Sacculinisation
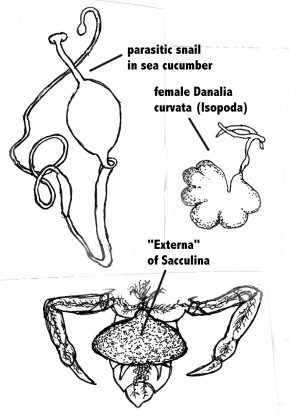
In discussing the question of directedness in evolution, The Nobel-prize winner Konrad Lorenz, one of the founders of the modern study of animal behaviour (ethology), pointed out that “from each already achieved stage of development evolution can go on in any direction whatever, blindly responding to every new selection pressure that turns up” [1]. He illustrated this by the process of retrograde evolution in certain parasites, a process for which he coined the term “sacculinisation”. The term is derived from the parasitic crustacean Sacculina, which has typical free-living crustacean larvae with complex articulated appendages like for example crayfish. After infecting crabs, it matures and all the appendages disappear. What is left is a sac-like appendage (the “Externa”) on the ventral side of the crab’s abdomen which contains the gonads, and a cancer-like growth in the interior of the crab which absorbs nutrients from the host (Fig .1). This “degenerate” evolution is common among crustaceans and certain snails. For example, another crustacean, the isopod Danalia curvata, a hyperparasite (i.e., a parasite of a parasite) of Sacculina, is also reduced to a sac-like appendage (Fig.1). Among snails, certain species have become endoparasitic in sea cucumbers; they have a worm-like appearance with no resemblance with typical snails (Fig.1).
Figure 1. A snail parasitic in a sea cucumber; the isopod Danalia curvata hyperparasitic on the parasitic crustacean Sacculina; and the “Externa” of the crustacean Sacculina parasitic on crabs. Note the cancer-like outgrowths of the Externa in the tissues of the crab. Only the most posterior parts of the crab are illustrated. Original Klaus Rohde. © Klaus Rohde
The Aspidogastrea. Increase in Complexity
Many people believe that parasites are indeed always in some way degenerate. However, parasitism does not always lead to reduction in complexity, on the contrary, it may have the opposite effect, as shown by the Aspidogastrea. Extensive light- and electron-microscopic studies of several species of Aspidogastrea, in particular Lobatostoma manteri and Multicotyle purvisi have demonstrated an amazing variety of sensory receptors, some of them occurring in very large numbers.This variety of receptors is as great or greater, and their number is considerably greater than in related free-living flatworms, the turbellarians. Likewise, the nervous system of the Aspidogastrea examined shows greater complexity than that of turbellarians.
The Juvenile/Adult, Sensory Receptors
Two species of Aspidogastrea in particular, Multicotyle purvisi from Malayan turtles, and Lobatostoma manteri from Australian marine fish, were examined using light microscopy, as well as scanning and electron-microscopy. It is important to understand that juveniles of Aspidogastrea from the intermediate host infective to the final host differ little from adults; either stage will therefore give identical results. Rohde (1966), based on the examination under the light microscope of serial sections impregnated with silver, drew attention to the great variety of sensory receptors and their great numbers in Multicotyle purvisi [2][3][4]. Subsequently, numerous studies also using scanning and transmission electron-microscopy, confirmed this not only for Multicotyle, but for Lobatostoma as well.
