastrea – the wide-spread interest is not restricted to specialists, to morphology and taxonomy, and will surely be lasting. The text contains many figures and is highly readable. The writer, author and editor of books on parasitology, is one of the major contributors of this group. Here he does its best to assist students and readers of related fields of study by traducing or describing terms in parentheses, for instance vas deferens (sperm duct), metacercariae (= encapsulated cercariae.) In short, this interesting and important group of parasites got an adequate and at the same time attractive treatment. Knols at its best. (Josef Alvermann, Baden-Baden)