Scanning electron-microscopy
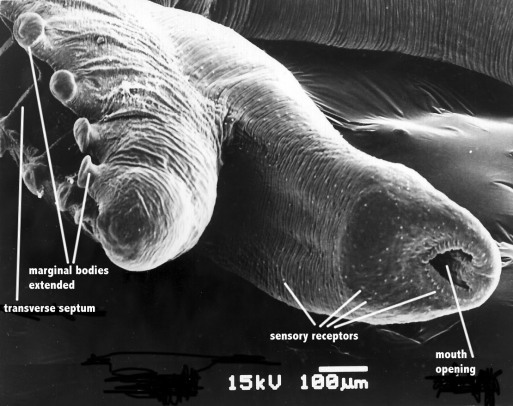
This technique shows only surface receptors (Fig.2). Rohde (1973) [5] counted the surface receptors on scanning electron-micrographs of one specimen of Lobatostoma manteri supplemented by counts of another specimen impregnated with silver, and reported numbers as follows: in each anterior marginal alveolus 35, in each marginal alveolus in middle of body 50 (total in all 60 marginal alveoli 2700, in all 29 median alveoli 870); in a marginal row of papillae just dorsal to the alveoli 780; on dorsal part of body 1200, along ventral margins of ventral head lobes 1600, on anterior side of median dorsal head lobe 140, on anterior side of ventral head lobes 300, on anterior sides of lateral dorsal head lobes 300, on posterior side of dorsal head lobes 200, on posterior side of ventral head lobe 150, on neck 200. The overall total is 8440. However, it must be stressed that subsurface receptors form only a small proportion of receptors, the total number of receptors therefore is far greater. Considering this, Rohde (1989) [6] [7] estimated that a fully grown worm of this species (4 mm length, unpressed) has a total of 20000-40000 receptors, which appears to be extraordinary for a worm of such a small size.
Figure 2. Scanning electron-micrograph of anterior end of Multicalyx elegans. Original Klaus Rohde. © Klaus Rohde
Transmission electron-microscopy
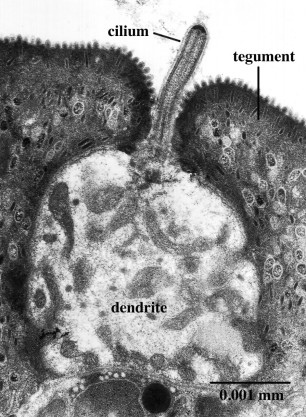
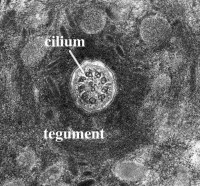
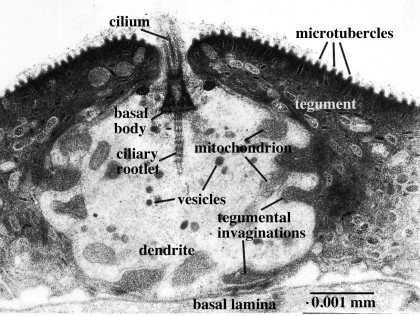
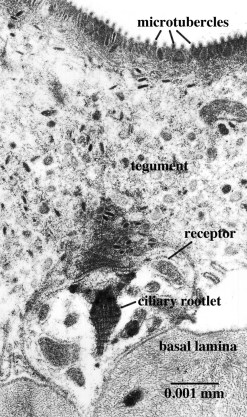
Transmission electron-microscopy is not restricted to the surface, but can be used to examine interior structures as well. Comparison of serial ultrathin sections has shown that juvenile/adult Lobatostoma manteri has at least 8 and possibly up to 14 types of receptors [6], distinguished by the presence or absence of a cilium and its length, by the absence or presence of ciliary rootlets and their shape, by the number of axonemal microtubules in the axoneme of the cilium, and whether they are part of a complex organ or not. Juvenile/adult Multicotyle has 7 and possibly up to 9 types. We illustrate a few major types of L. manteri in the following by single sections. However, for distinguishing different receptor types, in all cases serial sections were used. All receptors are differentiated endings of dendrites (nerve fibres), usually with ciliary structures within them. For example, the receptor illustrated in Fig. 3 has a short cilium at the end of the dendrite, which is embedded in the surface layer of the worm’s surface layer, its tegument. Typically, cilia have a 9+2 structure of the axoneme, i.e., it contains nine pairs (doublets) of microtubules in the periphery and two single microtubules in the centre (Fig.4), but there may be deviations from this pattern. Fig.5, a section through a similar receptor but through a slightly different plane, reveals structures not seen in Fig.3, such as a basal body at the base of the cilium, and a ciliary rootlet.
Figure 3. Ciliated surface receptor of Lobatostoma manteri. A single cilium arises from the terminal dendritic swelling. Original Klaus Rohde. © Klaus Rohde
Figure 4. Cross-section through cilium of sensory receptor. Note the 9+2 structure (nine doublets of microtubules in the periphery, two single microtubules in the centre). Original Klaus Rohde. © Klaus Rohde
Figure 5. Sensory surface receptor of Lobatostoma manteri. Note the cilium with a basal body and ciliary rootlet, mitochondria and vesicles, and deep invaginations of the tegument into the dendrite. Original Klaus Rohde. © Klaus Rohde
In another receptor type, a free cilium is lacking and the ciliary rootlet is expanded forming a disk-like structure (Fig. 6).
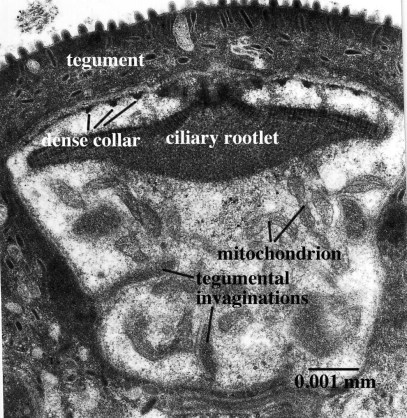
Figure 6. Receptor of Lobatostoma manteri without a free cilium, but with an expanded ciliary rootlet. Note also the sections through the dense collars around the upper part of the dendrite. Original Klaus Rohde. © Klaus Rohde
Figure 7. Subsurface receptor of Lobatostoma manteri. Note the well developed ciliary rootlet. Serial sections reveal that there is no cilium extending to and through the surface. Original Klaus Rohde. © Klaus Rohde
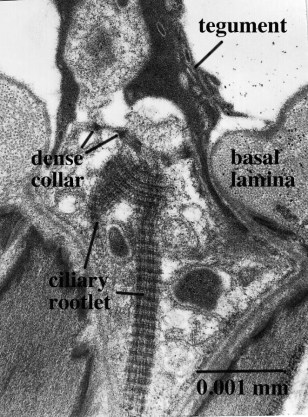
Figure 8. Non-ciliate receptor of Lobatostoma manteri located in a deep pit, with branching ciliary rootlet. Original Klaus Rohde. © Klaus Rohde
Electron-microscopic studies of Multicotyle purvisi [8] have shown the following receptor types, in many respects similar to those of Lobatostoma, but differing in some aspects:
1) disk-like receptor with many dense collars and a modified ciliary rootlet forming a disk;
2) non-ciliate receptor with long rootlet;
3) non-ciliate receptor with branching rootlet and dense mass of irregularly arranged microtubules;
4) non-ciliate receptor with rootlet fanning out from basal body, cross-striated in its upper and with electron-dense structures in its lower part;
5) uniciliate receptor with thick layer of cytoplasm around its axoneme;
6) receptor with short cilium, at base of deep invagination of tegument;
7) receptor with short cilium terminating in an electron-denser apical cap;
8) uniciliate receptor with long cilium.
In addition, there may be a small non-ciliate receptor with a long ciliary rootlet at the base of the thick dorsal tegument, and uniciliate receptors differing from the uniciliate receptor with a long cilium in the number of dense collars and the length of the cilium and ciliary rootlet.
The Juvenile/Adult, Nervous System
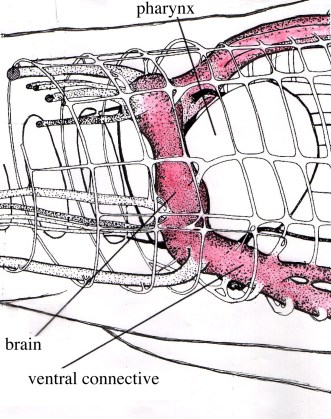
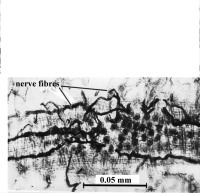
The nervous system of larval and adult Multicotyle purvisi was reconstructed in detail using serial sections impregnated with silver, supplemented by sections stained with various other stains, among them some specific for neurosecretion ([9][10][11] review in [4]). In most Platyhelminthes, the nervous system consists of longitudinal nerves (connectives) connected by transverse nerves (commissures); the dorsal part of one of the most anterior commissures is often particularly well developed, forming the cerebral commissure or brain. In Multicotyle, the number of anterior connectives is much greater than in any other species of the many turbellarians that have been examined, and there are two rings of commissures, one close to the tegument, the other deeper in the tissue. The dorsal part of an interior commissure just anterior to the pharynx is very large, forming the brain (Fig. 9). More posteriorly, the nerves form a typical system of connectives and commissures (with one pair of dorsal, one pair of lateral and one pair of ventral connectives), as well as a complex pattern innervating the ventral (adhesive) disk (Fig.10).
Figure 9. Nervous system of Multicotyle purvisi in the anterior part of the body. Some nerves on the right have been cut in order to show the arrangement of the nerves in cross-section. Note the two rings of commissures, and the very large brain (cerebral commissure) and main ventral connective (red). Original Klaus Rohde. © Klaus Rohde

Figure 10. Nervous system of Multicotyle purvisi in the middle part of the body, showing a typical arrangement of connectives and commissures in the dorsal part of the body, and an intricate pattern of nerves innervating the ventral (adhesive) disk. Original Klaus Rohde. © Klaus Rohde
Interestingly, a dense network of nerve fibres (nerve plexus) innervates the intestine (Fig.11), and also the connective tissue septum separating the dorsal part of the body from the ventral disk.
Figure 11. Nerve plexus around the intestine of Multicotyle purvisi. Section impregnated with silver as seen under the light-microscope. Original Klaus Rohde. © Klaus Rohde
Transmission electron-microscopy of the nerves of Multicotyle purvisi revealed the presence of a nerve sheath around parts of a posterior connectiv[12], a structure not known from other flatworms.
The Larva, Sensory Receptors and Nervous system
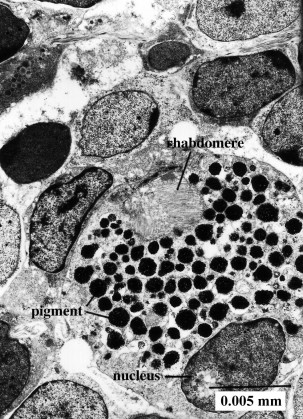
Sensory receptors of larvae of Multicotyle purvisi and Lobatostoma manteri were examined in several papers ([13][14][15][16] [17] reviews in [18][19]). In the former species, altogether 13 receptor types were found, including a paired eye and a paired receptor complex dorsal to the mouth cavity, each complex consisting of two dendrites, one forming a large liquid filled cavity with at least 10 short cilia lacking ciliary rootlets but possessing basal bodies and lamellate extensions of the ciliary membrane, the other penetrating the anterior wall of the cavity formed by the first dendrite and possessing a single cilium, star-shaped in cross section. Each eye (ocellus) consists of one pigment cell and two receptor cells with rhabdomeres (the light-sensitive dendritic endings, Fig.12).
Figure 12. Transmission electron-micrograph of ocellus (eye) of larval Multicotyle purvisi. Note pigment cell with pigment granules, nucleus of pigment cell, and rhabdomere (light-sensitive dendritic endings). Original Klaus Rohde. © Klaus Rohde
The larva of Lobatostoma manteri has only about nine types of receptors: eyes are lacking, and anterior receptor complexes were not found, either. The difference between the two larvae can be explained by the way of how they infect the intermediate host. The latter species does not hatch, it is ingested by a snail, the former hatches, swims in water, is attracted to the surface layer by light stimuli, and is then inhaled by a snail host.
The nervous system of larval Multicotyle was reconstructed using serial sections impregnated with silver. It shows the basic pattern also found in the adult, with nerves innervating the pharynx, intestine and posterior sucker, and a large number of anterior connectives [11].
The Complexity of Receptors and Nervous Systems in Free-living Flatworms
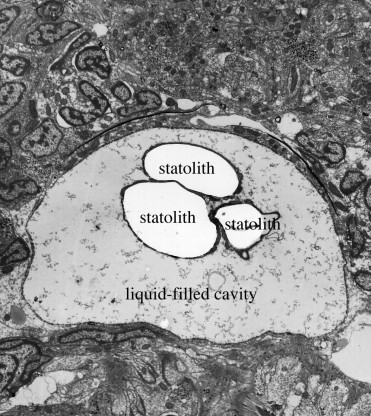
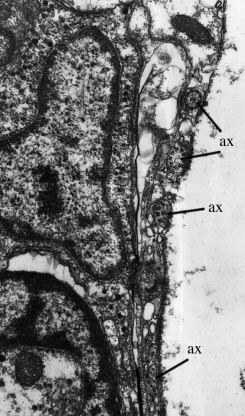
A considerable number of papers deal with the ultrastructure of various receptors in free-living turbellarians, but few authors have attempted to establish the complete “sensogram” (the complete spectrum of sensory receptors) of particular species. Examined in this respect were Luriculus australiensis, a small turbellarian from the meiofauna of Australian beaches [20], and Macrostomum cf. bulbostylum from freshwater [21]. An acoelan species examined by Smith and Tyler (1986) [22] was found to have nine types of receptors, but the Acoelomorpha are now considered to be a phylum of its own, i.e., they are not flatworms. Luriculus australiensis (length about 0.15mm) has nine types of uniciliate receptors; their number was not determined, but is certainly much smaller than that in the aspidogastreans. Macrostomum (length about 2 mm) has nine types of receptors including a photoreceptor (eye). The total number of receptors was estimated to “exceed 300”, i.e., it is far smaller than that of Lobatostoma (many 1000’s). The statocyst (a balancing organ) (Figs.13 and 14) [23] characteristic of Luriculus was not examined in [20]. It raises the number of receptor types in that species to ten. – The nervous system of the many free-living flatworms examined is not more but less complex than that of Multicotyle purvisi.
Figure 13. Statocyst of Luriculus australiensis, as seen under the transmission electron-microscope. Note the three large calcareous bodies (statoliths) in the liquid-filled cavity. Original Klaus Rohde. © Klaus Rohde
Figure 14. Transmission electron-micrograph of the statocyst wall of Luriculus australiensis. Note the axonemes (ax) which apparently are the sensory elements in the statocyst. Original Klaus Rohde. © Klaus Rohde
Conclusion
The evidence presented here clearly shows that the diversity of receptor types of some Aspidogastrea is at least as great and possibly greater than in free-living flatworms; the total number of receptors is far greater, only partly explained by the larger body size of the aspidogastreans examined, and the complexity of the nervous system is greater. Thus, not sacculinisation due to parasitism, but the opposite effect is apparent. Possible reasons are discussed in the third knol on the group: The Aspidogastrea: a Parasitological Model III.
References
- Lorenz, K. 1987. The Waning of Humaneness. Unwin Hyman, London, Sydney, Wellington. (German original 1983).
- Rohde, K. 1966. Sense receptors of Multicotyle purvisi Dawes (Trematoda, Aspidobothria). Nature, 211, 820-822.
- Rohde, K. 1968. Lichtmikroskopische Untersuchungen an den Sinnesrezeptoren der Trematoden. Zeitschrift für Parasitenkunde 30, 252-277.
- Rohde, K. 1972. The Aspidogastrea, especially Multicotyle purvisi Dawes, 1941. Advances in Parasitology, 10, 77-151.
- Rohde, K. 1973. Structure and development of Lobatostoma manteri sp. nov. (Trematoda, Aspidogastrea) from the Great Barrier Reef, Australia. Parasitology, 66, 63-83.
- Rohde, K. 1989. At least eight types of sense receptors in an endoparasitic flatworm: a counter-trend to sacculinization. Naturwissenschaften, 76, 383-385.
- Rohde, K. and Watson, N. 1989. Sense receptors in Lobatostoma manteri (Trematoda, Aspidogastrea). International Journal for Parasitology, 19, 847-858.
- Rohde, K. 1990. Ultrastructure of the sense receptors of adult Multicotyle purvisi (Trematoda, Aspidogastrea). Zoologica Scripta, 19, 233-241.
- Rohde, K. 1968. Vergleichende Untersuchungen über das Nervensystem der Trematoden (Digenea, Aspidogastrea, Monogenea). Zeitschrift für Parasitenkunde, 31, 12-13.
- Rohde, K. 1968. The nervous systems of Multicotyle purvisi Dawes, 1941 (Aspidogastrea) and Diaschistorchis multitesticularis Rohde, 1962 (Digenea). Implications for the ecology of the parasites. Zeitschrift für Parasitenkunde, 30, 78-94.
- Rohde, K. 1971. Untersuchungen an Multicotyle purvisi Dawes, 1941 (Trematoda, Aspidogastrea). III. Licht- und elektronenmikroskopischer Bau des Nervensystems. Zoologische Jahrbücher, Abteilung für Anatomie, 88, 320-363.
- Rohde, K. 1970. Nerve sheath in Multicotyle purvisi Dawes. Naturwissenschaften, 57, 502-503.
- Rohde, K. and Watson, N. 1990. Non-ciliate sensory receptors of larval Multicotyle purvisi (Trematoda, Aspidogastrea). Parasitology Research, 76, 585-590.
- Rohde, K. and Watson, N. 1990. Uniciliate sensory receptors of larval Multicotyle purvisi (Trematoda, Aspidogastrea). Parasitology Research, 76, 591-596.
- Rohde, K. and Watson. 1990. Paired multiciliate receptor complexes in larval Multicotyle purvisi (Trematoda, Aspidogastrea). Parasitology Research, 76, 597-601.
- Rohde, K. and Watson, N.A. 1992. Sense receptors of larval Lobatostoma manteri (Trematoda, Aspidogastrea). International Journal for Parasitology, 22, 35-42.
- Rohde, K., and Watson, N. 1991. Ultrastructure of pigmented photoreceptor of larval Multicotyle purvisi (Trematoda, Aspidogastrea). Parasitology Research, 77, 485-490.
- Rohde, K. 1994. The minor groups of parasitic Platyhelminthes. Advances in Parasitology, 33, 145-234.
- Rohde, K. 1999. Aspidogastrea, In Tree of Life (eds. Maddison, D.R. and Maddison, W.P.).http://tolweb.org/tree?group=Aspidogastrea&contgroup=Platyhelminthes
- Rohde, K. and Watson, N.A. 1993. Ultrastructure of sensory receptors of an undescribed species of Luridae (Platyhelminthes: Rhabdocoela). Australian Journal of Zoology, 41, 53-65.
- Xylander, W.E.R., Rohde K .and Watson, N.A. 1997. Ultrastructural investigations of the sensory receptors of Macrostomum cf. bulbostylum (Platyhelminthes, Macrostomida). Zoologischer Anzeiger, 236, 1-12.
- Smith, J.P.S. and Tyler, S. 1986. Frontal organs in the Acoelomorpha (Turbellaria): ultrastructure and phylogenetic significance. Hydrobiologia 132, 71-78.
- Rohde, K., Watson, N.A. and Faubel, A. 1993. Ultrastructure of the statocyst in an undescribed species of Luridae (Platyhelminthes: Rhabdocoela: Luridae) Australian Journal of Zoology, 41, 215-224.
Note
All figures are originals by the author. Further details, in particular reconstructions (diagrams) of the various receptor types and the nervous system, can be found on my Tree of Life page on the Aspidogastrea.